Introduction
The Hottentot Buttonquail Turnix hottentottus is one of 18 species of Turnicidae; a group of cryptic, small, terrestrial birds (Debus and Bonan Reference Debus, Bonan, del Hoyo, Elliott, Sargatal, Christie and de Juana2016). Hottentot Buttonquail is considered to be endemic to the fynbos biome of South Africa (Taylor et al. Reference Taylor, Peacock and Wanless2015), which is a fire driven Mediterranean-type ecosystem (Cowling et al. Reference Cowling, Richardson, Mustart, Cowling, Richardson and Pierce1997). It is the only Turnix species reported from the fynbos.
Remarkably little is known about the Hottentot Buttonquail. It is, like other buttonquails, assumed to be polyandrous (Dean Reference Dean, Hockey, Dean and Ryan2005). There is distinct sexual dimorphism, with males generally drab, but females with contrasting white belly and chestnut, reddish brown chest and face (Arizaga et al. Reference Arizaga, Deán, Vilches, Alonso and Mendiburu2011). Little is known about its breeding ecology, with published notes referring to Hottentot Buttonquail actually referring to Black-rumped Buttonquail T. nanus (e.g. Masterson Reference Masterson1973).
Taxonomically the species was considered conspecific with the Black-rumped Buttonquail (Dowsett and Dowsett-Lemaire Reference Dowsett and Dowsett-Lemaire1993), while Sibley and Monroe (Reference Sibley and Monroe1990) suggested the two taxa were separate species. The latter taxonomic treatment is supported by their allopatric ranges as well as differences in habitat preference and plumage (Dean Reference Dean, Hockey, Dean and Ryan2005) and currently accepted by BirdLife International (2016) as of 2014, BirdLife South Africa (Lotz 2014) and the International Ornithologists’ Union (Gill and Donsker Reference Gill and Donsker2014).
From the conservation perspective, the species has variously been described as: ‘on the brink of extinction’ (Brooke Reference Brooke1984); ‘possibly extinct’ (Debus Reference Debus, Del Hoyo, Elliott and Sargatal1996); ‘possibly critically endangered’ from c.2010–2013 (Lotz Reference Lotz2013). At the same time it was classified as ‘Least Concern’ globally while lumped with T. nanus (BirdLife International 2004); and as of 2014 ‘Endangered’ both globally (BirdLife International 2016) and nationally (Taylor et al. Reference Taylor, Peacock and Wanless2015). The most recent listings were partly based on an extrapolation by Lee (Reference Lee2013) of a density estimate obtained from point counts provided by Fraser (Reference Fraser1990) to a possible global population of 400 individuals. By contrast, a survey in 1994 by Ryan and Hockey (Reference Ryan and Hockey1995) on the Cape Peninsula suggested that area alone may hold 350 (100–560) birds, making it one of the most common bird species in restionaceous fynbos. However, the sparsity of records from the ongoing South African Bird Atlas Project (SABAP2) was highlighted by Lee (Reference Lee2013). Thus, informed decisions requiring population size, population trend and range, on the conservation status and for species management purposes, have been hampered by a general lack of knowledge of this species.
We conducted the first biome-wide survey of the Hottentot Buttonquail in order to estimate population size and range. We used occurrence records to conduct modelling using the maximum entropy or MaxEnt method (Phillips et al. Reference Phillips, Anderson and Schapire2006) to identify the species’ potential range. We used this model to identify climate and habitat variables that limit distribution. We then used encounter rates from our surveys to estimate density and modelled range to extrapolate population estimates.
Methods
Study area
The fynbos biome (fynbos; roughly synonymous with the Cape Floral Kingdom or Cape Floristic Region) comprises one of only six floral kingdoms in the world and is contained entirely within the political boundaries of South Africa (Cowling Reference Cowling1995). It is mostly restricted to the Western and Eastern Cape provinces in the Cape Fold Mountains (Figure 1). Owing to its exceptional plant species richness and high level of endemism, as well as high levels of animal diversity and endemism, it is recognised as one of the world’s 25 biodiversity ‘hotspots’ (Myers et al. Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000). Vegetation is dominated by three characteristic families: Proteaceae, Ericaceae and Restionaceae. The region experiences significant winter rainfall, although summer rainfall can predominate in the eastern regions (Cowling et al. Reference Cowling, Richardson, Mustart, Cowling, Richardson and Pierce1997). The fynbos biome is a fire-driven ecosystem, with most plant species adapted to an intermittent fire return interval of 6–40 years (Cowling Reference Cowling1992). Conversion to agriculture, urbanisation and the invasion of a variety of alien plant types pose major conservation threats to environmental integrity of the area (Rebelo and Siegfried Reference Rebelo and Siegfried1990).

Figure 1. Map of the study area in South Africa indicating biome types (Mucina and Rutherford Reference Mucina and Rutherford2006). Locations of Hottentot Buttonquail T. hottentottus encounters are indicated.
Surveys to determine presence and abundance of Hottentot Buttonquail
In order to determine the presence and abundance of Hottentot Buttonquail we conducted 131 ‘flush’ surveys across the fynbos biome from October 2015 to February 2016, with 275 km of survey lines covering a combined sample area of 802 ha. The survey period was planned to coincide with the breeding season, as birds have been reported to call during this period (Tarboton Reference Tarboton2011, Lee Reference Lee2013). The flush survey was a multiple-observer survey with observers walking in a line spaced ideally no more than 5 m apart. The length of the survey line was noted, and area calculated as the number of observers x 5 x length. Median transect length was 1.8 km (0.2–12.3 km). Due to a limited budget, we recruited participants on an opportunistic basis, and so teams ranged in size from two to 12. At least one of the authors was involved in all surveys to confirm species identification. We calculated density (individuals/ha) as the subset of all individuals observed within the transect line.
Range estimation through species distribution modelling using MaxEnt
We created a presence-only dataset based on our encounters as well as additional sighting information. A national public call for sightings through various media outlets yielded no responses. In addition to survey locations, we obtained 23 locations from previous fieldwork encounters and personal contacts, for a total of 61 presence locations. In order to address spatial autocorrelation, we randomly subsampled the data so that the distance between points was at least 1,000 m. This left 40 locations for model fitting and testing.
We examined the Worldclim database (Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2005) of 19 climatic variables, and excluded the derived variables with correlation coefficients of r > 0.5 for the area encompassing our modelling domain of the fynbos biome (Mucina and Rutherford Reference Mucina and Rutherford2006; approximately S30.4° to S34.7° and E17.8° to E26.5°; Figure 1), thus retaining the following variables: Bio 1 (Annual Mean Temperature), Bio 2 (Mean Diurnal Range), Bio 3 (Isothermality), Bio 4 (Temperature Seasonality), Bio 8 (Mean Temperature of Wettest Quarter), Bio 15 (Precipitation Seasonality) and Bio 18 (Precipitation of Warmest Quarter). In addition we derived slope from a digital elevation model that was created using 20 m contours and spot heights. We used the MODIS Collection 6 burned area product (MCD64A1; Giglio, et al. 2015) to determine vegetation age (years since last fire), with years since fire > 15 grouped into a single category. Habitat transformation was obtained from a reclassification of the 2014 National Landcover Layer (GTI South Africa 2015; https://egis.environment.gov.za/national_land_cover_data_sa) in which we defined landcover categories 1–9 as intact habitat and categories 10–72 as transformed habitat (Table S1 in the online supplementary material). All variables were prepared for input into MaxEnt by converting them to ASCII rasters with a 1,000 m resolution using the “Extract by Mask” tool in the Spatial Analyst extension for ESRI ArcMap 10.0.
We ran the models in MaxEnt version 3.3.3k using logistic output format. Twenty percent of the observations were set aside by means of random subsampling for model testing. The default “auto features” function was selected, which enabled the algorithm to select between linear, quadratic, product, threshold, and hinge features in fitting the models. The jackknife routine was used to measure variable importance and response curves were generated to show how each environmental variable influenced the prediction, with 100 replicates and the number of iterations for model convergence set to 500. The minimum, median and maximum distributions of the 100 replicate models were used for range calculations. We selected 5% as the value for parameter E (sensu Peterson et al. Reference Peterson, Papeş and Soberón2008), which is a threshold based on the amount omission error permissible. This parameter is determined based on the error characteristics of the occurrence data. We selected a relatively low value because our data consisted of GPS located field observations and we were confident of species identifications. We used the threshold calculator tool in the NicheA software package (Qiao et al. Reference Qiao, Peterson, Campbell, Soberón, Ji and Escobar2016) to determine the logistic threshold value at E = 5 to make binary predictions on habitat suitability. We also used this software package to construct partial Receiver Operating Characteristic curves (P-ROC) and to analyse mean AUC values for training and test data. In order to assess the one-tailed significance difference in the AUC from null expectations, we fitted a standard normal variate (z-statistic) and calculated the probability that the mean AUC ratio (model to null expectation) is ≤ 1.
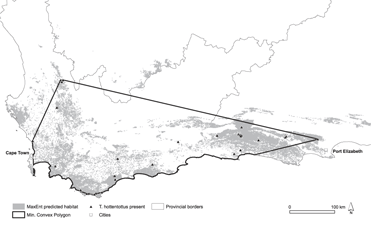
Estimates of population size were calculated as the product of the densities obtained from the surveys and the remaining habitat. We also determined extent of occurrence (EOO) for the species by creating a minimum convex polygon (MCP) around the buttonquail occurrence data using the “genmcp” command in Geospatial Modelling Environment (Beyer, 2012). Oceanic areas were clipped from this polygon.
Results
Abundance from flush surveys
During surveys across the fynbos we obtained 37 encounters with Hottentot Buttonquail, consisting of 31 individuals and six cases of two birds. The resulting density estimate was 0.032 ± 0.13 individuals per ha. Most birds were flushed from close proximity to observers (3.7 ± 4.4 m; n =31), and although the birds are sexually dimorphic, we were mostly unable to determine the sex of birds in flight.
Range estimation through species distribution modelling using MaxEnt
The range predicted by the models was 10,377–41,303 km2 (median 27,855 km2). The MaxEnt models produced a good fit to the training data as evidenced by the Receiver Operating Characteristic (ROC) with a mean fixed P-ROC AUC of 0.99 ± 0.004 (SD). The recorded mean AUC ratio was 1.49 ± 0.05 (range 1.38–1.71), and the probability of the mean AUC ratio being ≤ 1 was very small (P = 3.2 × 10-14). This was validated by test data, which had a mean AUC ratio of 1.47 ± 0.19 (range 1.15–1.95) and a probability of being better than a random model of P = 0.005. The logistic output maps are provided as Figures S1a–c in the supplementary information. Using the density estimate from flush surveys and our estimates of remaining suitable habitat, we obtained a median estimate of the population size of 89,136 individuals (range 33,206–132,169). The MCP representing EOO generated from buttonquail localities had a terrestrial extent of 79,886 km2 (see Figure 2).

Figure 2. Species distribution modelling map for Hottentot Buttonquail indicating suitable habitat from MaxEnt modelling using 5% training presence as a threshold (grey).
The variables that contributed the most to the distribution model were habitat transformation (27.4% permutation importance), followed by slope (16.1%), vegetation age (14.8%) and Bio 2 (Mean Diurnal Temperature Range; 17.1%). In the jackknife analysis Bio 2 and vegetation age produced both the highest training and test gain when used in isolation (Figure S2).
The model indicated buttonquail were associated with untransformed vegetation, 2–5 years after it was burnt, and where mean daily temperature was cooler. Suitability also appears to decrease with slope, being highest for relatively flat areas. Response curves for each of the environmental variables are presented in Figure S3.
Discussion
Our study, which is the first biome-wide survey for the Hottentot Buttonquail, derived a population estimate of 89,136 individuals and a median range of 27,855 km2. It was noted that Hottentot Buttonquails flushed only within very close proximity to observers, which may mean individuals were missed during the survey and the population estimate may be low. Despite these limitations, we are confident that the population is > 2,500 mature individuals and that the range is > 5,000 km2, being current IUCN conservation classification thresholds for ‘Endangered’ status (IUCN 2012).
Conventional survey techniques are unsuited to finding Hottentot Buttonquail. For example, no buttonquails were recorded during a comprehensive point count survey of the fynbos biome conducted in 2012 (Lee et al. Reference Lee, Barnard and Hockey2015 supplementary information). Likewise, little reliable inference on populations can be made from citizen science atlas data (Lee et al. Reference Lee, Altwegg and Barnard2017): our survey revealed their presence at locations with very high atlas coverage. In order to adequately survey for the presence of Hottentot Buttonquail, and likely other open landscape Turnix species, flush survey lines of a minimum of five people are required to walk substantial distances to cover large enough areas (Lee et al. Reference Lee, Wright and Reeves2018).
Variables associated with the presence of Hottentot Buttonquail
Modelling suggested Hottentot Buttonquail presence was associated with time-since-fire veld of 2-5 years; and negatively associated with steep slopes, which is in agreement with Lee et al. (Reference Lee, Wright and Reeves2018), as well as areas experiencing large mean diurnal or annual temperature fluctuations. The presence of the species was negatively associated with transformed landscapes.
Our knowledge of vital aspects of the life history and biology of the Hottentot Buttonquail, as well as many other Turnix species, remains to be clarified. It is possible for instance that dietary habits may be specialised, further restricting range and site occupancy. Movement and migrations of this species, while suspected (Blackshaw and Blackshaw Reference Blackshaw and Blackshaw1998), remain to be confirmed and explained. Our model outputs should be seen as an initial understanding of the likely distribution of suitable habitat for this species.
Implications for the conservation, and conservation status of Hottentot Buttonquail
The results of this survey and species population and distribution modelling lead us to suggest an IUCN Red List categorisation of ‘Vulnerable’ for the Hottentot Buttonquail, a downlisting from the current IUCN category of ‘Endangered’ (Taylor et al. Reference Taylor, Peacock and Wanless2015). The estimated extent of occurrence and population size both exceed the criteria thresholds for ‘Endangered’ (IUCN Standards and Petitions Subcommittee 2017). Our classification is primarily due to the lower estimates of range being less than 20 000 km2, which is a used for ‘Vulnerable’ categorisation – criterion B1. In addition, the remaining subpopulations of the species are both severely fragmented and likely to be experiencing ongoing decline in extent and quality of habitat – criteria B1a and B1biii of the Vulnerable categorisation. Loss of habitat in the species’ population strongholds in the fynbos biome is occurring due to habitat transformation for agriculture and encroachment of alien invasive vegetation.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270918000059
Acknowledgements
This study would not have been possible without the support of CapeNature and the Eastern Cape Parks and Tourism Agency. Thanks to all 80 survey participants, especially Wim de Klerk, Andrew de Blocq, Brian Haslett, Selengemurun Dembereldagva, Krista Oswald, Tom Barry, Johan Huisamen, and Marius Brand. Special thanks to Anton Odendaal and Brian van der Walt for fundraising initiatives. We would like to thank Graeme Buchanan, A. Townsend Peterson and two anonymous reviewers for input into early versions of this manuscript. This project was made possible by the generous contributions from a range of donors and organisations which included Club 300 Bird Protection of Sweden, Biosphere Expeditions, Avian Leisure Group, local bird club organisations in South Africa - BirdLife Overberg, Tygerberg bird club, Lakes bird club and additional funds from BirdLife South Africa, Bruce Ward-Smith and Geoff Crane.