Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease of unknown aetiology, which affects approximately 0·1 % of the population with a large variation across regions and ethnicity. Its pathogenesis is a combination of genetic, environmental and hormonal factors that lead to loss of balance control of cellular immune regulation( Reference Helmick, Felson and Lawrence 1 ). To date, the majority of studies conducted to understand the pathophysiology of this condition have focused on the auto-reactive B and T lymphocytes( Reference Peng 2 ). However, recently, attention has shifted to the role of the innate immune system, and particularly myeloid cells, in this disease. Monocytes/macrophages are critical effectors and regulators of many organ systems, including systemic metabolism, haematopoiesis, angiogenesis and malignancy( Reference Geissmann, Manz and Jung 3 , Reference Tugal, Liao and Jain 4 ). Both monocytes and macrophages are phenotypically altered in SLE; for example, macrophages show reduced uptake of apoptotic cells and enhanced activator status, with altered skew to a pro-inflammatory direction and an over-production of inflammatory cytokines such as TNF-α, IL-6 and IL-1β ( Reference Kavai and Szegedi 5 – Reference Sestak, Fürnrohr and Harley 7 ).
Several SLE animal models have been established to investigate SLE disease mechanisms. Among them, pristane (2,6,10,14-tetramethylpentadecane)-SLE model in BALB/c mice is widely used to test potential therapeutic agents( Reference Satoh and Reeves 8 , Reference Satoh, Yamagata and Watanabe 9 ) as it appears to mimic human idiopathic SLE syndrome closer than spontaneous strains( Reference Shaheen, Satoh and Richards 10 ). However, despite intensive research, no therapy to date has been found to cure SLE, and current treatments try to control signs and symptoms preventing the damage caused by disease activity and drugs.
In recent years, considerable interest has been given to the ability of diet and different nutritional factors for improving several immune-inflammatory diseases( Reference De Rosa, Galgani and Santopaolo 11 ). There is broad evidence that dietary therapy can be helpful in the management of SLE symptoms owing to its prophylactic effects without the side effects of classical pharmacology, thus contributing to reduce co-morbidities and to improve health and quality of life of patients with SLE. Current studies emphasised the importance of a diet with plenty of vitamin-rich foods and adequate supply of MUFA/PUFA and dietary fibre, with sodium restriction and moderate energy consumption. The potential contribution of phenols included in the diet for the management of SLE is also noteworthy( Reference Aparicio-Soto, Sanchez-Hidalgo and Alarcon-de-la-Lastra 12 ).
The consumption of virgin olive oil (VOO), the major source of MUFA in the traditional Mediterranean diet, is associated with a reduced risk of various chronic inflammatory pathologies( Reference Buckland, Mayen and Agudo 13 , Reference Alarcon de la Lastra, Barranco and Motilva 14 ). Many of the preventive properties of VOO have been previously ascribed to its high content of oleic acid. However, it is now generally recognised that minor compounds of VOO, such as the phenol fraction (PF), have also a biological relevance. In this regard, the high concentration of PF in VOO may contribute in concert with oleic acid to the health benefits of the Mediterranean diet, showing anti-inflammatory, antioxidant and anti-proliferative activities, as well as the ability to modulate relevant cellular signalling pathways( Reference Cardeno, Sanchez-Hidalgo and Alarcon-de-la-Lastra 15 – Reference Sanchez-Fidalgo, Cardeno and Sanchez-Hidalgo 17 ). A diet containing VOO has been shown to be effective in the prevention of kidney damage in mice with pristane-induced SLE and of abnormalities in other mouse models of immune-inflammatory diseases, including rheumatoid arthritis and ulcerative colitis( Reference Aparicio-Soto, Sanchez-Hidalgo and Cardeno 18 – Reference Sanchez-Fidalgo, Villegas and Cardeno 20 ). Recent studies reported that PF from VOO exerts immune-regulatory activity in vitro in peripheral blood mononuclear cells (PBMC) from patients with SLE and in peritoneal macrophages from wild-type mice( Reference Cardeno, Sanchez-Hidalgo and Aparicio-Soto 16 , Reference Aparicio-Soto, Sanchez-Hidalgo and Cardeno 21 ). However, the potential in vivo effects of VOO on inflammation in SLE and in vitro effects of PF from VOO in modulating the functional state of the monocyte–macrophage lineage have not been fully investigated.
This study was designed to evaluate the impact of a diet containing VOO on the inflammatory response in peritoneal macrophages from mice with pristane-induced SLE and of PF from VOO on the immune-inflammatory activity and plasticity of human primary monocytes and monocyte-derived macrophages.
Methods
Animals and diets
A total of sixty 11- to 12-week-old female BALB/c mice (17 (sem 2) g) were obtained (Harlan Interfauna Iberica) and maintained in the Animal Laboratory Centre of University of Seville under standard conditions: temperature, 24–25°C; humidity, 70–75 %; and lighting regimen of 12 h light–12 h dark cycle. They were fed standard rodent chow (Panlab A04) and water ad libitum until pristane induction of SLE-like disease. Experimental diets were formulated on the basis of the American Institute of Nutrition standard reference diet with the modification of varying sources of carbohydrates and the principal source for fats (10 % sunflower oil or VOO) (online Supplementary Table S1).
Pristane-systemic lupus erythematosus model and mouse peritoneal macrophages
At 3 months of age, SLE was induced in half of the animals by means of an intraperitoneal injection of 0·5 ml of pristane (99 % pure; Sigma-Aldrich) according to the procedure described by Satoh & Reeves( Reference Satoh and Reeves 8 ). The other half of the animals were subjected to an intraperitoneal injection of saline solution.
Mice were then randomised into the following four experimental groups (fifteen animals per group): (1) animals injected with saline solution and fed a diet containing a marketable sunflower oil (Koipesol-Deoleo) (SOD); (2) animals treated with pristane and fed SOD; (3) animals injected with saline solution and fed a diet containing a marketable VOO (Olea europaea L., picual variety; Oleoestepa SAC) (VOOD); and (4) animals treated with pristane and fed VOOD. During the entire duration of the experiment, mortality, weight and water and food consumption were monitored weekly. The composition of experimental diets and their content of fatty acids, sterols, squalene, triterpenic alcohols and tocopherols is available in online Supplementary Table S2.
After 24 weeks of the experimental period, animals were euthanised by overdoses of pentobarbital. Peritoneal exudate cells (macrophages) were then harvested by washing the peritoneal cavity with sterile ice-cold PBS according to the protocol described by Aparicio-Soto et al.( Reference Aparicio-Soto, Alarcon-de-la-Lastra and Cardeno 22 ). In brief, after centrifugation, cells were resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with l-glutamine (2 mm), glucose (4·5 g/l), heat-inactivated fetal calf serum (FCS, 10 %), penicillin (100 U/ml), streptomycin (100 mg/ml) and HEPES (10 mm) (PAA Laboratories GmbH), and then seeded in culture plates (1×106 cells/ml) for 2 h at 37°C in a 5 % CO2 humidified atmosphere. Non-adherent cells were removed by washing with PBS, and fresh RPMI 1640 medium supplemented with FCS (5 %) was added to cultured peritoneal macrophages for further experimentation (see below).
All animal care and experimental procedures were performed according to a protocol approved by the Animal Ethics Committee of the University of Seville, and all experiments were in accordance with the recommendations of the European Union regarding animal experimentation (Directive of the European Counsel 2010/630/EU) and Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for reporting experiments involving animals( Reference Kilkenny, Browne and Cuthill 23 , Reference McGrath, Drummond and McLachlan 24 ).
Human monocytes and their differentiation into macrophages
Healthy volunteers were recruited at Virgen del Rocio University Hospital for peripheral blood collection and isolation of monocytes. The study conformed to the principles outlined in the Helsinki Declaration of the World Medical Association. Donors (adult <35 years old) were non-smokers and not taking any medication. They were recognised as healthy according to medical history and routine laboratory test. Blood samples were collected in K3EDTA-containing Vacutainer tubes (Becton Dickinson), and PBMC were isolated by centrifugation over Ficoll Histopaque gradient (Sigma-Aldrich). Monocytes were isolated from PBMC using positive selection with CD14 MicroBeads according to the manufacturer’s instructions (MACS; Myltenyi Biotec). The purity of monocytes was tested by CD14 fluorescein isothiocyanate labelling and fluorescence-activated cell sorting (FACS) analysis using a FACScanto II flow cytometer (BD Biosciences). After isolation, cells were suspended in RPMI 1640 medium supplemented with l-glutamine, penicillin, streptomycin and heat-inactivated FCS (10 %) at a density of 5×105 cells/ml.
Monocytes were induced to differentiate during 6 d in RPMI 1640 supplemented with l-glutamine, penicillin, streptomycin, heat-inactivated FCS (10 %) and recombinant human macrophage colony-stimulating factor (rhM-CSF, 25 ng/ml) to obtain naïve M0 macrophages. Every 2 d, fresh medium containing rhM-CSF was added. M0 macrophages were then exposed (for additional 24 h) to lipopolysaccharide (LPS) (100 ng/ml) and interferon (IFN)-γ (20 ng/ml) for M1 polarisation or to IL-4 (20 ng/ml) for M2 polarisation in the presence or absence of PF from VOO (25 and 50 µg/ml).
Extraction and chemical characterisation of phenol fraction from virgin olive oil
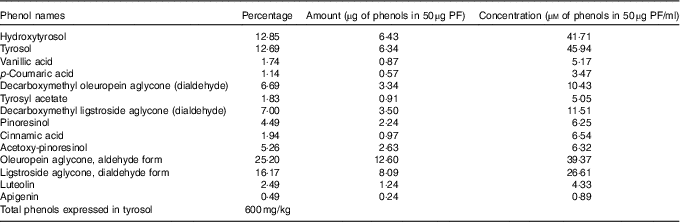
The same VOO used above for animal diets was used to extract PF according to the procedure described by Vazquez Roncero et al.( Reference Vazquez Roncero, Janet del Valle and Janet del Valle 26 ), with some modifications( Reference Cardeno, Sanchez-Hidalgo and Aparicio-Soto 16 ). Quantitative and qualitative analysis of PF was performed according to COI/T20/29doc (International Olive Council) by high-performance ternary gradient liquid chromatography. The content of total phenols was calculated by measuring the sum of the areas of the related chromatographic peaks and expressed in mg/kg of tyrosol. The composition of the isolated PF from VOO is detailed in Table 1.
Table 1 Composition of the phenol extract (PF) from virgin olive oil using COI/T20/29doc
Treatments with lipopolysaccharide
Mouse peritoneal macrophages were treated with 5 μg/ml LPS in the presence or absence of PF (25 or 50 µg/ml) diluted in dimethylsulphoxide for 18 h. In the case of human monocytes/macrophages, the concentration of LPS was 100 ng/ml and the incubation time was 24 h.
Measurement of nitrite production
Cell culture supernatants were transferred to a ninety-six-well assay plate and mixed with Griess reagent (Sigma-Aldrich). The amount of nitrite, as an index of NO generation, was determined by a spectrophotometric method according to the Griess reaction( Reference Vazquez Roncero, Janet del Valle and Janet del Valle 26 ) and by extrapolation from a standard curve with sodium nitrite as standard. The absorbance at 540 nm was measured by using a microtitre plate reader (BioTek).
Cytokine assay
The concentration of IL-1β, IL-6, IL-17 and TNF-α in cell culture supernatants was determined using appropriate commercial ELISA kits (Diaclone). Values were expressed in pg/ml, as calculated from calibration curves after serial dilutions of human recombinant standards for each assay. The intensity of each sample was read at 450 nm in a microtitre plate reader.
Cell viability assay
Cells seeded in ninety-six-well plates (1×105 cells/well) were incubated in the presence or absence of PF for 24 h. At the end of the exposure time, the effect on cell growth/viability was analysed by the mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to formazan (Sigma-Aldrich)( Reference Denizot and Lang 27 ). Cell survival was measured as the percentage of absorbance compared with that obtained in control, non-treated cells.
RNA isolation and real-time quantitative PCR analysis
Total RNA was extracted from cells by using TRIsure reagent (Bioline), as instructed by the manufacturer. RNA quality was assessed by A
260:A
280 ratio in a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific). RNA (1 µg) was subjected to reverse transcription (iScript; Bio-Rad) according to the manufacturers’ protocol. An amount of 20 ng of the resulting complementary DNA (cDNA) was used as a template for real-time PCR amplifications. The mRNA levels for specific genes were determined by real-time PCR in a MX3000P system (Stratagene). For each PCR reaction, cDNA template was added to Brilliant SYBR green QPCR Supermix (Bio-Rad) containing the primer pairs for either gene or for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 18S ribosomal (18S) as housekeeping genes. The sequence and additional information for the primers used are in online Supplementary Table S3. All amplification reactions were performed in triplicate, and average threshold cycle (C
t) numbers of the triplicates were used to calculate the relative mRNA expression of candidate genes. The magnitude of change of mRNA expression for candidate genes was calculated by using the standard
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
method. All data were normalised to endogenous reference (GAPDH and 18S) gene content and expressed as fold of controls.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
method. All data were normalised to endogenous reference (GAPDH and 18S) gene content and expressed as fold of controls.
Flow cytometry analysis
Surface membrane expression of CD14 (PE anti-human CD14; Miltenyi Biotec), CD68 (FITC anti-human CD68; Miltenyi Biotec), CD16 (APC-Cy7 anti-human CD16; Miltenyi Biotec) and C-C chemokine receptor type 2 (CCR2) (APC anti-human CCR2; BD Biosciences) on monocytes was assessed by FACS analysis. According to the manufacturer’s instructions, 5×105 purified monocytes after in vitro treatment in the presence or absence of LPS were incubated with antibodies at room temperature, in the dark, for 15 min, followed by fixation and lysing of erythrocytes with 20× volume of FACS lysing solution (BD Biosciences). Fluorescence intensity was measured by a FACScanto II flow cytometer and calibrated using CellQuest™ software (BD Biosciences). Results were analysed using the Win-List software package (Verity Software House). Mean fluorescence intensity (MFI) of 104 counted cells was measured in each sample. Monocytes were gated as forward scatterhigh (FSChigh)–side scatterhigh (SSChigh) cells. Expression levels were presented as MFI corrected for non-specific binding of isotype control antibodies.
Isolation and immunoblotting detection of proteins
Cells were rinsed, collected and processed as described by Sanchez-Hidalgo et al.( Reference Sanchez-Hidalgo, Martin and Villegas 28 ). Protein concentration was measured for each sample using a protein assay reagent (Bio-Rad) according to Bradford’s method using γ-globulin as a standard( Reference Bradford 29 ). Aliquots of supernatant containing equal amount of proteins (20 µg) were separated on 10 % acrylamide gel by SDS-PAGE, and the proteins were electrophoretically transferred into a nitrocellulose membrane and incubated with specific primary antibodies, such as polyclonal mouse anti-human PPARγ (Abcam), rabbit anti-inducible nitric oxide synthase (iNOS) (Cayman Chemical) (1:100 000) and monoclonal mouse anti-human β-actin (Sigma-Aldrich) antibodies, overnight at 4°C. After rinsing, the membranes were incubated with a horseradish peroxidase-labelled secondary antibody anti-rabbit (Cayman Chemical) (1:50 000) or anti-mouse (Dako) (1:2000) containing blocking solution for 1–2 h at room temperature. Immunodetection was performed using enhanced chemiluminescence light-detecting kit (Pierce). The signals were captured using an LAS-3000 Imaging System (Fujifilm), and densitometry data were studied following normalisation to the housekeeping loading control. The signals were analysed and quantified by Image Processing and Analysis in Java (Image J; Softonic) and expressed in relation to LPS-treated cells.
Statistical analysis
All values in the figures and text are expressed as arithmetic means with their standard errors. Experiments were carried out in triplicate. Data were evaluated with GraphPad Prism version 6.01 software. The statistical significance of any difference in each parameter among the groups was evaluated by one-way ANOVA using Tukey’s multiple comparisons test as post hoc test. P values <0·05 were considered statistically significant. In the experiments involving densitometry, figures are representative of at least three different experiments performed on different days.
Results
Effects of diet containing sunflower oil and virgin olive oil on nitrite production in peritoneal macrophages from pristane-systemic lupus erythematosus mice
As shown in Fig. 1(a), nitrite production was induced in peritoneal macrophages from pristane-SLE mice when fed SOD but not with VOOD. The in vitro treatment of peritoneal macrophages from vehicle control mice with LPS did not induce nitrite production; however, nitrite was produced in LPS-treated peritoneal macrophages from pristane-SLE mice, with this effect being significantly less pronounced in animals feeding on VOOD when compared with animals feeding on SOD.
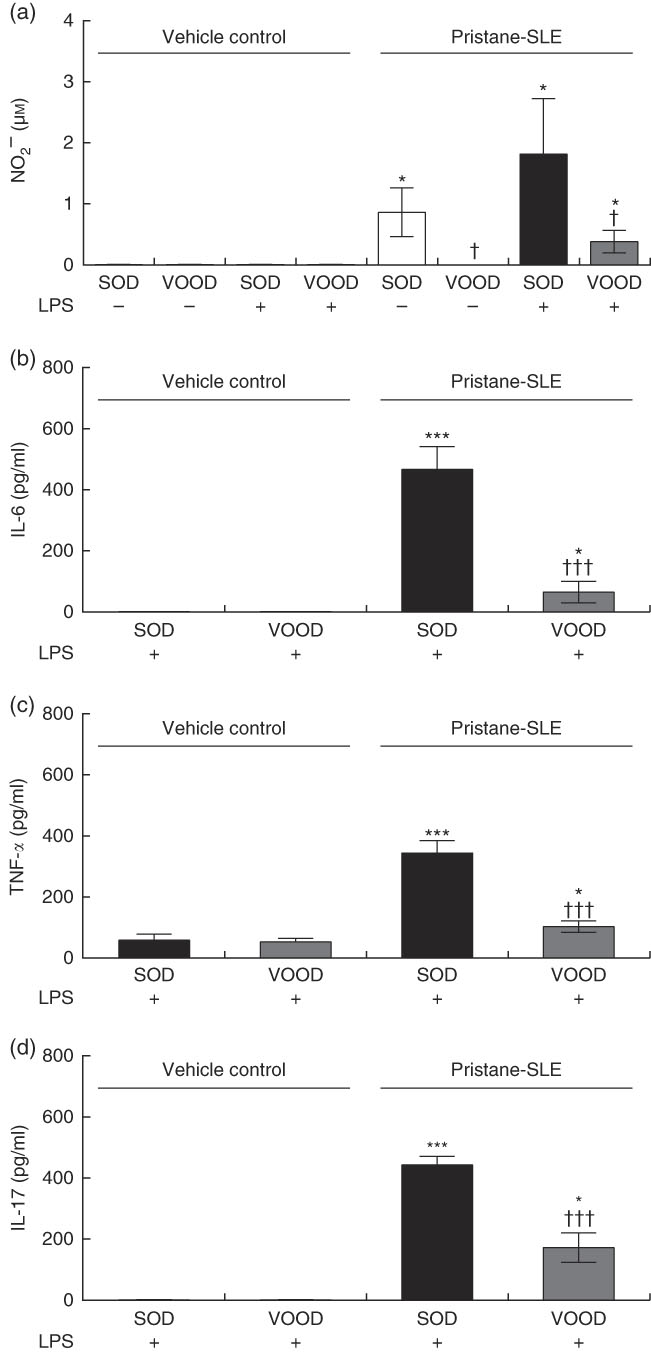
Fig. 1 Sunflower oil and virgin olive oil (VOO) diets on nitrite and pro-inflammatory cytokine production in lipopolysaccharide (LPS)-treated peritoneal macrophages from pristane-systemic lupus erythematosus (SLE) mice. Animals were injected with saline solution or pristane and then fed a diet containing sunflower oil (SOD) or VOO (VOOD) as indicated. After isolation, peritoneal macrophages were treated or not treated with LPS for 24 h, after which the supernatant was collected. (a) Nitrite, (b) IL-6, (c) TNF-α and (d) IL-17 concentration. Values are means (n 10 per group), with their standard errors represented by vertical bars. * P<0·05 and *** P<0·001 v. vehicle control (saline solution); † P<0·05 and ††† P<0·001 v. SOD.
Effects of diet containing sunflower oil and virgin olive oil on pro-inflammatory cytokine production in peritoneal macrophages from pristane-systemic lupus erythematosus mice
The production of IL-6, TNF-α and IL-17 was virtually absent in peritoneal macrophages from vehicle control mice fed either SOD or VOOD (data not shown). In addition, no significant differences were found for the production of IL-6 (Fig. 1(b)), TNF-α (Fig. 1(c)) and IL-17 (Fig. 1(d)) between LPS-treated peritoneal macrophages from vehicle control mice fed SOD and VOOD. However, the production of these cytokines was increased in LPS-treated peritoneal macrophages from pristane-SLE mice, with this effect being significantly less pronounced in animals feeding on VOOD when compared with animals feeding on SOD.
Effects of phenol fraction from virgin olive oil on viability of human monocytes
After 24 h of treatment with PF (6·25–50 µm), the viability of human monocytes (>95 % were alive) was not affected (data not shown).
Effects of phenol fraction from virgin olive oil on nitrite production and inducible nitric oxide synthase expression in lipopolysaccharide-treated human monocytes
LPS is a strong activator of the inflammatory response and immune regulation, which induces human monocytes to secrete different cytokines, including TNF-α and IL-1β, among others( Reference Feng, Goodridge and Harnett 30 ). As shown in Fig. 2(a), nitrite production was induced in LPS-treated human monocytes. However, this effect was significantly reduced and accompanied by a down-regulation in the protein (Fig. 2(b)) and gene (Fig. 2(c)) expression of iNOS by PF from VOO in a dose-dependent manner.
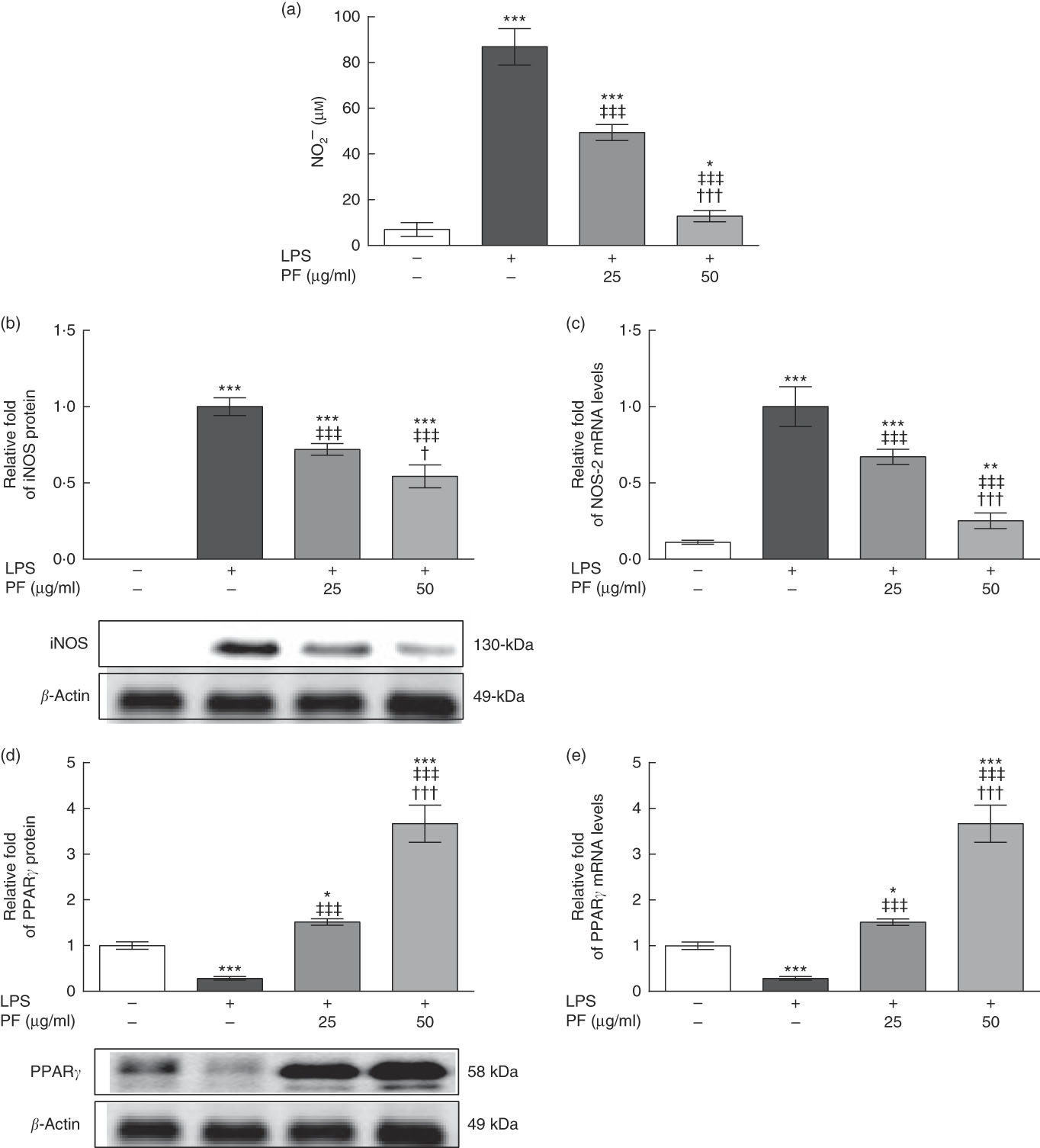
Fig. 2 Phenol fraction (PF) from virgin olive oil (VOO) on nitrite production and inducible nitric oxide synthase (iNOS) and PPARγ expression in lipopolysaccharide (LPS)-treated human monocytes. Cells were isolated from peripheral blood samples of healthy volunteers and immediately treated or not treated with LPS in the presence (25 and 50 µg/ml) or absence of PF from VOO for 24 h, after which the supernatant, cellular proteins and RNA were collected. (a) Nitrite concentration. (b) Relative fold change in band intensity of iNOS protein. (c) Relative fold change in mRNA level of NOS-2 gene. (d) Relative fold change in band intensity of PPARγ protein. (e) Relative fold change in mRNA level of PPARγ gene. β-Actin was served as an equal loading control for normalisation of protein levels. Values are means for three independent experiments in triplicate, with their standard errors represented by vertical bars. * P<0·05, ** P<0·01 and *** P<0·001 v. control non-LPS-treated cells; † P<0·05 and ††† P<0·001 v. other PF concentration; ‡‡‡ P<0·001 v. LPS-treated cells.
Effects of phenol fraction from virgin olive oil on PPARγ expression in lipopolysaccharide-treated human monocytes
While protein (Fig. 2(d)) and gene (Fig. 2(e)) expression of PPARγ was down-regulated in LPS-treated human monocytes, this effect was opposed by PF from VOO, which up-regulated protein and gene expression of PPARγ in a dose-dependent manner.
Effects of phenol fraction from virgin olive oil on pro-inflammatory cytokine and Toll-like receptor 4 expression in lipopolysaccharide-treated human monocytes
Both secretion (Fig. 3(a)–(c)) and gene expression (Fig. 3(d)–(f)) of IL-6, TNF-α and IL-1β were induced in LPS-treated human monocytes. These effects were significantly reduced by PF from VOO in a dose-dependent manner.
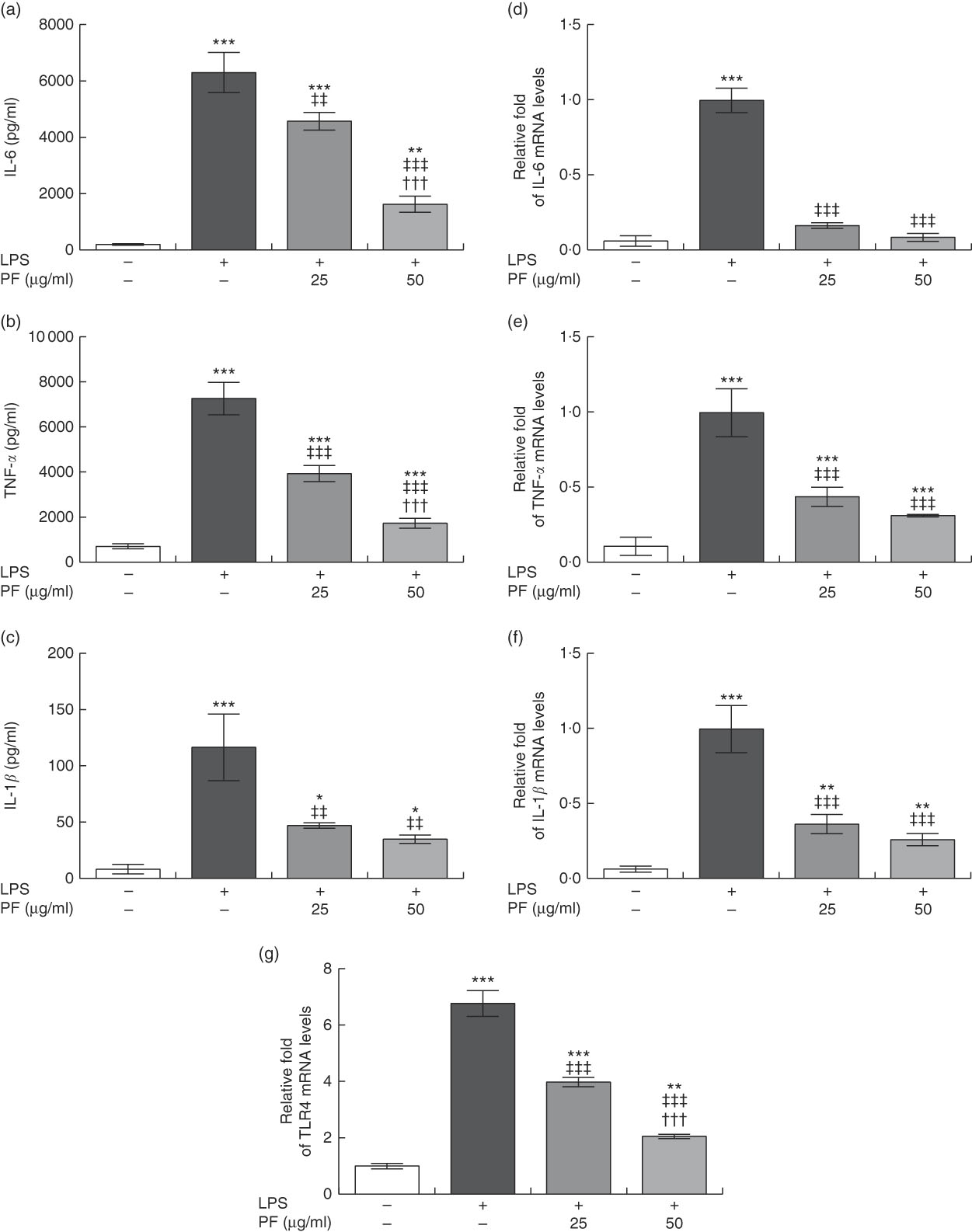
Fig. 3 Phenol fraction (PF) from virgin olive oil (VOO) on pro-inflammatory cytokine production and gene expression, and Toll-like receptor 4 (TLR4) gene expression in lipopolysaccharide (LPS)-treated human monocytes. Cells were isolated from peripheral blood samples of healthy volunteers and immediately treated or not treated with LPS in the presence (25 and 50 µg/ml) or absence of PF from VOO for 24 h, after which the supernatant and cellular RNA were collected. (a) IL-6, (b) TNF-α and (c) IL-1β concentration. (d–g) Relative fold change in mRNA level of IL-6, TNF-α, IL-1β and TLR4 genes, respectively. β-Actin was served as an equal loading control for normalisation of protein levels. Values are means of three independent experiments in triplicate, with their standard errors represented by vertical bars. * P<0·05, ** P<0·01 and *** P<0·001 v. control non-LPS-treated cells; ††† P<0·001 v. other PF concentration; ‡‡ P<0·01 and ‡‡‡ P<0·001 v. LPS-treated cells.
Toll-like receptor 4 (TLR4) is extensively expressed in immune cells and plays a critical role in recognition and signalling of bacterial LPS, inducing cytokine release( Reference Chow, Young and Golenbock 31 , Reference Cole, Georgiou and Monaco 32 ). TLR4 gene expression (Fig. 3(g)) was induced in LPS-treated human monocytes. However, this effect was significantly reduced by PF from VOO in a dose-dependent manner.
Effects of phenol fraction from virgin olive oil on polarisation in human monocyte-derived macrophages
Monocytes from healthy donors were cultured with rhM-CSF to mature into naïve M0 macrophages. FACS analysis showed that approximately 97 % of M0 macrophages were CD68-positive cells (data not shown). Then, cells were treated with canonical stimuli for polarisation into M1 or M2 macrophages in the presence or absence of PF from VOO. In comparison with M1 macrophages with increased prototypical markers (CD80, CD64 and monocyte chemoattractant protein-1 (MCP-1)), those macrophages polarised to M1 in the presence of PF from VOO had a significant decreased gene expression of CD80 (Fig. 4(a)), CD64 (Fig. 4(b)) and MCP-1 (Fig. 4(c)). These effects of PF from VOO appeared to be dose-dependent. In addition, the relative gene expression of CD200R (Fig. 4(d)), MRC-1 (Fig. 4(e)) and CD36 (Fig. 4(f)) (as prototypical M2 markers) was significantly increased by PF from VOO, mainly at high dose (50 μg/ml), after M2 polarisation. The effects of PF from VOO in reducing gene expression of CD80 and MCP-1 in M1-polarised macrophages were not comparable to those observed in M2-polarised macrophages; however, PF from VOO potentiated the transcriptional activity of CD200R, MRC-1 and CD36 genes involved in the M2 phenotypic change.
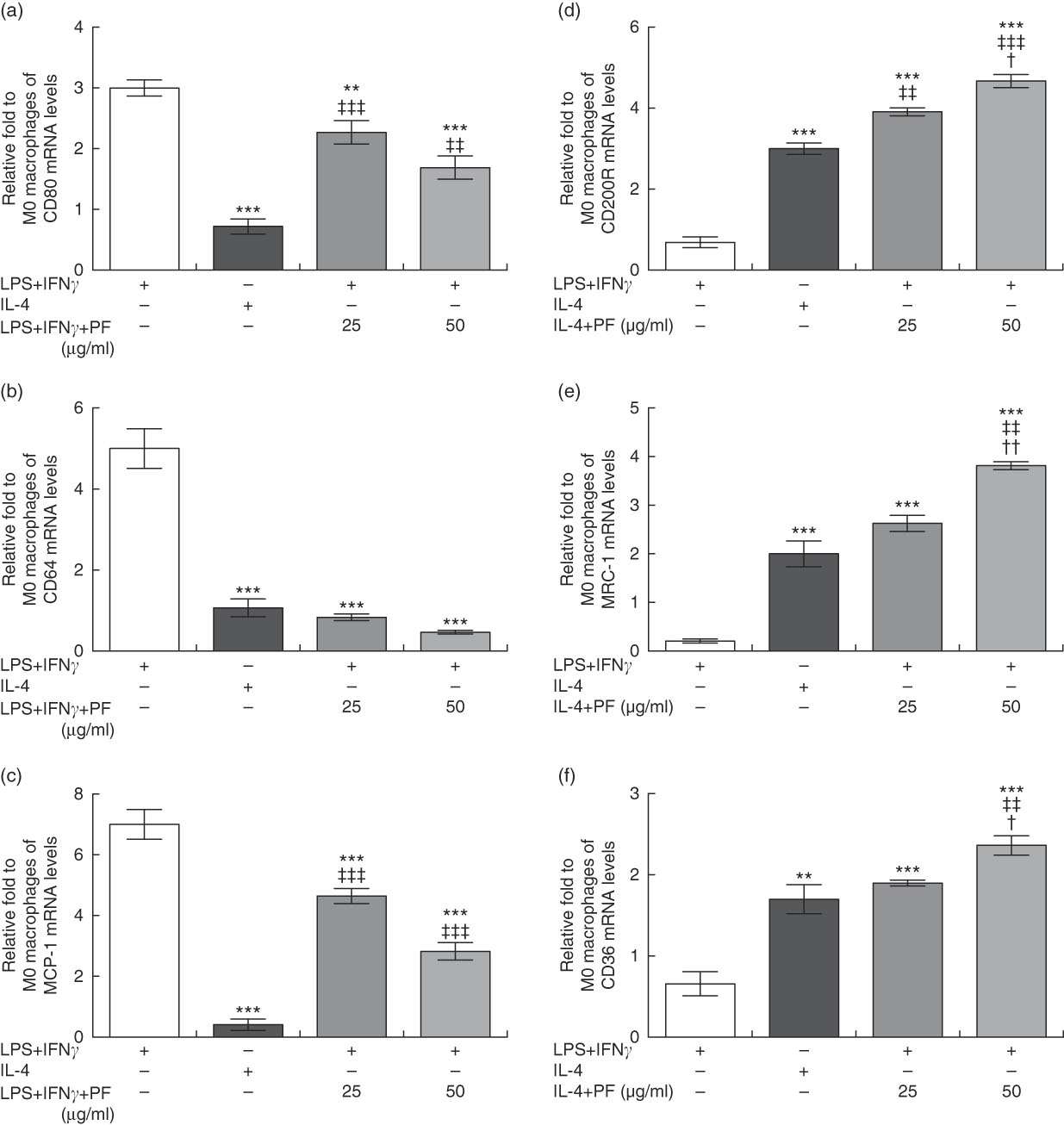
Fig. 4 Phenol fraction (PF) from virgin olive oil (VOO) on polarisation in human monocyte-derived macrophages. Monocytes were isolated from peripheral blood samples of healthy volunteers and immediately cultured with macrophage colony-stimulating factor for 6 d to differentiate into naïve M0 macrophages. These cells were then treated with lipopolysaccharide (LPS) and interferon (IFN)-γ to polarise into M1 or with IL-4 to polarise into M2 macrophages in the presence (25 and 50 µg/ml) or absence of PF from VOO for 24 h, after which the cellular RNA was collected. (a–c) Relative fold change in mRNA level of CD80, CD64 and MCP-1 genes in M1/M2 compared with M0 macrophages. (d–f) Relative fold change in mRNA level of CD200R, MRC-1 and CD36 genes in M1/M2 compared with M0 macrophages. Values are means for three independent experiments by triplicate, with their standard errors represented by vertical bars. ** P<0·01 and *** P<0·001 v. M1 macrophages; † P<0·05 and †† P<0·01 v. other PF concentration; ‡‡ P<0·01 and ‡‡‡ P<0·001 v. M2 macrophages.
Effects of phenol fraction from virgin olive oil on distribution of human monocyte subsets during lipopolysaccharide challenge
Human monocyte subsets are based on their expression of cell surface markers CD14 (LPS co-receptor) and CD16 (Fc gamma receptor II)( Reference Ziegler-Heitbrock 33 ). Classical monocytes are defined as CD14++CD16– cells, intermediate monocytes as CD14++CD16+ cells and non-classical monocytes as CD14+CD16++ cells. Classical monocyte subsets were increased in LPS-treated human monocytes (Fig. 5(a) and (b)). This effect was counteracted by PF from VOO in a dose-dependent manner, with a monocyte subset distribution at 50 μg/ml of PF similar to that observed in untreated human monocytes. In agreement, the increased surface expression of the prototypical classical monocyte marker CCR2 in LPS-treated human CD14++CD16– cells (Fig. 5(c)) was less pronounced by PF from VOO in a dose-dependent manner. Similar effects of PF from VOO were observed on gene (Fig. 5(d)) and surface (Fig. 5(e)) expression of CCR2 in the entire monocyte population treated with LPS.
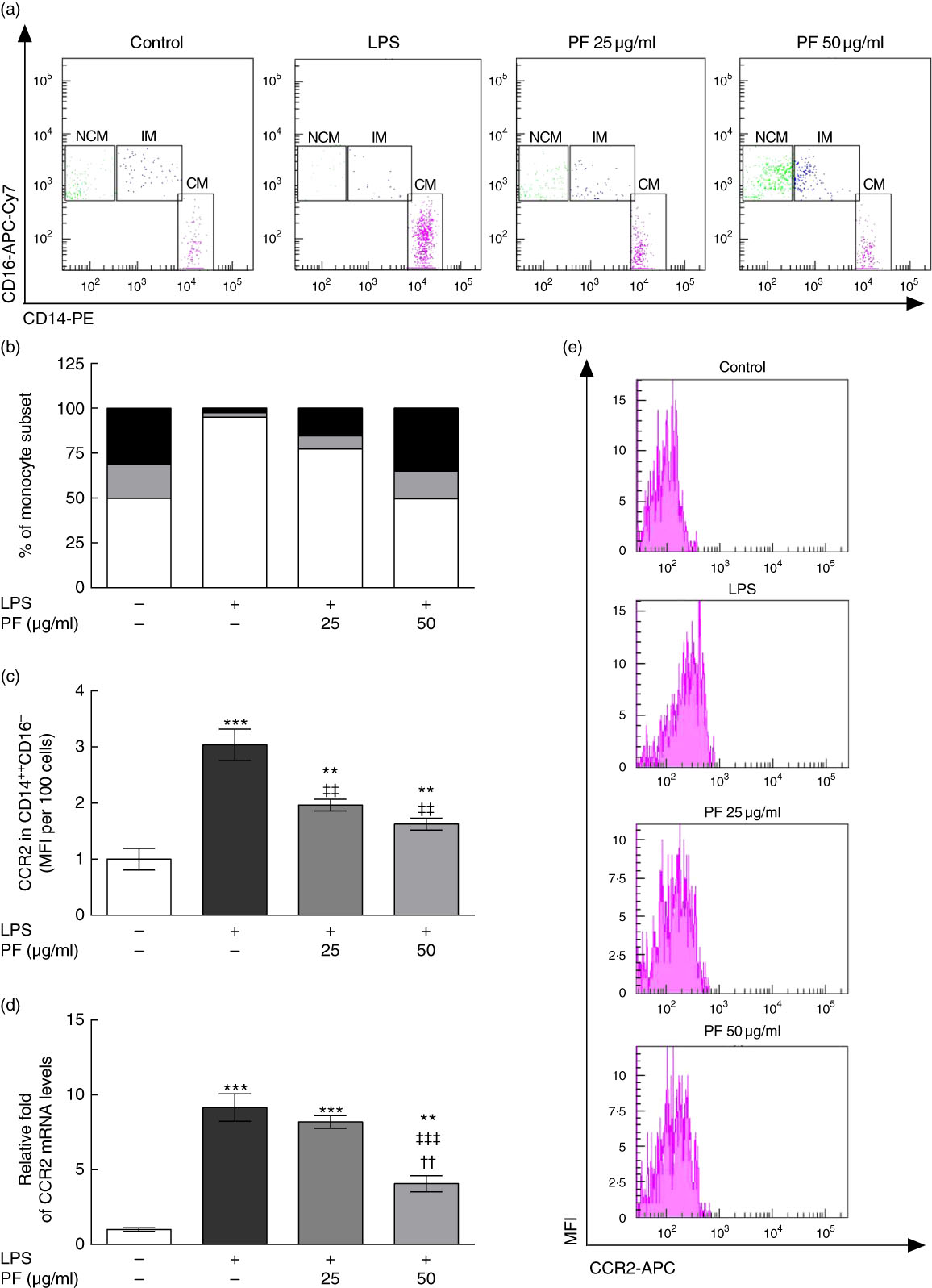
Fig. 5 Phenol fraction (PF) from virgin olive oil (VOO) on distribution of human monocyte subsets during lipopolysaccharide (LPS) challenge. Monocytes were isolated from peripheral blood samples of healthy volunteers and immediately treated or not treated with LPS in the presence (25 and 50 µg/ml) or absence of PF from VOO for 24 h, after which they were stained for fluorescence-activated cell sorting analysis of surface markers CD14 and CD16 or the cellular RNA was collected. (a) Flow cytometry analysis of monocyte subsets according to their CD14 and CD16 surface expression (CM, classical monocytes as CD14++CD16– cells; IM, intermediate monocytes as CD14++CD16+ cells; NCM, non-classical monocytes as CD14+CD16++ cells). (b) Percentage of classical (
![]() ), intermediate (
), intermediate (![]() ) and non-classical (
) and non-classical (![]() ) monocytes. (c) C-C chemokine receptor type 2 (CCR2) surface expression in classical, CD14++CD16– monocytes. (d) Relative fold change in mRNA level of CCR2 gene. (e) Representative flow cytometry plots of CCR2 surface expression. Values are means of three independent experiments in triplicate, with their standard errors represented by vertical bars. ** P<0·01 and *** P<0·001 v. control non-LPS-treated cells; †† P<0·01 v. other PF concentration; ‡‡ P<0·01 and ‡‡‡ P<0·001 v. LPS-treated cells.
) monocytes. (c) C-C chemokine receptor type 2 (CCR2) surface expression in classical, CD14++CD16– monocytes. (d) Relative fold change in mRNA level of CCR2 gene. (e) Representative flow cytometry plots of CCR2 surface expression. Values are means of three independent experiments in triplicate, with their standard errors represented by vertical bars. ** P<0·01 and *** P<0·001 v. control non-LPS-treated cells; †† P<0·01 v. other PF concentration; ‡‡ P<0·01 and ‡‡‡ P<0·001 v. LPS-treated cells.
Discussion
SLE is an autoimmune disease characterised by autoantibody production and chronic inflammation in multiple organs, whose aetiology still remains unclear. In the past, most of the SLE pathogenesis studies considered the adaptive immune system to be the primary cause of autoimmunity, focusing on the primary abnormalities of B and T lymphocyte functions. More recent studies are now centred on the innate immunity and on how the SLE autoimmune response is initiated and maintained( Reference Li, Lee and Reeves 34 ). Of note, monocytes and macrophages play a pivotal role in the innate immune system with widespread immunological function( Reference Unanue 35 ), and abnormalities in its phenotype and functions have been associated with a variety of autoimmune disorders, including SLE( Reference Katsiari, Liossis and Sfikakis 36 ).
Currently, SLE therapy includes corticosteroids and immunosuppressants, with varying success and usually severe side effects( Reference van Vollenhoven, Parodis and Levitsky 37 ). For this reason, new therapeutic strategies continue to be investigated and the interest in dietary supplements and nutritional therapy may be considered as a safe therapeutic strategy for SLE patients. Importantly, recent epidemiologic, clinical and experimental studies have suggested that VOO and especially its phenols might possess preventive effect on immune-inflammatory-related diseases, including SLE( Reference Alarcon de la Lastra, Barranco and Motilva 14 , Reference Cardeno, Sanchez-Hidalgo and Alarcon-de-la-Lastra 15 , Reference Sanchez-Fidalgo, Cardeno and Sanchez-Hidalgo 17 , Reference Aparicio-Soto, Sanchez-Hidalgo and Cardeno 18 , Reference Aparicio-Soto, Sanchez-Hidalgo and Cardeno 21 ). However, the effects of VOO or its PF on immune-inflammatory functions of the monocyte–macrophage lineage remain to be elucidated.
Oxidative stress plays a substantial role not only in the pathogenesis of autoimmune rheumatic diseases and their complications, but also on organ/system-specific disease activity. Thus, a deregulation of redox homoeostasis may lead to over-production of pro-inflammatory cytokines and NO, and thereby to a condition of oxidative stress, which plays an important role in SLE( Reference Sukkar and Rossi 38 ). Furthermore, the modifications of lipids, proteins and DNA associated with increased iNOS activity have been shown to be involved in the pathogenesis of SLE( Reference Oates and Gilkeson 39 ). This highlights that targeted therapies for iNOS and oxidative stress may provide the means to reduce the pathogenic consequences of SLE.
Here we found that a diet containing VOO contributed to prevent NO and pro-inflammatory cytokine production in peritoneal macrophages of mice with pristane-induced SLE. The amount of VOO administered to animals was equivalent to 20 g daily consumption for a person of 70 kg body weight. A lower production of NO and pro-inflammatory cytokines was also observed in LPS-treated peritoneal macrophages from pristane-induced SLE mice fed a diet containing VOO when compared with SOD. These observations suggest that VOO may be beneficial for attenuating pristane-induced inflammation in the mouse model of SLE, in agreement with a previous study reporting lower paw swelling, weight of spleen and thymus, urinary level of proteins, blood level of matrix metalloproteinase-3 (MMP-3) and kidney damage in mice with pristane-induced SLE and fed VOO when compared with those fed sunflower oil( Reference Aparicio-Soto, Sanchez-Hidalgo and Cardeno 18 ). VOO was a dietary fat rich in oleic acid and minor compounds such as phenols, squalene and triterpenic alcohols, whereas sunflower oil was rich in linoleic acid, sterols and tocopherols. However, the component or components that make VOO different from sunflower oil in the above conceptual framework are still unknown.
On the basis of this interest and owing to the success of VOO against renal injury in mice with pristane-induced SLE( Reference Aparicio-Soto, Sanchez-Hidalgo and Cardeno 18 ) and of PF from VOO against joint inflammation in mice with collagen-induced arthritis( Reference Rosillo, Alcaraz and Sanchez-Hidalgo 40 ) and activation of T cells from patients with SLE and healthy subjects( Reference Aparicio-Soto, Sanchez-Hidalgo and Cardeno 21 ), we focused on effects of PF from VOO on pro-inflammatory mediators and plasticity in human circulating monocytes and monocyte-derived macrophages. Our study demonstrated the ability of PF from VOO to dampen NO production and iNOS protein and gene expression induced by LPS in human peripheral blood monocytes in vitro. These findings show that VOO and its PF can target iNOS activity in myeloid cells from either mice or humans. They are also in good agreement with those previously reported in LPS-treated peritoneal macrophages from mice with acute experimental colitis, where the PF from VOO inhibited the increase of iNOS protein expression through NF-κB (NF-κ-light-chain enhancer of activated B cells) and mitogen-activated protein kinases signalling pathways( Reference Cardeno, Sanchez-Hidalgo and Aparicio-Soto 16 , Reference Sanchez-Fidalgo, Cardeno and Sanchez-Hidalgo 17 ).
Modulation of pro-inflammatory mediators in the monocyte–macrophage lineage is considered one of the strategies to develop therapeutic compounds against several inflammatory diseases. In this regard, TLR4 plays an important role in monocyte and macrophage activation and in macrophage polarisation by the recognition of LPS, which triggers the release of cytokines with a predominant role in the inflammatory response( Reference Chen, Yang and Zumbrun 41 ). Our results revealed that PF from VOO dampens TLR4 and pro-inflammatory cytokine gene expression, as well as pro-inflammatory cytokine production induced by LPS in human monocytes in vitro. This provides evidence to suggest that PF from VOO may be effective in reducing LPS-mediated inflammation by interfering with the LPS/TLR4 axis.
Despite the limited available data related to PPARγ and SLE, some studies have shown the potential therapeutic benefits of PPARγ agonists on this disease( Reference Venegas-Pont, Sartori-Valinotti and Maric 42 ). According to previous studies, the activation of PPARγ may cause an inhibition in the expression of pro-inflammatory cytokines and may drive the differentiation of immune cells to anti-inflammatory phenotypes( Reference Chinetti, Fruchart and Staels 43 , Reference Martin 44 ). In our study, we found that PF from VOO not only avoided the LPS-induced decreases of PPARγ gene expression but demonstrated its potential in augmenting the transcriptional activity of this gene in human peripheral blood monocytes, which probably contributed to the observed lower inflammatory phenotype after LPS challenge.
Macrophages are key modulator and effector cells in the immune response because their activation influences and responds to other arms of the immune system. In vitro, they can be classified into different subsets, including naïve M0, by the treatment of peripheral blood monocytes with M-CSF and macrophages polarised from M0 by LPS and IFN-γ (M1 macrophages, which express a spectrum of pro-inflammatory molecules such as those mentioned above) or by IL-4 (M2 macrophages, which express a wide array of anti-inflammatory molecules)( Reference Perez-Jimenez, Alvarez de Cienfuegos and Badimon 45 ). Therefore, we were interested in examining whether PF from VOO had any influence on polarisation of M0 macrophages in terms of their phenotype towards pro- or anti-inflammatory direction. It was noteworthy that PF from VOO blocked the expression of M1 signature genes and favoured the phenotype of M2 macrophages induced by IL-4, probably suggesting the beneficial influence of PF from VOO on both polarisation channels. The skewing into a subset with an M2-like phenotype, even upon treatment with LPS and IFN-γ, consistently emphasises that PF from VOO may be also beneficial for maintaining tissue homoeostasis under inflammatory conditions. The effects of PF from VOO on LPS-induced deregulation of human peripheral blood monocyte subsets in vitro, by retaining proportions of classical, intermediate and non-classical subsets to values found in untreated cells, further support this notion. A deregulation of peripheral blood monocytes based on an increase in the proportion of the classical subset and a decrease in the proportion of the non-classical subset has been reported in patients with active SLE( Reference Burbano, Vasquez and Rojas 46 ). Our study also found an inhibitory effect of PF from VOO on CCR2 surface and gene expression in LPS-treated human peripheral blood monocytes, which is indicative of a reduction in their migratory capacity and traffic to sites of inflammation( Reference Moser, Kelly and Lessard 47 , Reference Yang, Zhang and Yu 48 ).
The effects of chemicals or diets in animal models are not always predictive for humans. Therefore, it is of crucial importance to investigate the potential benefits of VOO and PF from VOO, the latter as a non-synthetic anti-inflammatory dietary complement, in human disease. Unfortunately, we do not provide data on comparative effects of individual components of PF, appropriate doses or long-term intake of this fraction from VOO in healthy and/or diseased volunteers, which of course will need to be assessed in future studies. However, for the first time, our study provides several lines of in vivo and in vitro evidence that VOO and PF from VOO target and counteract inflammatory pathways in the monocyte–macrophage lineage of mice with pristane-induced SLE and of healthy subjects, which is a meaningful foundation for further development and application in preclinical and clinical use of PF from VOO in patients with SLE. Thus, we anticipate that VOO, and particularly its PF, can be helpful in reducing SLE activity and be part of the armamentarium in the management of SLE.
Acknowledgements
M. A.-S. gratefully acknowledges support from a Postgraduate National Program of FPU fellowship and financial sponsorship from the Spanish Ministerio de Educación, Cultura y Deporte. S. M.-d. l. P. has the benefit of a FPI fellowship (BES-2012-056104) of MICINN. B. B. and S. M.-d. l. P. acknowledge support from ‘V Own Research Plan’ (University of Seville). The authors gratefully acknowledge the assistance of Centre for Technology and Innovation Research, University of Seville (CITIUS). The authors thank I+D+i of Oleoestepa SAC for kindly providing the VOO.
This study was supported by research grants AGL2011-26949 and AGL2011-29008 (Spanish Ministry of Science and Innovation, MICINN) and P-10AGR-6609 (Junta de Andalucía).
M. A.-S. and S. M.-d. l. P. performed cell cultures, cytokine measurements and RT-qPCR and western blot experiments. S. M.-d. l. P. and B. B. performed flow cytometry experiments and analysed the data. M. A.-S., S. M.-d. l. P. and F. J. G. M. wrote the main manuscript. C. A. d. l. L., M. S.-H. and F. J. G. M. designed and supervised the project and revised the paper. All authors discussed the results and implications and commented the manuscript at all stages.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518001976











