Introduction
Field experiments assessing the impact of weeds on crop growth and yield have been conducted for many crops and a wide range of weeds. These experiments generally fall into two categories: first, those using naturally occurring weeds, often hampered by uneven distribution and density of weed species and by staggered germination (Cardoso et al. Reference Cardoso, Alves, Severino and Vale2011; Elezovic et al. Reference Elezovic, Datta, Vrbnicanin, Glamoclija, Simic, Malidza and Knezevic2012); and second, those using sown weeds, often of a single weed species and usually at specific densities (Ma et al. Reference Ma, Yang, Wu, Jiang, Ma and Ma2016; MacRae et al. Reference MacRae, Webster, Sosnoskie, Culpepper and Kichler2013).
The second approach using sown weeds, often of a single species, offers the advantage of giving detailed information on the impacts of individual weed species. However, this approach can be challenging to undertake, as it can be difficult to obtain the large quantities of weed seed that may be required for field experiments. In addition, it can be difficult to get weeds to germinate at the densities and times required for an experiment, often because of low germination rates and variable seed dormancy (Strydhorst et al. Reference Strydhorst, King, Lopetinsky and Harker2008). These issues can be overcome by transplanting weeds into the field (Ma et al. Reference Ma, Yang, Wu, Jiang, Ma and Ma2016; MacRae et al. Reference MacRae, Webster, Sosnoskie, Culpepper and Kichler2013), but this process is labor-intensive and the survival rate of transplanted weeds can be poor in hot, dry conditions, as is often the case over much of the period when cotton is grown in Australia.
An alternative approach for competition experiments is to use a “mimic” weed, which is normally a readily available cultivated species with a high germination rate and low seed dormancy. Among the many species used as mimic weeds are barley (Hordeum vulgare L.) (Strydhorst et al. Reference Strydhorst, King, Lopetinsky and Harker2008), common sunflower (Charles and Taylor Reference Charles and Taylor2007), Japanese millet (Wu et al. Reference Wu, Walker, Osten and Robinson2010), oats (Avena sativa L.) (Brain et al. Reference Brain, Wilson, Wright, Seavers and Caseley1999), perennial ryegrass (Lolium perenne L.) (Afifi and Swanton Reference Afifi and Swanton2012), rapeseed (Brassica napus L.) (Vollmann et al. Reference Vollmann, Wagentristl and Hartl2010), white mustard (Sinapis alba L.) (Didon and Boström Reference Didon and Boström2003; Lotz et al. Reference Lotz, Christensen, Cloutier, Fernandez Quintanilla, Légère, Lemieux, Lutman, Pardo Iglesias, Salonen, Sattin, Stigliani and Tei1996), and winter wheat (Triticum aestivum L.) (Cerrudo et al. Reference Cerrudo, Page, Tollenaar, Stewart and Swanton2012).
Mimic weeds are often chosen from crop species of the same genus as the real weeds for which they substitute—oats substituting for wild oat (Avena fatua L.) (Brain et al. Reference Brain, Wilson, Wright, Seavers and Caseley1999) and Japanese millet for junglerice (Wu et al. Reference Wu, Walker, Osten and Robinson2010). When closely related species are not available, species of different genera that display similar morphological characteristics have been used, such as common sunflower substituting for fierce thornapple (Charles and Taylor Reference Charles and Taylor2007).
Where nutrients and water are not limiting, weeds primarily compete for light (Didon and Boström Reference Didon and Boström2003; Ma et al. Reference Ma, Yang, Wu, Jiang, Ma and Ma2016). Consequently, plant height, leaf structure and orientation, photosynthetic rate, and growth rate are the primary morphological characteristics associated with plant competitiveness in well-fertilized, fully irrigated crops (Didon Reference Didon2002; MacRae et al. Reference MacRae, Webster, Sosnoskie, Culpepper and Kichler2013), as is the situation for most cotton grown in Australia. Thus, mimic weeds are generally chosen to have a similar height and structure to the real weed, although other factors can be important, such as a similarity in sensitivity to herbicides (Brain et al. Reference Brain, Wilson, Wright, Seavers and Caseley1999).
The use of a mimic weed in competition studies offers many advantages, such as greater experimental controllability and repeatability (Strydhorst et al. Reference Strydhorst, King, Lopetinsky and Harker2008). Mimic weed cultivation generally involves the ability to achieve more uniform weed germination, growth, density, and distribution (Lotz et al. Reference Lotz, Christensen, Cloutier, Fernandez Quintanilla, Légère, Lemieux, Lutman, Pardo Iglesias, Salonen, Sattin, Stigliani and Tei1996; Vollmann et al. Reference Vollmann, Wagentristl and Hartl2010). Experiments utilizing mimic weeds can be conducted to examine aspects of competition such as the effects of crop variety (Didon and Boström Reference Didon and Boström2003), soil nutrition, and herbicide tolerance (Brain et al. Reference Brain, Wilson, Wright, Seavers and Caseley1999), without the confounding effects of uneven weed emergence, growth, density, and distribution. Alternatively, mimic weeds can be valuable to examine the effects of weed emergence, growth, density, or distribution, while keeping the other factors constant (Charles and Taylor Reference Charles and Taylor2007). Post-experiment issues, such as the ongoing germination of hard-seeded weeds from seeds produced during the experiments (Walker et al. Reference Walker, Wu and Bell2010), may also be reduced or largely eliminated by using mimic weeds.
The studies thus far, however, have relied on the often-unstated assumption that the competitive effect of the mimic weed is similar to that of a real weed. This assumption is central to these studies but has not previously been examined in the scientific literature. The aim of the current study was to test this assumption (that a mimic weed can have a competitive effect comparable to that of a real weed), comparing the morphological traits of the mimic and real weeds, and their competitive effects on cotton morphology and lint yield.
The weeds used in the study—bladder ketmia, fierce thornapple, and junglerice—are species commonly problematic in cotton fields in Australia (Werth et al. Reference Werth, Boucher, Thornby, Walker and Charles2013) that represent a range of weed morphological types: a medium-sized broadleaf weed, a large broadleaf weed, and a grass weed, respectively. Bladder ketmia, fierce thornapple, and junglerice were compared with the mimic weeds: mungbean, common sunflower, and Japanese millet, respectively.
Materials and methods
Experiments were conducted in the three growing seasons, 2004–2005, 2006–2007, and 2007–2008, in bins constructed from fibrous cement sheets, each bin with surface dimension 1.0 m by 0.9 m, and 0.5 m deep, fully exposed to the external environment, at the Australian Cotton Research Institute, Narrabri (30.12°S, 149.36°E, elevation 201 m). Bins were filled with a sand and peat moss mix (2:1 by volume), with a drainage hole in each bin. Bins were arranged in eight rows, oriented north–south, and irrigated to saturation by overhead sprinklers in rows on a weekly basis. A slow-release complete plant fertilizer was applied at planting at the recommended rate, and bins were top-dressed every 2 wk with nitrogen at 18 kg ha–1 in the form of granulated urea (46% N). Weed seeds of bladder ketmia, fierce thornapple, and junglerice were collected from multiple plants from nearby cotton fields over the 2002–2003 and 2003–2004 seasons.
Experimental design
The experiments used a factorial design with six species and five weed densities, with four replicates. Cotton and weeds were planted in a single row 0.9 m in length running north to south along the center of each bin on October 13 of each year. All bins were planted with cotton at 15 seeds m–1, and weeds were planted to achieve 0, 1, 2, 5, and 10 plants m–1 for common sunflower and fierce thornapple; 0, 3, 6, 15, and 30 plants m–1 for mungbean and bladder ketmia; and 0, 10, 20, 50, and 100 plants m–1 for Japanese millet and junglerice—one real or mimic weed species per bin. These target populations represented moderate to heavy levels of weed pressure, as typically seen in Australian cotton fields. Populations were thinned to the target numbers of weeds 6 wk after planting. The removed weeds were selected to optimize spacing between the remaining weeds. Bins were hand-weeded regularly to remove other weeds.
Data collection
The number of cotton plants and corresponding weed plants were recorded in each bin at mid-season, 90 d after planting (DAP), and at cotton harvest, 150 DAP. Plant height, the number of leaves, and the node number (broadleaf plants only) were recorded at mid-season on five cotton and five weed plants in each bin chosen randomly (all weeds were measured in treatments with fewer than five weeds per bin). Two weed plants were removed from each bin at this stage, 90 DAP, after cutting at ground level. Leaf area, leaf size, and aboveground plant weight of the weeds was recorded (a single weed was removed and measured in treatments with only one weed per bin). This removal was done after the end of the critical period for weed control (Korres and Norsworthy Reference Korres and Norsworthy2015). Leaf area was measured using a Li Cor LI-3100 area meter (LI-COR Inc., Lincoln, NE). Plants were weighed after drying at 70 C for at least 72 h in a forced-air oven. Weed and cotton height, cotton node number, and the aboveground plant dry weight of the cotton were recorded at cotton harvest. Once all bolls had opened, seed cotton was hand-picked from all cotton plants in each bin and was ginned using a single-saw gin to determine cotton lint yield.
Statistical analysis of paired data sets
Data for each of the morphological traits of both weeds and cotton, the cotton lint yield, and the ginning percentage were compared for the effects of year, density, and species by ANOVA for each real weed and mimic weed pair separately (junglerice vs. Japanese millet, bladder ketmia vs. mungbean, and fierce thornapple vs. common sunflower) using R statistical software (R version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria), with a significance level of P ≤ 0.05. Treatment means for each weed pair were averaged over density and separated using Fisher’s protected LSD test at P ≤ 0.05, where density was not a significant effect in the analysis. Data were presented separately where density was a significant effect without an effect of species. Figures were used to present relationships where a significant effect of both density and species was indicated (P ≤ 0.05). Linear (Equation 1), allometric (Equation 2), exponential (Equation 3), and hyperbolic (Equation 4) models were fit to these data and the Akaike information criterion used to select the model with best fit. These equations were as follow:
where y is the morphological trait, ginning percentage, or lint yield; x is the weed density; a is the intercept of the line; and b is the slope of the line,
where y is the morphological trait, ginning percentage, or lint yield; x is the weed density; a and b are constants,
where y is the morphological trait, ginning percentage, or lint yield; x is the weed density; a,b, and c are constants, and
where y is the morphological trait, ginning percentage, or lint yield; x is the weed density; a is the upper asymptote;b is a constant.
Standard errors of the means were determined using the delta method in R. The quality of the fit of the models was assessed using the coefficient of determination (r 2).
Statistical analysis of combined data set
The data sets for the six species were combined. The effects of weed species, density, and year on the cotton lint yield were examined for the combined data set using ANOVA. Density and year were not significant effects in the analyses, with differences in lint yield related only to species. To explore any associations between the morphological traits and cotton lint yield, a linear model (Equation 1) was developed, relating cotton lint yield per meter to weed species and the combination of each of the morphological traits per meter. Data for each trait were first tested for normality and log transformed where indicated by the analysis. Both transformed and nontransformed traits were included in the regression, allowing the results of the regression to select the relationship with the best fit. Traits that were not significantly correlated with cotton lint yield were progressively deleted from the analysis using the stepwise linear regression method. A minimal model derived from the analysis containing only those traits significantly (P < 0.05) correlated with lint yield was fit to the data. This process, using stepwise linear regression, was repeated to explore the association between the morphological traits and relative cotton lint yield. Linear (Equation 1) and exponential (Equation 3) regression models were fit to the relationships for lint yield and relative lint yield, and the Akaike information criterion used to select the model with best fit. The quality of the fit of the models was assessed using the coefficient of determination (r 2).
Results and discussion
The species used as mimic weeds in this study were chosen because they had at least some physical resemblance to the real weeds selected for the study, with broad similarities in plant height, dry weight, and architecture. However, in-season measurements showed many differences between the real and mimic weeds, as described below.
Japanese millet vs. junglerice
Junglerice plants were taller than Japanese millet plants at mid-season, but no difference in plant height was observed at cotton harvest (Table 1). There were no effects of species or density on leaf number, leaf area, or leaf size at mid-season (Table 1). Dry weight at mid-season decreased with increasing weed density, but there was no effect of species (Table 2).
Table 1. Comparisons of morphological traits for each weed and mimic weed pair at mid-season (90 d after planting), and plant height at cotton harvest (150 d after planting).
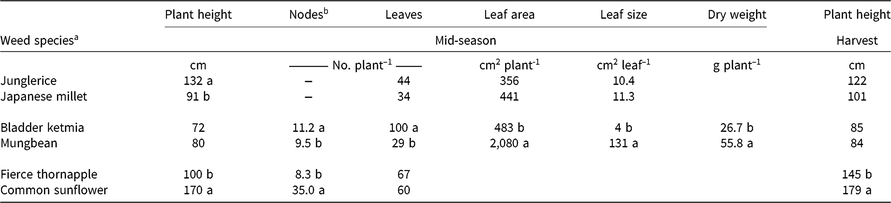
a There was no significant (P ≥ 0.05) effect of weed density. Within each pair, means followed by differing letters are significantly different according to Fisher’s protected LSD test at P ≤ 0.05.
b Node number was not recorded for the grasses.
Increasing weed density reduced the height of the accompanying cotton plants at mid-season, with no difference between the species (Table 2). There was no effect of weed species or density on cotton leaf number at mid-season, or cotton height, node number, dry weight, or ginning percentage at harvest (Table 3). However, junglerice reduced cotton’s node number at mid-season by one node when compared with Japanese millet (Table 3). The presence of either weed caused a reduction in lint yield of 30% or more at all densities, with junglerice reducing lint yield by 16% more than Japanese millet at the highest weed density—a 53% yield reduction compared with a 37% reduction for Japanese millet (Figure 1). The greater reduction in lint yield at the highest density may be related to the greater height at mid-season of the junglerice plants when compared with Japanese millet.
Table 2. Comparisons of Japanese millet and junglerice dry weight, and the effect of these weeds on cotton height at mid-season (90 d after planting), over weed densities of 10 to 100 plants m–1 of crop row.

a The analysis showed no effect of species. Means followed by differing letters are significantly different according to Fisher’s protected LSD test at P ≤ 0.05.
Table 3. Responses of cotton morphological traits and ginning percentage to competition from weed and mimic weed pairs. Measurements were taken at mid-season, and at cotton harvest, 90 and 150 d after planting, respectively.

a There was no significant (P ≥ 0.05) effect of weed density. Within each pair, means followed by differing letters are significantly different according to Fisher’s protected LSD test at P ≤ 0.05.
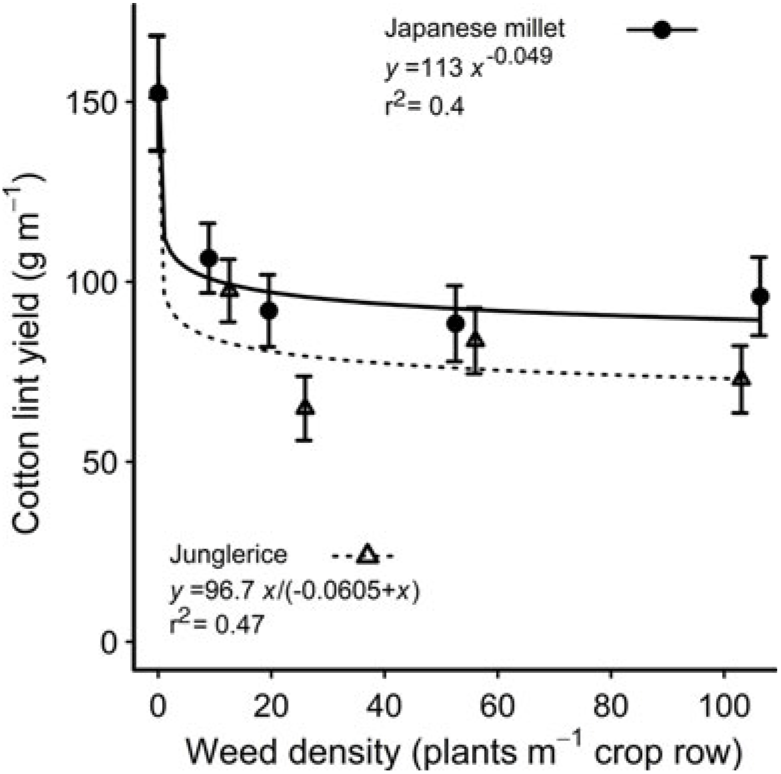
Figure 1. Reduction in cotton lint yield from competition with Japanese millet and junglerice over densities of 10 to 100 weeds m–1 of crop row. The parameters of the equations are shown within each figure. Vertical bars indicate 1 SEM.
The junglerice in this study was quite different from the plants reported by Awan et al. (Reference Awan, Chauhan and Cruz2014), who used junglerice collected from fields in Indonesia. At a similar weight, the Indonesian junglerice plants had many more leaves and much greater leaf area than the plants in our study. This disparity in leaf number and leaf area can be attributed to the plasticity of this species, to the very different selection environments of rice production in Indonesia, and to the more open canopy of the broad-acre cropping system of the Australian cotton industry. Phenotypic plasticity among Echinochloa spp. has been reported elsewhere (Claerhout et al. Reference Claerhout, Dewaele, De Riek, Rerheul and De Cauwer2016; Danquah et al. Reference Danquah, Johnson, Riches, Arnold and Karp2002; Tabacchi et al. Reference Tabacchi, Mantegazza and Ferrero2006). Altop and Mennan (Reference Altop and Mennan2011), for example, reported variations of up to 100% in plant height, leaf area, and dry weight of E. crus-galli (L.) Beauv. ECHCG samples from Turkey.
Given the known plasticity of the Echinochloa genus, the similarity of morphological traits between the Japanese millet and the junglerice used in our study indicates that Japanese millet has value as a mimic weed to substitute for junglerice. However, the variety of Japanese millet we used was less competitive than our junglerice and therefore not an ideal mimic weed to be substituted for junglerice. A more aggressive millet variety may be more suitable for future studies.
Mungbean vs. bladder ketmia
Mungbean and bladder ketmia plants remained similar in height throughout the season but varied in node number, leaf number, leaf area, leaf size, and dry weight at mid-season (Table 1). Bladder ketmia had two more nodes and three times more leaves at mid-season than did mungbean. However, the mungbean leaves were 34 times larger than the bladder ketmia leaves, and mungbean had 4 times the leaf area compared with bladder ketmia at mid-season. Mungbean plants were double the weight of the bladder ketmia plants.
There was no effect of weed species or density on plant height, node number, leaf number, or ginning percentage of the cotton plants (Table 3). However, mungbean reduced the dry weight of the cotton plants at harvest by 15% when compared with the bladder ketmia, with no effect of weed density. The presence of both weed species reduced cotton lint yield compared with the weed-free plots, but there was no effect of species (Table 4).
Table 4. Response in cotton lint yield to the presence of bladder ketmia and mungbean at densities of 0 to 30 m–1 of crop row.

a The analysis showed no effect of species. Means followed by differing letters are significantly different according to Fisher’s protected LSD test at P ≤ 0.05.
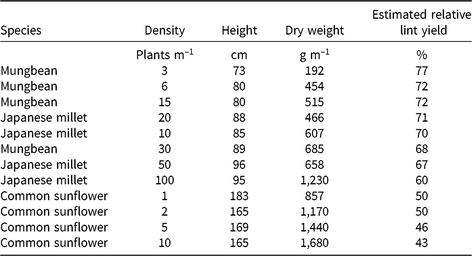
Table 5. The relationship between weed height and dry weight at mid-season (90 d after planting) of the mimic weeds, mungbean, Japanese, millet and common sunflower, and estimated relative yield of irrigated cotton using the regression equation y = 91.3 – 0.167 x – 0.0122 w, where y is the lint yield relative to the weed-free yield, x is the weed height, and w the weed dry weight
The bladder ketmia used in this study had a large number of small, deeply lobed leaves, with low specific leaf area compared with mungbean. Bladder ketmia is a variable species (Johnson and Craven Reference Johnson and Craven2013), with plants from different regions varying greatly in their morphological traits (Johnson Reference Johnson2003). The large number of small leaves is typical of bladder ketmia, with the plants in our study mid-range in size (height, leaf number, and leaf area) when compared with those in the study of Johnson (Reference Johnson2003). Mungbean was more similar in leaf size to Hibiscus verdcourtii Craven, another closely related weed also common in Australian cotton fields (Werth et al. Reference Werth, Boucher, Thornby, Walker and Charles2013).
Given the differences in node number, leaf number, leaf size, leaf area, and dry weight between the two species, mungbean would not seem to be a suitable mimic weed to substitute for bladder ketmia. However, the real and mimic weeds had similar competitive effects on the cotton, with no differences in their effect on cotton lint yield or their impact on other cotton morphological traits, other than a small difference in cotton dry weight at harvest. On this basis of a similar competitive effect, mungbean is an acceptable substitute for bladder ketmia.
Common sunflower vs. fierce thornapple
Fierce thornapple plants were shorter than common sunflower both at mid-season and at cotton harvest, although the difference in height diminished with time (Table 1). Fierce thornapple was a more open, branched plant and had only a quarter the number of nodes compared with common sunflower at mid-season, but the same number of leaves (Table 1). The common sunflower used in these experiments was a commercial variety (‘Hysun 38’) and produced a single head on the main stem, with no branching. There was a large disparity in leaf size at mid-season, with common sunflower leaves 18 times larger than fierce thornapple leaves at the lowest weed density, diminishing to 9 times larger at the highest density (Figure 2A). Plant density had almost no effect on fierce thornapple leaf size at mid-season, whereas common sunflower leaf size declined by 60% as weed density increased. The disparity in leaf size resulted in common sunflower having 11 times the leaf area of fierce thornapple at the lowest density at mid-season, declining to 4 times the leaf area at the highest density (Figure 2B). Weed dry weight was affected by both species and density. Common sunflower plants were four times heavier than fierce thornapple plants at mid-season for the lowest weed density, with no difference at the highest weed density (Figure 2C).
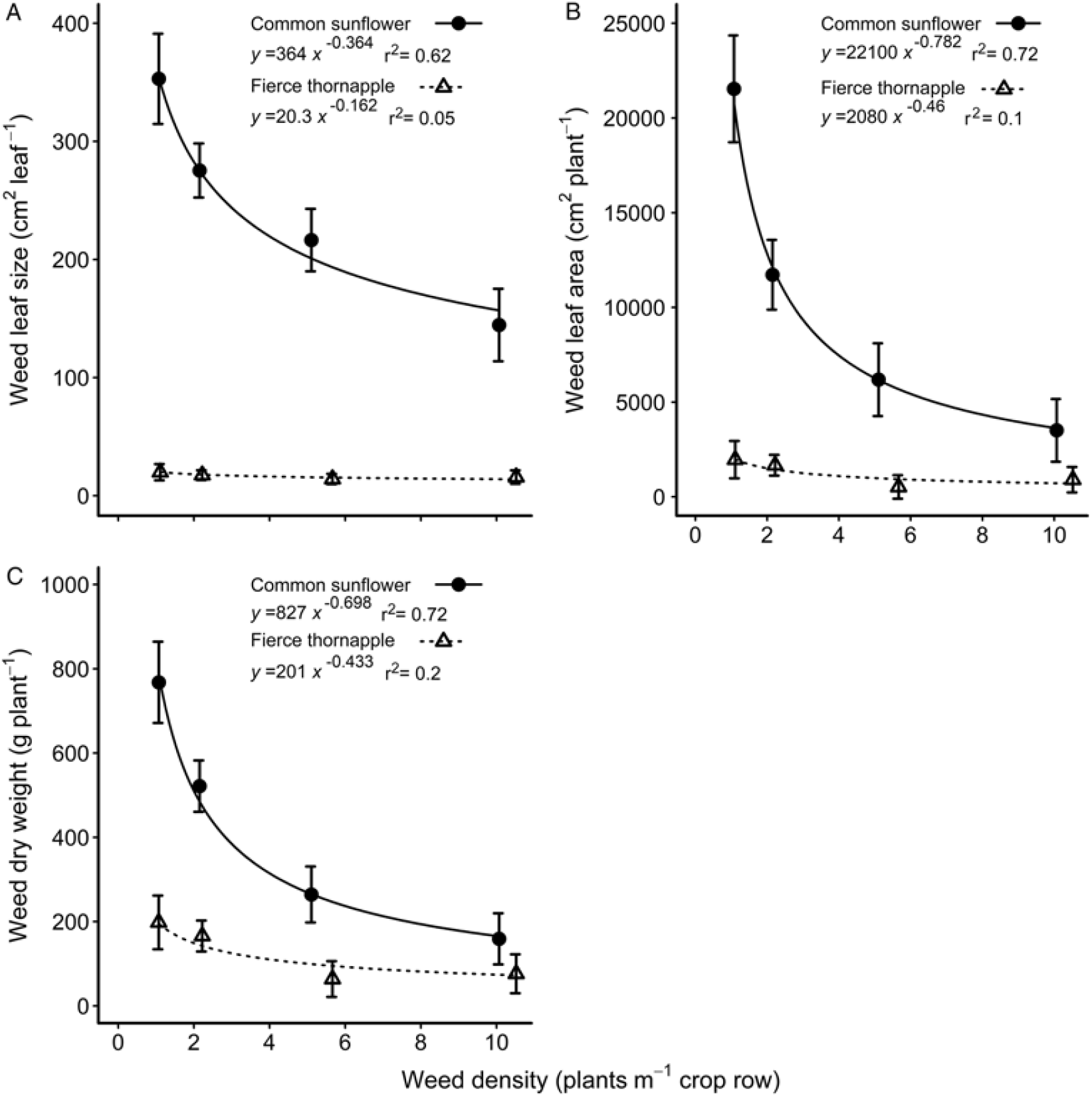
Figure 2. Comparisons of common sunflower and fierce thornapple leaf size (A), leaf area (B), and aboveground dry weight (C) at mid-season (90 d after planting) over densities of 1 to 10 weeds m–1 of crop row. The parameters of the equations are shown within each figure. Vertical bars indicate 1 SEM.
The presence of common sunflower had greater negative impact on the morphological traits of the accompanying cotton than did fierce thornapple at all weed densities and over the range of traits measured. Crop height at mid-season was reduced 47% more by one common sunflower per meter, than by one fierce thornapple, declining to 58% more at 10 weeds m–1 of crop row (Figure 3A). At cotton harvest, the reduction in crop height was still 19 cm, or 24%, with no effect of density remaining (Table 3). Fierce thornapple had little impact on the node number of the cotton. At mid-season, the cotton plants had four fewer nodes when competing with common sunflower compared with fierce thornapple at 1 weed m–1, declining to six fewer nodes at 10 weeds m–1 (Figure 3B). Cotton competing with common sunflower still had approximately four fewer nodes than cotton competing with fierce thornapple at cotton harvest (Table 3). Common sunflower reduced the leaf number per meter of the cotton by around 57% more than did fierce thornapple at mid-season over the range of weed densities (Figure 3C). Cotton plants were 60% lighter at harvest when competing with common sunflower, compared with plants competing with fierce thornapple (Table 3). These differences combined to cause a 41% greater reduction in lint yield when cotton competed with common sunflower compared to cotton competing with fierce thornapple at 1 weed m–1, declining to a 50% yield reduction at 10 weeds m–1 (Figure 3D).
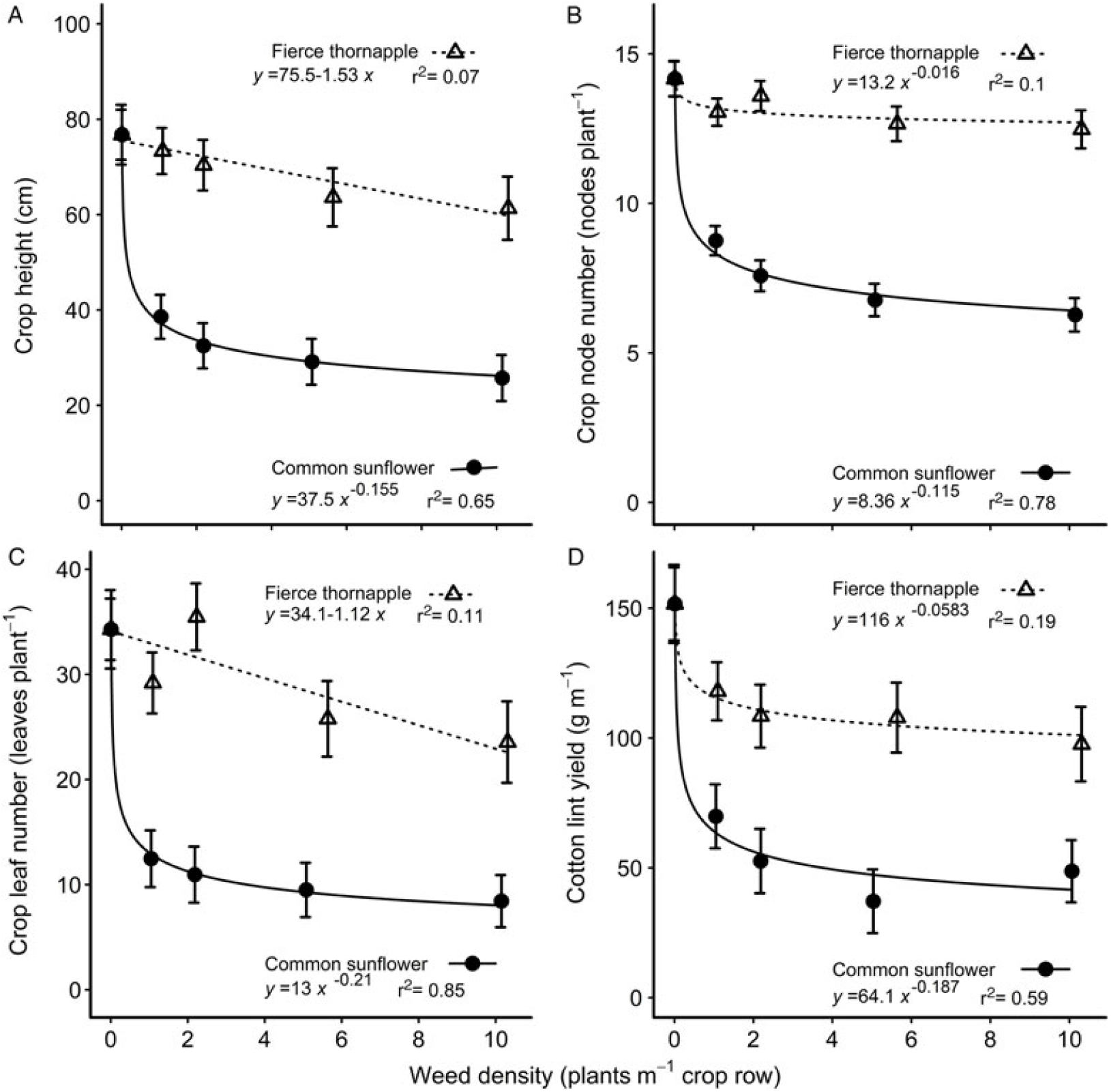
Figure 3. Effect of competition from common sunflower and fierce thornapple on cotton height (A), node number (B), and leaf number (C) at mid-season (90 d after planting), and the lint yield at cotton harvest (D) over densities of 1 to 10 weeds m–1 of crop row. The parameters of the equations are shown within each figure. Vertical bars indicate 1 SEM.
The fierce thornapple plants in our study weighed 91 to 236 g plant–1 at mid-season for 10 and 1 plant m–1 of crop row, respectively—much less than the plants reported in Charles et al. (Reference Charles, Murison and Harden1998), which averaged 1,100 ± 300 g dry weight plant–1—but were similar in height to the plants in Charles et al. (Reference Charles, Murison and Harden1998) at 140 ± 10 cm (Table 1). This difference in weight was associated with heavy insect injury suffered by the plants in the current study, especially later in the season. This level of insect injury was not seen in fierce thornapples in the previous study, as insecticides were heavily used to manage insects in the cotton crop where these weeds were located. As a consequence of this heavy insect injury, the fierce thornapple in our study was less competitive than the plants in the earlier study and less competitive than the common sunflower, which suffered minimal insect injury. The common sunflower plants were similar in weight to the plants reported by Massignam et al. (Reference Massignam, Chapman, Hammer and Fukai2009), which at maturity ranged from 200 to 400 g plant–1, but smaller in size than the fierce thornapple plants reported in Charles et al. (Reference Charles, Murison and Harden1998).
Given these differences in node number and branching, leaf size and area, and impact on cotton yield, the common sunflower variety chosen was not an ideal mimic weed to substitute for fierce thornapple. It is likely that a shorter, branched variety of common sunflower would have been more suitable for this study.
Cotton lint yield and the morphological traits
Selecting a mimic weed that closely resembles a real weed in both its morphological traits and its impact on the crop is an ideal that may be difficult to achieve in practice. Japanese millet, for example, is closely related to junglerice and appears to be a good substitute for this weed, but in our study, it caused around 15% less reduction in cotton lint yield compared with junglerice. This difference between Japanese millet and junglerice could not be overcome by adjusting the density of the mimic weed within the range of densities used in our study (Figure 1). Yet, for the cotton grower, it is the impact of the weed on the crop’s yield that is the most important measure of the weed’s competitive effect on the crop. Hence, in practical terms, a mimic weed that has the same impact on crop yield as the real weed is arguably a suitable substitute in competition experiments even if it differs in other morphological traits. Such was the case with bladder ketmia and mungbean, where despite the many differences in their morphological traits, the two species had similar impacts on cotton lint yield (Table 4). From this observation, we conjectured that differences or similarities in some of the morphological traits we measured were more strongly associated with reductions in cotton lint yield than were differences in other traits.
To explore associations between morphological traits of a weed and reductions in crop yield, data sets over all weeds (real and mimic weeds) were combined and stepwise linear regression was used to determine which of the morphological traits was most significantly correlated with the reductions in cotton lint yield (P ≤ 0.05). Of the traits measured, a combination of weed height and weed dry weight at mid-season were most significantly correlated (P < 0.001) with reductions in cotton lint yield, with neither species (real or mimic weed), plant density, nor year significant components of the regression. The relationship between cotton lint yield and a competition index combining weed height and weed dry weight at mid-season is shown in Figure 4. These relationships were further generalized by comparing relative cotton lint yield (lint yield as a percentage of the weed-free yield) with weed height (Figure 5A) and weed dry weight (Figure 5B) at mid-season, and developing a new combined competition index incorporating weed height and dry weight (Figure 5C).
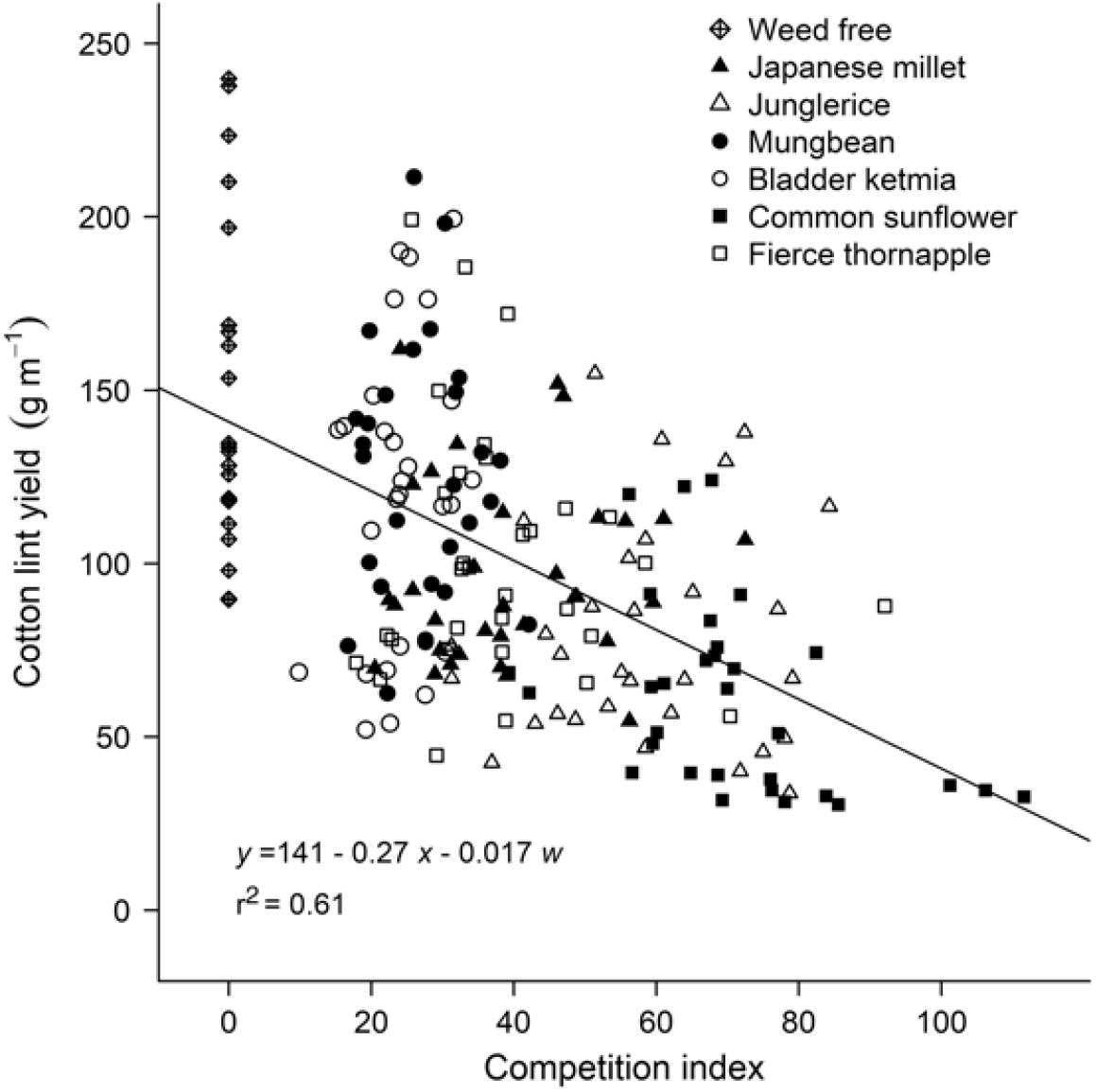
Figure 4. Cotton lint yield (y) was correlated with a competition index derived using stepwise regression. The competition index combined weed height (x) and dry weight in g m–1 (w) at mid-season, 90 d after planting. The parameters of the equation are shown within the figure.
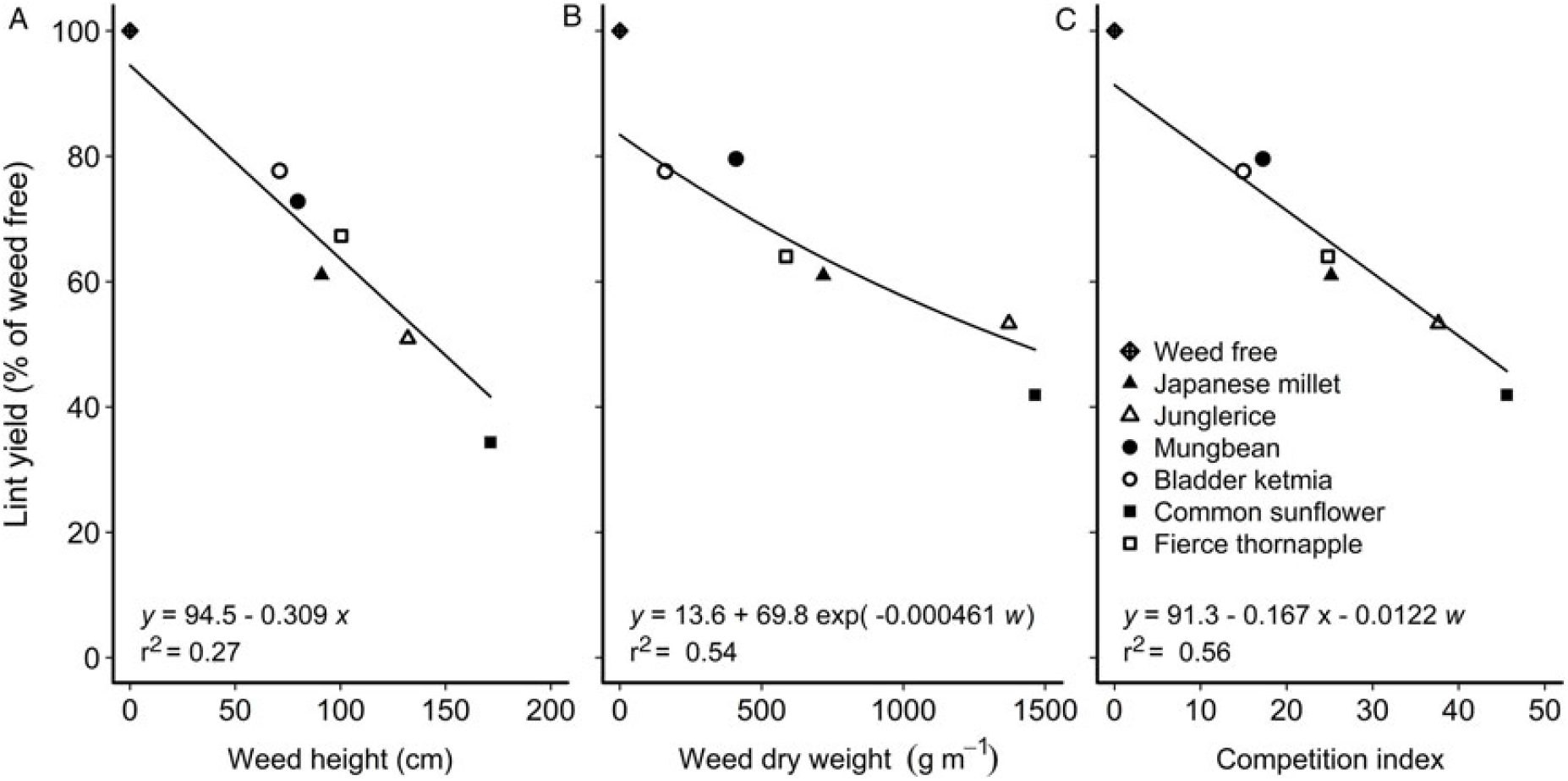
Figure 5. Cotton lint yield (y) as a percentage of the weed-free yield (y) was correlated with (A) weed height (x), (B) weed dry weight (w), and (C) the combination of height (x) and dry weight (w) at mid-season, 90 d after planting. The equations of the lines are shown within each figure.
The importance of weed dry weight in our findings was consistent with the observations of Charles et al. (Reference Charles, Murison and Harden1998), Goldberg and Landa (Reference Goldberg and Landa1991), and Ma et al. (Reference Ma, Yang, Wu, Jiang, Ma and Ma2016), and effectively integrates many of the other components of competitiveness. Dry weight is often used as a measure of competitive ability (Awan et al. Reference Awan, Chauhan and Cruz2014; Brain et al. Reference Brain, Wilson, Wright, Seavers and Caseley1999; Cerrudo et al. Reference Cerrudo, Page, Tollenaar, Stewart and Swanton2012). Although weed density was not directly an important effect in our combined analysis, it had a significant effect on weed dry weight (gm–1) of all weed pairs, with dry weight per meter increasing as density increased. Hence, weed density was incorporated in the relationship as a component of weed dry weight, with dry weight a more significant indicator of reductions on crop yield than weed density per se. Plant height also significantly correlated with weed competitiveness (Didon Reference Didon2002; MacRae et al. Reference MacRae, Webster, Sosnoskie, Culpepper and Kichler2013), as plants primarily compete for light in situations where water and nutrients are not limiting. This correlation emphasizes the need to ensure that real weeds and their mimic analogues are as similar in height throughout the season as possible. Didon (Reference Didon2002) also observed that early growth rate and leaf angle can be important traits in competition for light, and these may have been additional factors in the differing responses of junglerice and Japanese millet. Our measurements were less detailed than that of Didon (Reference Didon2002), not allowing this level of scrutiny.
Selecting mimic weeds
From our findings, we conclude that mimic weeds should be chosen that match as closely as possible the height and dry weight at mid-season of the real weeds, with attention to changes in these traits throughout the season. Varietal variation in these traits may provide for closer matching among commercial crop varieties. In a fully irrigated cotton crop with good crop nutrition, the competitive differences between plants as dissimilar as common sunflower and junglerice could be largely related to differences in plant height and dry weight. This difference allows the possibility of mimic weeds being substituted for a range of weeds in irrigated cotton, provided one can account for the differences in these traits. The mimic weeds in our study covered a range from a height of 73 cm and dry weight of 192 g m–1 for 3 mungbeans m–1 at mid-season, reducing the estimated yield of the accompanying cotton to 77% relative to weed-free cotton, through a height of 165 cm and dry weight of 1,680 g m–1 for 10 common sunflower plants m–1, reducing the cotton yield to 43% (Table 5). The results from these mimic weeds could be used to estimate the yield loss for any weed in this height and dry-weight range. Data from other mimic weeds would be needed to define the relationship outside this range.
When comparisons are being made where seasonal variation can have a larger impact on crop production, such as in rain-fed cropping, this accounting for differences in height and dry weight may require comparisons between real and mimic weeds in each experimental season to allow for seasonal variations (Halford et al. Reference Halford, Hamill, Zhang and Doucet2001). Where seasonal and environmental conditions are not limiting, such as with fully irrigated cotton, it seems likely that mimic weeds can be satisfactorily substituted for real weeds, provided the plants have similar height and dry weight at mid-season, or one can account for the differences in height and dry weight. From our results in fully irrigated cotton, it would seem that it is unnecessary to study the competitive effects of a full range of weed species, as results could be extrapolated from fewer species. However, differences in root biomass and architecture were not considered in our study where rooting depth was constrained. Differences between weeds in root biomass and architecture may be of more importance in the field, especially where soil moisture and nutrition are limiting. Future studies should be undertaken in the field where rooting depth and architecture are not constrained by artificial barriers, as may have occurred in our study.
This work has implications for competition experiments in well-fertilized, fully irrigated cotton, where competition studies have previously often been undertaken on a species-by-species basis. It may be possible to extend our experimental approach using mimic weeds to develop a multi-species weed competition model, where species differences are accounted for using differences in plant height and dry weight. This possibility warrants further testing in the field.
Author ORCIDs
Graham William Charles https://orcid.org/0000-0003-0691-5335
Acknowledgments
We gratefully acknowledge the support for this work by the NSW Department of Primary Industries (DPI), the University of New England, the Cotton Research and Development Corporation (CRDC), and the Australian Cotton Cooperative Research Centre. No conflicts of interest have been declared. We also gratefully thank Dr. Ian Taylor (CRDC) who initiated this work, Bruce McCorkell, Biometrician, DPI, for his help analyzing the data, and the reviewers who contributed to the paper.














