INTRODUCTION
Ageing of the immune system is characterized by complex changes including decreased proportions of naive CD8 T cells, reduced overall levels of T-cell proliferative capacity and altered patterns of cytokine secretion in response to antigens or mitogens, accumulations of clonal expansions of CD8 T cells with limited antigen specificity, and impaired generation of effector B- and T-cell responses. These differences in immune signatures are together termed immunosenescence because they are believed to be associated with increased morbidity and mortality caused by infectious disease in the elderly.
Unlike viruses that induce acute infection, viruses that induce a lifelong persistent infection such as herpesviruses establish an evolving equilibrium with the host and stimulate lymphocytes repeatedly upon reactivation. The herpesvirus cytomegalovirus (CMV) has been identified as a driving force behind the clonal expansion and altered T-cell function found in several studied populations of elderly people [Reference Looney1–Reference Almanzar3] and is part of an ‘immune risk profile’ associated with increased mortality established in longitudinal studies of the very elderly (for reviews, see [Reference Pawelec4, Reference Derhovanessian, Larbi and Pawelec5]). Recent prospective studies have reported high CMV antibody levels to be associated with increased mortality in the elderly [Reference Roberts6, Reference Wang7].
Commonly, more than half of the middle-aged populations in Western countries are infected with CMV, which can be shed in the bodily fluids of any infected person. Common factors related to exposure or susceptibility have been found to be related to CMV seroprevalence, including socioeconomic factors and ethnicity [Reference Dowd, Aiello and Alley8, Reference Staras9]. Underlying mechanisms influencing susceptibility to CMV infection are unknown.
To study the mechanisms influencing susceptibility to CMV infection we measured CMV seroprevalence in two genetically informative cohorts. First, in long-living sib pairs (men aged ⩾89 years and women aged ⩾91 years) and their offspring (who are middle-aged subjects enriched for familial longevity) together with the offspring's partners with whom they share daily living. This enabled us to study the influence of genes and shared environment (both early and adult) on the susceptibility to CMV infection. Second, elderly monozygotic (MZ) and dizygotic (DZ) twins were studied to disentangle further genetic and early environmental factors in the susceptibility to CMV infection. These study designs enable us to shed light on the influence of genetic factors, shared (early) family environment and the later-life environment on the susceptibility to CMV infection.
MATERIALS AND METHODS
Leiden Longevity Study (LLS)
Data were obtained from the LLS, where long-lived siblings were recruited together with their offspring and the partners of their offspring. Families were eligible for the studies if (i) at least two long-lived, full siblings were alive, where men were considered long-lived if they were aged ⩾89 years and women if they were aged ⩾91 years, and (ii) the parents of the siblings were of Caucasian descent. All sibling pairs were recruited between 2002 and 2006. For the current analysis, we included 844 long-lived siblings, 754 of their offspring and 754 partners of the offspring with information on CMV serostatus and sex. For all subjects, blood samples were taken at baseline for extraction of deoxyribonucleic acid and ribonucleic acid and the determination of non-fasting serum and plasma parameters. The offspring of the long-lived siblings have been previously shown to have a survival benefit, with an average 30% lower mortality rate compared to their birth cohort [Reference Schoenmaker10]. There were no selection criteria on health or demographic characteristics. Further information on the design of the study and characteristics of the cohort has been published elsewhere [Reference Schoenmaker10, Reference Westendorp11]. The Medical Ethical Committee of the Leiden University Medical Center approved the study, and informed consent was obtained from all participants.
Longitudinal Study of Aging Danish Twins (LSADT)
The LSADT is based on the Danish Twin Registry, which has been described in detail previously [Reference Skytthe12]. The registry was established in 1954 as the first nationwide twin registry in the world and includes twin pairs born in Denmark between 1870 and 1910 and same-sex pairs born between 1911 and 1930. From this registry, 2172 twins with a median age of 77 years (range 72–102 years) participated in the 1997 LSADT survey, whether or not the co-twin was still alive. The survey included an extensive face-to-face interview, comprising questions on self-rated health, alcohol consumption, and height and weight. A total of 456 same-sex pairs participated. Blood samples (20 ml EDTA blood) were collected from all same-sex pairs where both members were willing to provide a blood sample. Blood was collected from 699 subjects within a 6-month period [Reference Bathum13]. CMV status was successfully determined for a total of 302 same-sex twin pairs.
Virological analysis
CMV serostatus was determined by ELISA using the CMV-IgG ELISA PKS assay (Medac GmbH, Germany) in offspring and partners of the LLS, the CMV-IgG kit (ETI-CYTOK-G PLUS DiaSorin, Italy) in long-living nonagenarians and the anti-CMV-IgG kit (Dade Behring Marburg GmbH, Germany) in the Danish cohort. Herpes simplex virus and varicella zoster virus serostatus in offspring and partners of the LLS cohort were determined by ELISA using the Enzygnost anti-HSV/IgG and Enzygnost anti-VZV/IgG assay, respectively (Siemens Healthcare Diagnostics GmbH, Germany). Liaison Epstein–Barr virus nuclear antigen (EBNA) IgG chemiluminescence immunoassay (CLIA) was used for determination of Epstein–Barr virus (EBV) serostatus (DiaSorin). EBNA IgG-negative sera were additionally tested for viral capsid antigen (VCA) IgG and considered EBV seropositive if VCA IgG was detectable. All the immunoassays are commercially available and were performed according to the manufacturer's instructions.
Statistical analysis
For the within-partnership, within sibship, and twin-pair analyses of concordance in CMV serostatus, the conditional probability that one member of a pair had a given CMV status given that the other pair member had the given CMV status was calculated. In twin research, this probability is called the probandwise concordance rate.
The concordance rate was calculated separately for long-living sibling pairs, for offspring enriched for familial longevity, and their partners and MZ and DZ twins. Using logistic regression, it was tested if the probabilities of offspring enriched for familial longevity were the same as those obtained for their partners and similarly for the MZ and DZ twins. Finally, the CMV concordance between the long-living sibling pairs and DZ twins was compared to test the impact of age similarity within the sib pairs as DZ twins have the same degree of genetic similarity as siblings, but have a higher degree of shared early environment due to identical age.
Analyses of twin similarity
The similarity of MZ and DZ twins was assessed not only using probandwise concordance rates for seropositivity and seronegativity but also tetrachoric correlations for CMV serostatus (which is the same for seropositivity and seronegativity). The classic twin-study methodology is based on the assumption that MZ twins have identical genotypes, whereas DZ twins share, on average, half of their segregating genes, and thus are no more genetically related than biological full siblings. A greater phenotypic similarity in MZ than in DZ twins is expected if there is a substantial genetic component in the aetiology of the disease. The probandwise concordance rate is defined as the proportion of affected twin partners of probands. It reflects the probability of one twin being infected, given that the partner twin is infected. Thus, it is directly comparable with risk rates reported for other relatives [Reference McGue14]. The correlations attributable to the dichotomous outcome, i.e. CMV seropositive, were investigated by assuming an underlying normally distributed liability (negative susceptibility) to a condition (CMV infection) because of genetic and environmental factors. The manifestation of a condition is established when an individual exceeds the threshold of affliction on the liability distribution, and the impact of genetic and environmental effects are reflected in the similarity of the other twin's liability to the condition [Reference Neale and Cardon15]. We estimated the correlations in liability by a multifactorial threshold model [Reference Falconer16] and the Mx software package [Reference Neale17].
Heritability
According to standard biometric practice when estimating heritability as the proportion of total phenotypic variance attributable to genetic variance in the population, no epistasis (gene–gene interaction), no gene–environment interaction or correlation and no assortative mating with respect to loci affecting the risk of CMV infection was assumed. The phenotypic variance can then be separated into four variance components: variance attributable to additive genetic effects (A), genetic dominance (D), shared environment (C), and non-shared (individual-specific) environment (E) [Reference Neale and Cardon15]. Only non-shared environments contribute to dissimilarity within MZ twin pairs because of their presumed genetic identity, whereas the effects of additive genetic factors and genetic dominance may also contribute to dissimilarity within DZ pairs, who share, on average, half of the additive and one-quarter of the dominant genetic factors. The method for selecting the best model followed standard procedures (structural equation analyses). Because the effects of genetic dominance (D) and shared environment (C) are completely confounded in the classical study of twins reared together, it is not possible to estimate all of the parameters simultaneously in a single model [Reference Neale and Cardon15]. Thus, five models (ACE, ADE, AE, CE, E) were fitted to the data. The best model is considered to be one that fits the data well (by a χ2 goodness-of-fit test based on log-likelihood difference of nested models) and is the most parsimonious (i.e. none of the parameters in the model can be deleted without a substantial increase in the χ2 value). For comparison of non-nested models Akaike's Information Criterion (AIC; −2*log-likelihood minus twice the degree of freedom) was used. The model with the lowest AIC represents the best balance of goodness of fit and parsimony [Reference Lindsey and Jones18]. For comparison among nested models, the χ2 difference test (i.e. Δχ2=2*Δlog-likelihood of the nested models) was used. The difference in χ2 of the models is itself distributed as a χ2 statistic, with the degrees of freedom equal to the difference in the degrees of freedom of the models being compared.
RESULTS
The baseline health characteristics of subjects of both study cohorts are shown in Table 1. The offspring enriched for familial longevity of the LLS had a lower prevalence of hypertension, diabetes mellitus and myocardial infarction.
Table 1. Characteristics for the participants in the Leiden Longevity Study (LLS) and in the Longitudinal Study of Aging Danish Twins (LSADT)

n.a., Not available.
Table 2 shows the herpesvirus seroprevalence in both cohorts. In the LLS, the overall seroprevalence of CMV in offspring and partners was 46%. Offspring enriched for familial longevity were significantly less likely to be CMV infected compared to their partners (offspring: 42% vs. partners: 51%, P=0·003). There was no difference in seroprevalence of the herpesviruses varicella zoster, herpes simplex and EBV. In the LSADT the overall prevalence of CMV was significantly higher at 76%. No differences in seroprevalence were found between MZ and DZ twins (MZ: 73% vs. DZ: 78%, P=0·15). Although the long-living siblings were older than the twins, they had lower CMV seroprevalence (60%, P<0·001).
Table 2. Herpesvirus seroprevalence in subjects of the Leiden Longevity Study (LLS) and the Longitudinal Study of Aging Danish Twins (LSADT)

n.a., Not available.
Within the long-lived sib-pairs the concordance rate in CMV seropositivity was 75% (Table 3). The intra-pair tetrachoric correlation in CMV serostatus for the long-lived sib-pairs was 0·62 [95% confidence interval (CI) 0·49–0·73] in same-sex siblings and 0·56 (95% CI 0·46–0·66) in all siblings.
Table 3. Concordance rates of cytomegalovirus (CMV) serostatus in 604 same-sex twins (302 pairs) aged 73–94 years in the Longitudinal Study of Aging Danish Twins (LSADT) and 844 long-lived sibs (574 pairs within sibships) from the Leiden Longevity Study (LLS)

CI, Confidence interval; MZ, monozygotic; DZ, dizygotic.
In the LSADT, the MZ and DZ twins had high and similar CMV concordance rates (MZ: 90% vs. DZ: 88%, P=0·51) (Table 3). The intra-pair tetrachoric correlation in CMV serostatus was 0·85 (95% CI 0·70–0·94) for MZ twins and 0·70 (95% CI 0·49–0·84) for DZ twins. The correlations were not different at conventional levels of significance (P=0·17). When applying an ACE model to the data, we found that the model including common environment (C) and non-shared environment (E) was the best fit with estimates of c2=0·78 and e2=0·22 where c2 is the proportion of variance in the underlying liability to CMV infection explained by shared environment, and e2 is the proportion explained by non-shared environment. There was no evidence for a genetic component in this cohort. The intra-pair tetrachoric correlation in DZ twins did not differ from that observed in long-lived sib-pairs at conventional levels of significance.
The offspring enriched for familial longevity were less susceptible to CMV infection in adulthood than their partners. Of the 372 offspring enriched for longevity living with a CMV-positive partner, only 58% were infected (Table 4). Of the 304 partners who lived with a CMV-positive offspring, 71% were infected (test for equal proportions: P<0·001). The intra-pair tetrachoric correlation in CMV serostatus for the offspring-partner pairs was 0·56 (95% CI 0·42–0·67).
Table 4. Cytomegalovirus (CMV) serostatus concordance between subjects enriched for longevity and partners, Leiden Longevity Study (726 pairs, n=1452)
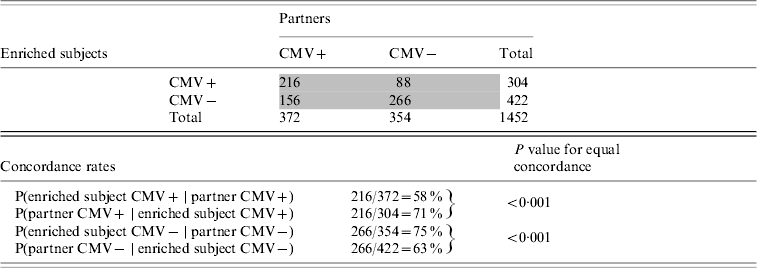
Numbers in grey cells refer to number of pairs, numbers in white cells refer to number of subjects.
DISCUSSION
When compared with their partners, offspring enriched for familial longevity had lower susceptibility to CMV infection. They were more likely to live with a CMV-infected partner if they themselves were seropositive for CMV and less likely to live with a seronegative partner if they themselves were seronegative. This pattern suggests that subjects enriched for familial longevity infect their partners more frequently, whereas the partners are less likely to infect the subjects enriched for longevity. The causes for this difference in CMV susceptibility might be genetic factors or environmental factors established before the partnership is formed, such as early-life environment. The prevalence of CMV infection increases with age although most evidence for this comes from cross-sectional studies which are unable to disentangle age effect from cohort effect as later cohorts may be less susceptible [Reference Dowd, Aiello and Alley8, Reference Staras9]. The long-lived siblings had significantly lower CMV prevalence than the twins (mean age 93 years and 79 years, respectively). The annual seroconversion in the general population is likely to be in the region of 1% [Reference Schottstedt19]. However CMV prevalence in the long-lived siblings is comparable to that of female day-care workers in their thirties in The Netherlands [Reference Stelma20] and approximately 30 percentage points lower than the contemporary 90·8% US prevalence estimates for persons aged >80 years [Reference Staras9], which illustrates the extremely low prevalence in LLS long-lived siblings. This indicates that the long-lived siblings are less susceptible to infection either due to genetic factors or advantageous early-life conditions. Family studies are not able to disentangle the effect of shared environmental factors and genetic influence, but twin studies are, and the present combination with both twin design and LLS design provides unique analytical opportunities.
The great degree of similarity in CMV concordance between MZ and DZ twins suggests that genetic factors are unlikely to play a major role in susceptibility to CMV infection in the general population. MZ twins share all their genetic variants while DZ twins share only half and still there is no higher similarity in CMV serostatus in MZ twins than in DZ twins. This suggests that susceptibility to CMV infection is driven to a great extent by shared environment, not shared genes. It is theoretically possible that the low susceptibility to CMV infection observed in LLS long-lived siblings and their offspring has a substantial genetic component. The LSADT findings of an absence of genetic effects in twins pertain to the general population, but the findings do not rule out the existence of families with a genetic predisposition for low susceptibility to CMV. The LLS participants were selected on the basis of familial longevity. If selection on familial longevity induces co-selection for genes that decreases susceptibility to CMV at any episode in life, then the findings of the LSADT may not apply to LLS families. The lower rate of CMV infection may have contributed to survival to high ages in these families. Recent studies have shown that CMV is associated with mortality [Reference Roberts6, Reference Wang7], but there is no conclusive evidence yet that CMV negativity has a causal effect on longevity. For example, no association was observed with mortality in the two samples included in this study. The comparison of LSADT DZ twins and LLS long-lived siblings also supports the interpretation that shared environment may be responsible for CMV susceptibility. These two groups are full siblings and share an average of 50% of their genes. These two groups also share early-life environment. As LSADT DZ twins are closer in age than LLS long-lived siblings the degree to which they share early-life environment is probably somewhat higher. The intra-pair tetrachoric correlations in CMV serostatus were slightly higher in LSADT DZ twins than in same-sex LLS long-lived siblings, but not different at conventional levels of significance. So, despite the fact that LLS long-lived siblings have an unexpectedly low prevalence of CMV they look like the general population – as represented by the DZ twins – within pairs. This is exactly what would be expected if shared environment in early life was responsible for CMV susceptibility. Therefore, our findings are best explained by a persisting influence of early-life environment on CMV susceptibility that extends beyond childhood. The factors responsible are still unknown.
This study is based on two cohort studies that are among the largest of their kind. The combination of these cohorts uniquely enables us to separate the effects of different types of influences on CMV susceptibility. Mismeasurement of exposure and outcome is probably a minor source of bias for our study. The measurement of CMV serostatus in both cohorts was performed using standard assessment techniques, which carry only a very small risk of mismeasurement. The validity of the assignment of zygosity in the LSADT group has been shown to be higher than 95% [Reference Christiansen21].
This study rests upon an assumption of comparability between the two cohorts in terms of underlying CMV susceptibility. The differences in CMV seroprevalence between the various groups could reflect age effects (CMV susceptibility increases between midlife and old age) or cohort effects (susceptibility is different in cohorts born in the 1910s and 1920s compared to cohorts born later). It is possible that the relative importance of genetics, early- and later-life environment varies by age or cohort, which would limit the comparability of the two cohorts and the generalizability of our findings. We propose that genetic factors and adult environment play a lesser role in susceptibility to CMV inflection than early-life environment. However, genetics and adult environment may still play an important role in determining susceptibility to infection as well as moderating the effects of CMV infection on the immune system, but this cannot be explored in this present study as data on immune function would be needed for both cohorts.
ACKNOWLEDGEMENTS
This study was partially supported by the LifeSpan Network of Excellence, which was funded by the European Union 6th Framework Programme. The first and corresponding author is the guarantor for the integrity of the article as a whole.
DECLARATION OF INTEREST
None.








