Mg is a cofactor of numerous enzymes and plays an essential role in a wide range of fundamental cellular reactions. Insufficient Mg intake therefore induces numerous abnormalities in rodents(Reference Mazur, Maier and Rock1). Mg deficiency induced oxidative stress, which was evaluated by lipid peroxidation, and apoptosis in rat liver(Reference Vormann, Günther and Höllriegl2, Reference Martin, Uring-Lambert and Adrian3). In addition, TAG and total cholesterol concentrations were increased in the liver and serum of Mg-deficient rats(Reference Akiyama, Uehara and Katsumata4). These features resemble the altered metabolism in the liver of rats fed a high-Fe diet; Fe overload enhanced lipid peroxidation, increased apoptotic cell number and elevated liver fat concentration and serum lipid concentrations, including TAG and total cholesterol(Reference Wang, Eriksson and Xia5–Reference Silva, Silva and de Paula8). In view of the accumulation of hepatic Fe in Mg-deficient rats(Reference Vormann, Günther and Höllriegl2, Reference Kimura and Yokoi9, Reference Sanchez-Morito, Planells and Aranda10), increased hepatic Fe content may cause various Mg-deficiency-related abnormalities in the liver.
Hepcidin was originally isolated from human urine as an anti-microbial peptide(Reference Park, Valore and Waring11) and is currently recognised as a hormone secreted from the liver in response to the Fe overload; it negatively regulates intestinal Fe absorption through internalisation and degradation of an Fe transporter, ferroportin(Reference Lee and Beutler12). Considering that hepatic Hepcidin transcription is triggered by excess Fe(Reference Pigeon, Ilyin and Courselaud13, Reference Kautz, Meynard and Monnier14), Mg deficiency is expected to increase Hepcidin expression in the liver; however, a previous study revealed an increase in the intestinal absorption of Fe in Mg-deficient rats(Reference Sanchez-Morito, Planells and Aranda10), suggesting the failure of regulatory Fe metabolism by Hepcidin. The present study examined the expression of hepatic Hepcidin in Mg-deficient rats.
Materials and methods
Animals and diets
A total of twelve 5-week-old male Sprague–Dawley rats were purchased from SLC Japan (Shizuoka, Japan) and cared for according to the Guide for the Care and Use of Laboratory Animals (Animal Care Committee, Kyoto University, Kyoto, Japan). They were individually housed in stainless-steel cages in a temperature-, humidity- and light-controlled room (24°C, 60 %, 12 h light–12 h dark cycle). All rats were fed a control diet (American Institute of Nutrition-93G diet)(Reference Reeves15) for a 5 d adaptation period, followed by feeding either the control diet or an Mg-deficient diet (American Institute of Nutrition-93G-based diet with Mg-free mineral mixture). The Mg content determined in the control diet and the Mg-deficient diet was 49·6 and 4·2 mg/100 g, respectively. Rats were pair-fed their respective experimental diets and were allowed free access to demineralised water for 4 weeks. After the feeding trial, the rats were killed by collecting blood from the abdominal aorta under isoflurane anaesthesia, and the liver was collected.
Measurement of dietary magnesium and calcium, serum magnesium, liver iron and liver thiobarbituric acid-reactive substances
Dietary sample, and serum and liver samples were digested with trace element-grade HNO3 and H2O2 (Wako, Osaka, Japan), and dietary and serum Mg and liver Fe were determined by atomic absorption spectrophotometry (AA-6600F; Shimadzu, Kyoto, Japan). Analytical accuracy of liver Fe was confirmed by analysis of a certified reference material of bovine liver (Standard Reference Material 1577b; National Institute of Standards and Technology, Gaithersburg, MD, USA). The liver samples were also homogenised in chilled saline by Polytron (PT1600E; Kinematica, Lucerne, Switzerland), and the homogenate was centrifuged at 105 000 g for 30 min at 4°C. The concentration of thiobarbituric acid-reactive substances in the supernatant was determined using a commercial kit (OXI-TEK TBARS Assay Kit; ZeptoMetrix, Buffalo, NY, USA) according to the manufacturer's instructions.
RNA isolation and quantitative RT-PCR
Total RNA was isolated from the liver samples using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Absorbance at 260 nm was measured to quantify RNA concentration, and simultaneously the ratio of absorbance at 260 nm to that at 280 nm was monitored to assess the purity of RNA. Quantitative RT-PCR was carried out as described previously(Reference Furutani, Murakami and Funaba16, Reference Suenaga, Matsui and Funaba17). The following oligonucleotides were used as PCR primers: 5′-gggcagaaagcaagactgat-3′ and 5′-ttacagcatttacagcagaagagg-3′ for Hepcidin (GenBank accession no. NM_053469.1); 5′-gacagcagagtcgcaatcg-3′ and 5′-agctcacgtaaagctcatgc-3′ for bone morphogenetic protein (Bmp) 6 (GenBank accession no. NM_013107); 5′-gcgagatcagtgccttgg-3′ and 5′-ttttcctcttgcctcctgaa-3′ for the inhibition of DNA binding 1 (Id1) (GenBank accession no. NM_012797.2); 5′-actacctgcagagggactgc-3′ and 5′-actttcaccaaagtaggcacttg-3′ for haemochromatosis (Hfe, GenBank accession no. NM_053301.4); 5′-gtagcatcgggagccaac-3′ and 5′-tcaaaggctgcaggaagatt-3′ for Hemojuvelin (GenBank accession no. NM_001012080.1); 5′-gagttcactgacatcatcaagca-3′ and 5′-tccagcctcacgaggagtat-3′ for transferrin receptor 1 (Tfr1) (GenBank accession no. NM_022712); 5′-tcagtaacatctttgcgtgcat-3′ and 5′-gccccgataacgacatagtg-3′ for Tfr2 (GenBank accession no. NM_001105916). PCR primers for activin receptor-like kinase 2 (Alk2), activin receptor-like kinase 3 (Alk3), activin receptor type IIA (Actr2a), activin receptor type IIB (Actr2b), Bmp type II receptor (Bmpr2) and glyceraldehyde-3-phosphate dehydrogenase (G3pdh) were described previously (Reference Nishino, Ooishi and Kurokawa18). The relative mRNA level is expressed as a ratio of the G3pdh mRNA level.
Statistical analyses
Data are expressed as means with their standard errors. Differences between the treatments were examined by Student's t test. Differences of P < 0·05 were considered significant.
Results and discussion
Consistent with the previous results(Reference Vormann, Günther and Höllriegl2, Reference Kimura and Yokoi9, Reference Sanchez-Morito, Planells and Aranda10), the serum concentration of Mg was significantly lower in rats fed the Mg-deficient diet (Table 1). In addition, liver concentrations of Fe and thiobarbituric acid-reactive substances, an index of oxidative stress, were higher in the Mg-deficient group. Expression of hepatic Tfr1 was significantly lower in Mg-deficient rats than in control rats, whereas that of hepatic Tfr2 was comparable between the groups. These results were consistent with the results of Fe-overloaded mice(Reference Fleming, Migas and Holden19, Reference Theurl, Ludwiczek and Eller20). Fe-responsive elements within the untranslated region are present for Tfr1 but not for Tfr2 mRNA, which explains why the mRNA level of Tfr1 but not Tfr2 was negatively regulated by Fe status(Reference Trinder and Baker21). Thus, effects of Mg deficiency on the expression of Tfr1 and Tfr2 could reflect Fe status in the liver.
Table 1 Effect of magnesium deficiency on the serum concentration of magnesium, liver concentration of iron and thiobarbituric acid-reactive substances (TBARS), and hepatic expression of iron-related molecules
(Mean values with their standard errors, n 6)
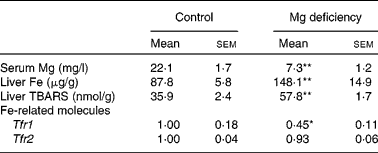
Tfr, transferrin receptor.
Mean values were significantly different from those of the control group: * P < 0·05, ** P < 0·01.
Mg deficiency did not affect the gene transcript level of Hepcidin in the liver (Table 2). Hepcidin is a hormone that regulates intestinal Fe absorption negatively(Reference Lee and Beutler12). Hepcidin expression is transcriptionally induced in response to the elevation of hepatic Fe(Reference Lee and Beutler12). The present study revealed that the expression of Hepcidin in the liver is not up-regulated by Mg deficiency, irrespective of the enhanced accumulation of hepatic Fe. Thus, it is suggested that the lack of response of the Hepcidin expression is at least partly responsible for Mg-deficiency-induced dysregulation of Fe homeostasis.
Table 2 Effect of magnesium deficiency on the hepatic expression of Hepcidin, bone morphogenetic protein (Bmp) 6, inhibition of DNA binding 1 (Id1), haemochromatosis (Hfe), Hemojuvelin and Bmp receptors
(Mean values with their standard errors, n 6)
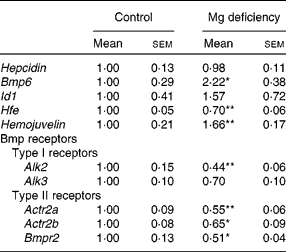
Alk, activin receptor-like kinase; Actr2, activin receptor type II; Bmpr2, Bmp type II receptor.
Mean values were significantly different from those of the control group: * P < 0·05, ** P < 0·01.
Expression of Bmp6 was significantly higher in Mg-deficient rats than in control rats, but Id1 expression was not different between the dietary groups (Table 2). In the liver, Hepcidin is transcriptionally regulated by Bmp6(Reference Babitt, Huang and Xia22, Reference Andriopoulos, Corradini and Xia23), and Id1 is a representative Bmp-responsive gene regulated at the transcription level(Reference Korchynskyi and ten Dijke24). Previous studies revealed that Fe overload up-regulated the expression of Bmp6 and Id1 in the liver(Reference Kautz, Meynard and Monnier14, Reference Kautz, Meynard and Besson-Fournier25). Exogenous Bmp6 increased Hepcidin expression in Hep3B cells(Reference Babitt, Huang and Xia22) as well as in the liver(Reference Andriopoulos, Corradini and Xia23). Furthermore, targeted disruption of the Bmp6 gene decreased the expression of Hepcidin and accumulated Fe in the liver(Reference Andriopoulos, Corradini and Xia23, Reference Meynard, Kautz and Darnaud26). Thus, Bmp6 is a signal mediator linking Fe accumulation and Hepcidin expression, although transcriptional activation of the Bmp6 gene by excess Fe accumulation is currently unclear at the molecular level(Reference Camaschella27). In the present study, the expression of Bmp6 was increased 2·2-fold in rats fed the Mg-deficient diet. The extent of the response was comparable with a previous result; feeding a high-Fe diet for 7 weeks resulted in a 1·8-fold increase in Bmp6 expression and sevenfold increase in Hepcidin expression in DBA/2 mice(Reference Kautz, Meynard and Monnier14). Mg deficiency may blunt the Bmp pathway by altering the function of factors involved in hepatic Hepcidin induction.
The gene transcript level of Hfe was significantly lower in Mg-deficient rats than in control rats, whereas that of Hemojuvelin was higher in Mg-deficient rats (Table 2). Upon Bmp binding to the two types of receptors, i.e. type I and type II serine/threonine receptors, the receptor complex phosphorylates and activates Smad1/5/8, leading to transcriptional activation of the target genes such as Id1 (Reference Miyazono, Kamiya and Morikawa28). The strength and duration of the Bmp signal are regulated at multiple steps; expression of co-receptors for Bmp is involved in the fine-tuning of Bmp signalling(Reference Miyazono, Kamiya and Morikawa28). Previous studies revealed that Hemojuvelin, which is a gene product of Hfe2 and a co-receptor of Bmp, including Bmp6, enhances Hepcidin expression both in vitro and in vivo (Reference Babitt, Huang and Xia22, Reference Babitt, Huang and Wrighting29, Reference Xia, Babitt and Sidis30). In view of the up-regulation of Hemojuvelin expression in Mg-deficient rats, the co-receptor is unlikely to be involved in the unresponsiveness to Bmp6.
Recently, Kautz et al. (Reference Kautz, Meynard and Besson-Fournier25) revealed that the expression of Bmp6 was enhanced in Hfe-null mice, but hepatic Bmp signalling, such as phosphorylation of Smad1/5/8 and Id1 expression, was not accelerated. Similar results were also recently obtained in patients with hereditary haemochromatosis with mutation of the HFE gene(Reference Ryan, Ryan and Fabre31). In the liver of Fe-overloaded mice, both Hfe and Hemojuvelin expressions were increased(Reference Theurl, Ludwiczek and Eller20). Therefore, the blunting of Bmp signalling at the gene transcript level of Hepcidin may be explained by the result that Mg deficiency down-regulated Hfe expression in the liver, although up-regulation of Hepcidin expression in response to Bmp2, Bmp4 and Bmp9 in primary hepatocytes from wild-type mice was comparable with those from Hfe-null mice(Reference Truksa, Peng and Lee32).
Down-regulation of the expression of Bmp receptors is possibly related to blunting of Bmp signalling in Mg-deficient rats. Among Bmp receptors, expression of hepatic Alk2, Actr2a, Actr2b and Bmpr2 was significantly lower in Mg-deficient rats than in control rats (Table 2); expression of activin receptor-like kinase 6 (Alk6), a Bmp type I receptor, was not significant (data not shown). Receptor expression level also determines the strength of Bmp signalling(Reference Miyazono, Kamiya and Morikawa28, Reference Murakami, Kawachi and Ogawa33).
In conclusion, the accumulation of hepatic Fe by Mg deficiency is a stimulant inducing Bmp6 expression but not Hepcidin expression by blunting Bmp signalling possibly resulting from down-regulation of the receptor expression. Unresponsive Hepcidin expression may have a role in Mg-deficiency-induced changes related to increased liver Fe.
Acknowledgements
The present study received no specific grant from any funding agency in the public, commercial or not-for profit sectors. N. I. and T. M. designed the study; N. I. and M. K. performed the study; N. I., M. F. and T. M. analysed the data and wrote the manuscript. All authors discussed the results and approved the manuscript in its final version. The authors declare no conflict of interest.






