Introduction
In recent years, adoption of conservation tillage practices, including cover crops, has increased substantially in the southern United States for benefits related to increased soil organic matter (OM) and improved soil health properties, such as retained soil moisture, increased nutrient holding capacity, reduced erosion potential, increased soil microbial diversity, and potentially lower crop production inputs (Gallaher Reference Gallaher1977; Gianessi Reference Gianessi2005; Keeling et al. Reference Keeling, Segarra and Abernathy1989; Liebl et al. Reference Liebl, Simmons, Wax and Stoller1992; Reeves Reference Reeves1997; Sainju and Singh Reference Sainju and Singh1997). Aside from soil health benefits, plant residues left undisturbed on soil surfaces from species like cereal rye (Secale cereale L.) can suppress emergence of certain weedy species, particularly those within the genus Amaranthus, such as Palmer amaranth (Amaranthus palmeri S. Watson) (Barnes et al. Reference Barnes, Putnam and Burke1987; Barnes and Putnam Reference Barnes and Putnam1983; Chou and Patrick Reference Chou and Patrick1976; Creamer et al. Reference Creamer, Bennett, Stinner, Cardina and Regnier1996; Liebl et al. Reference Liebl, Simmons, Wax and Stoller1992; Putnam Reference Putnam1988; Webster et al. Reference Webster, Simmons, Culpepper, Grey, Bridges and Scully2016; Wiggins et al. Reference Wiggins, McClure, Hayes and Steckel2015, Reference Wiggins, Hayes and Steckel2016, Reference Wiggins, Hayes, Nichols and Steckel2017). Otherwise, crop residues commonly provide only 3 to 5 wk of weed suppression, and that suppression is highly dependent on residue species and residue breakdown degradation rates (Mohler and Callaway Reference Mohler and Callaway1995; Moore et al. Reference Moore, Gillespie and Swanton1994; Teasdale Reference Teasdale1996; Williams et al. Reference Williams, Mortensen and Doran1998; Khalil et al. Reference Khalil, Flower, Siddique and Ward2018).
Despite the utility of Amaranthus suppression with crop residues, this cultural method of weed control alone does not provide broad-spectrum control of velvetleaf (Abutilon theophrasti Medik.), barnyardgrass, giant foxtail (Setaria faberi Herrm.), and large crabgrass [Digitaria sanguinalis (L.) Scop.] (Buhler and Daniel Reference Buhler and Daniel1988; Reddy et al. Reference Reddy, Zablotowicz, Locke and Koger2003; Steinsiek et al. Reference Steinsiek, Oliver and Collins1982; Teasdale et al. Reference Teasdale, Beste and Potts1991; Teasdale and Mohler Reference Teasdale and Mohler2000). Additionally, crop residue efficacy is dependent on weed density. For example, redroot pigweed (Amaranthus retroflexus L.) and common lambsquarters (Chenopodium album L.) at low densities (20 to 40 weeds m−2) were suppressed with cereal rye residue; however, at high densities (150 to 170 weeds m−2), little to no suppression was observed (Zasada et al. Reference Zasada, Linker and Coble1997). Therefore herbicides are still needed for sufficient weed control.
The combination of conservation tillage practices and postemergence herbicide applications is not only insufficient for season-long weed control but also results in high selection pressure to postemergence herbicides, of which few options exist due to widespread resistance (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012; Wiggins et al. Reference Wiggins, McClure, Hayes and Steckel2015, Reference Wiggins, Hayes, Nichols and Steckel2017). Consequently, residual herbicides must be integrated with conservation tillage and postemergence herbicides to provide sufficient, season-long weed control while reducing selection pressure on postemergence herbicides (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012). Residual herbicides have traditionally been applied directly to bare soil surfaces in conventional tillage systems. However, residual herbicides applied to crop residue–covered soil surfaces can result in reduced herbicide entry into soil due to spray interception and herbicide adsorption to crop residue (Alletto et al. Reference Alletto, Benoit, Bolognesi, Couffignal, Bergheaud, Dumeny, Longueval and Barriuso2013; Brown et al. Reference Brown, Hayes, Tyler and Mueller1994; Schmitz et al. Reference Schmitz, Witt and Mueller2001). For example, applications to straw mulch reduced oryzalin amounts in soil by 15% to 43% compared to applications to bare soil (Banks and Robinson Reference Banks and Robinson1984). Banks and Robinson (Reference Banks and Robinson1982, Reference Banks and Robinson1986) also reported that no more than 45% of metribuzin or 20% of acetochlor, alachlor, or metolachlor applied to soil covered with wheat straw reached the soil surface. However, Crutchfield et al. (Reference Crutchfield, Wicks and Burnside1985) reported that despite significant reductions in metolachlor soil concentrations after applications to wheat straw–covered soil, acceptable levels of control of witchgrass (Panicum capillare L.), tumble pigweed (Amaranthus albus L.), and wild-proso millet (Panicum miliaceum L.) were achieved. As such, although application of residual herbicides to crop residue–covered soil surfaces often results in reduced herbicide soil concentrations, reduced efficacy is not always observed.
To provide sufficient weed control and protection from photodegradation, residual herbicides must be moved into the top few centimeters of soil, where germinating weed seeds reside (Knake et al. Reference Knake, Appleby and Furtick1967; Savage and Barrentine Reference Savage and Barrentine1969; Weise and Hudspeth Reference Weise and Hudspeth1968). Increasing the carrier volume of residual herbicide application to crop residue–covered soil has been shown to increase efficacy; however, greater carrier volumes require increased application costs and time (Borger et al. Reference Borger, Riethmuller, Ashworth, Minkey, Hashem and Powles2013, Reference Borger, Riethmuller, Ashworth, Minkey and Hashem2015). Soil incorporation of residual herbicides in conservation tillage systems primarily relies on rainfall or overhead irrigation. Consequently, a potential alternative method for increasing residual herbicide efficacy from applications to crop residue–covered soils could be to apply a greater amount of overhead irrigation. According to Smith et al. (Reference Smith, Ferrell, Webster, Fernandez, Dittmar, Munoz and MacDonald2016), 1.3 cm is considered the standard rainfall amount to incorporate most residual herbicides. In the aforementioned studies with metribuzin and acetochlor, alachlor, or metolachlor, Banks and Robinson (Reference Banks and Robinson1982, Reference Banks and Robinson1986) included various amounts of overhead irrigation in their treatments; however, no more than 1.3 cm was applied. Therefore the objective of this study is to determine if increased levels of simulated overhead irrigation can improve residual herbicide efficacy when applied to wheat straw–covered soil.
Materials and Methods
Greenhouse experiments were conducted in October and December 2018 at Mississippi State University in Starkville to evaluate the effect of differential levels of overhead irrigation on the efficacy of acetochlor, pyroxasulfone, and pendimethalin on barnyardgrass applied to soil covered with wheat residue. Greenhouse trays (25 × 51 cm) were filled to approximately 6 cm depth with a locally sourced silt loam consisting of 3% clay, 55% silt, and 43% sand with a pH of 5.7, cation exchange capacity (CEC) of 8.3, and 1.7% OM, then transferred to a greenhouse maintained at 30/24 C day/night temperatures and a 14-h photoperiod with supplemental lighting. Seven days prior to herbicide application, volumetrically measured barnyardgrass seeds (Azlin Seed Services, Leland, MS, USA) roughly equivalent to 3,000 seeds tray−1 were shallowly planted by hand in the trays. This constant seeding rate was chosen to ensure that barnyardgrass densities were uniform and high enough to collect measurable amounts of biomass in the study. Barnyardgrass was chosen as a representative weed species because it is commonly ranked among the top ten most common and troublesome weeds in cotton (Gossypium hirsutum L.) and soybean [Glycine max (L.) Merr.] systems in the mid-southern United States and has been shown to be tolerant of crop residue suppression (Steinsiek et al. Reference Steinsiek, Oliver and Collins1982; Webster Reference Webster2013). After planting, the soil surfaces of half the trays in each experimental run were covered with locally sourced wheat straw, which had been oven-dried at 50 C for 3 d prior to use, at a constant rate of 3,000 kg ha−1. This wheat straw rate was based on the average dry weight of wheat residue found in local fields after wheat harvest. Furthermore, the wheat straw used in these studies was a homogenous mixture of pieces ranging from ∼0.2 to 25 cm in length that had been dried, baled, and shaken loose prior to weighing and implementing in the study. Trays were then adequately watered and left to drain for 48 hr (5 d after planting) to reach field capacity, at which time, herbicides were applied. After watering, wheat residue was approximately 2 to 5 cm deep, which covered the soil surface 90% to 100%.
The setup for each experimental run was a completely randomized design with a factorial arrangement of wheat residue (present or absent) × herbicide (acetochlor, pyroxasulfone, or pendimethalin) × simulated irrigation amount (0.3, 0.6, 1.3, 2.5, or 5.1 cm) plus a bare soil nontreated control (NTC) and a wheat residue–covered NTC. Acetochlor (Warrant®, Bayer CropScience, Research Triangle Park, NC, USA), pyroxasulfone (Zidua®, BASF Corp., Research Triangle Park, NC, USA), and pendimethalin (Prowl® H2O, BASF Corp.) were applied at 1,260, 119, and 1,120 g ai ha−1, respectively, with a compressed air-pressurized dual-nozzle track sprayer (Generation IV Spray Booth, Devries Manufacturing, Hollandale, MN, USA) calibrated to deliver 94 L ha−1 at 276 kPa with AIXR 110015 nozzles (TeeJet® Technologies, Spraying Systems Co., Wheaton, IL, USA). The track sprayer was also equipped with an overhead irrigation simulator fitted with two DR11010 nozzles (Wilger Inc., Lexington, TN, USA) on a water-only boom calibrated to deliver 28,234 L ha−1 at 172 kPa to achieve the desired amount. The nozzles produced ultra-coarse spray quality (volume median diameter > 665 μm) (ASABE 2009). All simulated overhead irrigation treatment amounts greater than 0.6 cm were made in increments of 0.6 cm to allow sufficient time for water to soak into soil and minimize puddling. After the simulated overhead irrigation applications, no further overhead water was applied to trays, and trays were subsurface irrigated as needed (daily) to ensure sufficient soil moisture for barnyardgrass emergence and growth. Weed species other than barnyardgrass that emerged during the experiment were clipped weekly to ensure that the soil or wheat straw surface was not disturbed.
Data Collection and Analysis
At 28 d after treatment (DAT), experimental units were visually evaluated for barnyardgrass control on a scale from 0 to 100, 0 being similar to the bare soil NTC and 100 being no plants present. Additionally, at 28 DAT, all barnyardgrass plants in the center 213 cm2 of each tray were counted and cut at the soil surface level, dried at 50 C for 5 d, and weighed to determine density and aboveground biomass.
All data were first subjected to analysis of variance (ANOVA) to test for main effects and interactions under the agricolae package in R (version 0.98.1091, RStudio Inc., Boston, MA, USA). All assumptions of ANOVA were met; therefore no data transformations were necessary. Significant interactions between main effects and experimental runs were not detected (P ≥ 0.13); therefore data were pooled across two runs. Based on R 2 values and lack-of-fit tests, a simple linear regression model in the R stats package best explained the data. Data were then grouped by herbicide and wheat residue type (present or absent) and regressed against simulated overhead irrigation level using a linear quadratic regression model:
where Y is the response (barnyardgrass control, density, or biomass 28 DAT), x is the level of simulated overhead irrigation (expressed in centimeters), C is the intercept, and B is the slope. Data were then plotted graphically under the ggplot2 package in R and fitted with a 95% confidence band.
Results and Discussion
Barnyardgrass control from acetochlor applications to bare soil increased with simulated overhead irrigation (B = 2.9) (Table 1; Figure 1). Despite this positive relationship with acetochlor, barnyardgrass control was greater from pyroxasulfone and pendimethalin applied to bare soil compared to acetochlor, regardless of irrigation level (Figure 1). Among the herbicides tested, pyroxasulfone provided the greatest barnyardgrass control when applied to bare soil and was not influenced by irrigation level, because the slope was similar to zero (B = 0.2; C = 85.4) (Table 1; Figure 1). Barnyardgrass control from pendimethalin applied to bare soil was negatively influenced by increased irrigation (B = −2.1). The solubilities of pyroxasulfone and pendimethalin are less than that of acetochlor (Shaner Reference Shaner2014; Westra et al. Reference Westra, Shaner, Westra and Chapman2014); therefore increasing simulated overhead irrigation when pyroxasulfone or pendimethalin was applied to bare soil most likely did not increase the availability of either herbicide in the soil–water solution, whereas acetochlor availability did increase as irrigation amount increased. When applied to wheat straw, all three herbicides resulted in decreased barnyardgrass control (Figure 1). Barnyardgrass control by both acetochlor and pyroxasulfone increased with simulated overhead irrigation; in contrast, control with pendimethalin applied to wheat straw–covered soil decreased with increasing simulated overhead irrigation amounts (B = −4.6). However, reduced barnyardgrass control with acetochlor and pyroxasulfone in applications to wheat straw–covered soil was not alleviated to levels similar to bare soil applications by increasing irrigation (Figure 1). Barnyardgrass response to pendimethalin applied to wheat straw–covered soil was peculiar in that it was the only herbicide that responded negatively to increased irrigation. Consequently, barnyardgrass control by pendimethalin applied to wheat straw–covered soil was similar to control by bare soil applications followed by simulated overhead irrigation amounts of 0.3 to 1.2 cm based on 95% confidence intervals (Figure 1). Furthermore, barnyardgrass control by pendimethalin applied to wheat straw–covered soil was more variable than bare soil applications based on 95% confidence intervals.
Table 1. Regression parameters from experiments investigating the effect of differential simulated overhead irrigation amounts on barnyardgrass control, density, and biomass 28 d after treatment from acetochlor, pyroxasulfone, and pendimethalin applied to bare soil or wheat straw–covered soil under greenhouse conditions in Mississippi in 2018.a
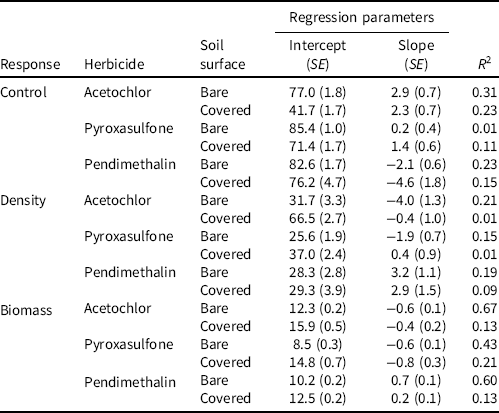
a Regression model: Y = Bx + C, where Y is the response, x is the explanatory variable (simulated rainfall expressed in centimeters), C is the intercept, and B is the slope.
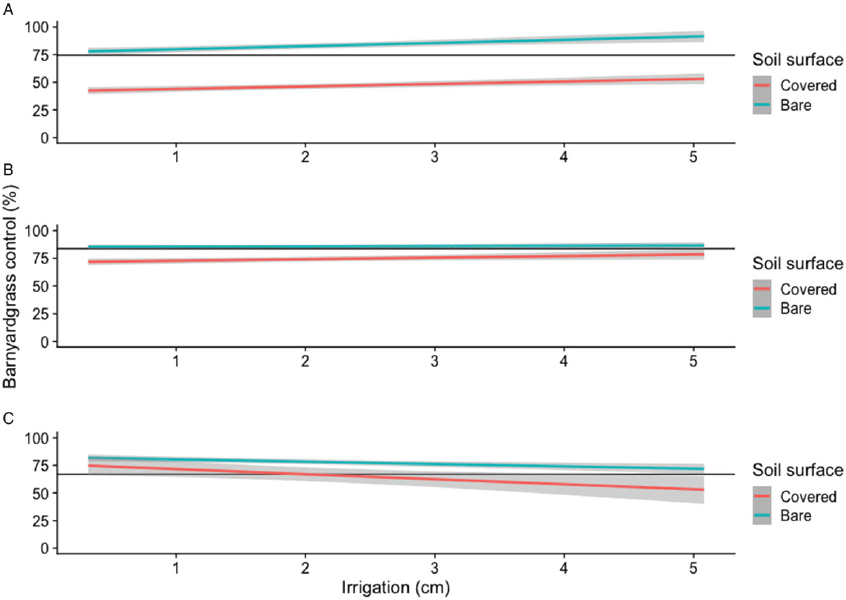
Figure 1. Regression of barnyardgrass control 28 DAT as affected by acetochlor (A), pyroxasulfone (B), or pendimethalin (C) applied to bare soil or wheat straw–covered soil and simulated overhead irrigation amount in greenhouse experiments conducted in Mississippi in 2018. (A) Gray bands represent 95% confidence intervals. (B) Horizontal black lines represent the lower limit of the 95% confidence interval for the maximum of bare soil applications. (C) Mean barnyardgrass control of the bare and wheat residue–covered soil nontreated control was 0% and 16%, respectively.
Barnyardgrass densities resulting from acetochlor applications to bare soil decreased by approximately 4 plants 213 cm−2 for every increase in 1 cm of simulated overhead irrigation (Table 1; Figure 2). However, pyroxasulfone applied to bare soil resulted in a decrease of only 1.9 plants 213 cm−2 for every increase in 1 cm of simulated overhead irrigation. Similar to visually evaluated control, the negative effect of increasing simulated overhead irrigation on pendimethalin was apparent for barnyardgrass densities, as densities increased by 3.2 plants 213 cm−2 for every increased in 1 cm of simulated overhead irrigation. When applied to wheat straw–covered soil, neither acetochlor nor pyroxasulfone treatment reduced barnyardgrass densities, similar to bare soil applications, and no effects of irrigation amount were detected, as the slopes were similar to zero (Table 1; Figure 2). However, barnyardgrass densities after applications of pendimethalin to wheat straw–covered soil were similar to bare soil applications across all simulated overhead irrigation levels. Smith et al. (Reference Smith, Ferrell, Webster, Fernandez, Dittmar, Munoz and MacDonald2016) reported no differences among Palmer amaranth densities from acetochlor applications to bare sandy soil receiving 0 to 1.3 cm of irrigation.
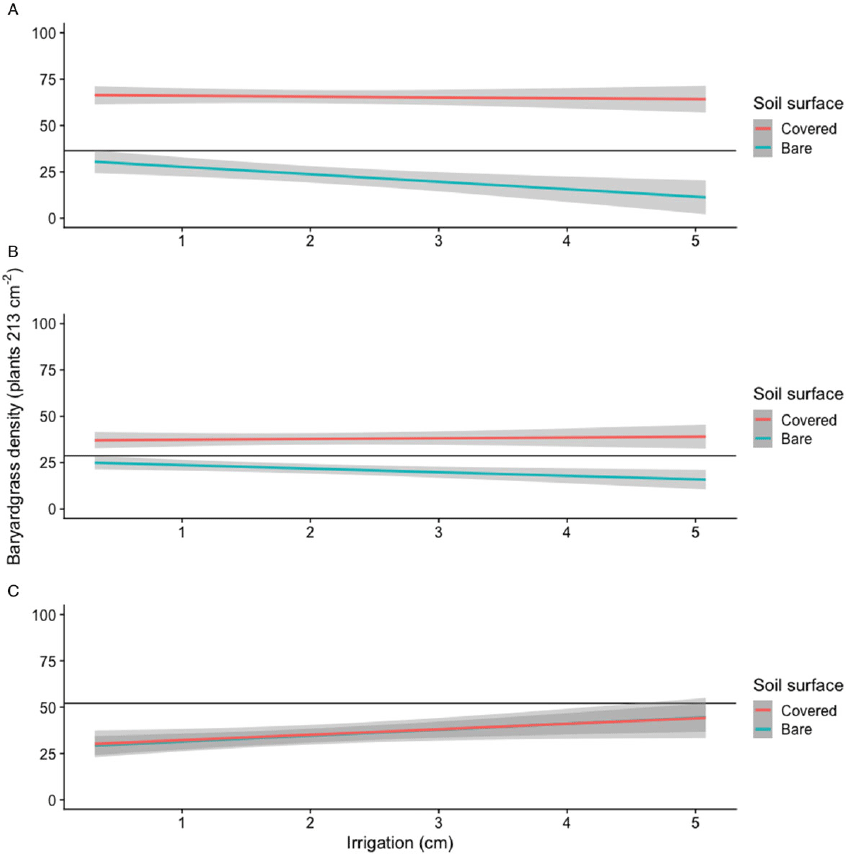
Figure 2. Regression of barnyardgrass densities (plants 213 cm−2) 28 DAT as affected by acetochlor (A), pyroxasulfone (B), or pendimethalin (C) applied to bare soil or wheat straw–covered soil and simulated overhead irrigation amount in greenhouse experiments conducted in Mississippi in 2018. (A) Gray bands represent 95% confidence intervals. (B) Horizontal black lines represent the upper limit of the 95% confidence interval for the maximum of bare soil applications. (C) Mean density of bare and wheat residue–covered soil nontreated controls was 88 and 81 plants 213 cm−2, respectively.
Increasing amounts of simulated overhead irrigation led to decreased barnyardgrass biomass in acetochlor and pyroxasulfone applications to both bare soil and wheat straw–covered soil (Table 1; Figure 3). Conversely, biomass reductions by pendimethalin treatments was negatively influenced by increasing irrigation levels (Table 1; Figure 3). However, greater biomass was observed from pendimethalin in wheat straw–covered soil applications compared to bare soil applications until at least 3.2 cm of irrigation was applied where biomass levels were similar among the two soil surface types. It is also important to note that in terms of barnyardgrass biomass data, applications of acetochlor and pyroxasulfone to wheat straw–covered soil resulted in more inconsistent responses than bare soil applications, as can be seen from the width of the 95% confidence band of the fitted regression lines (Figure 3).
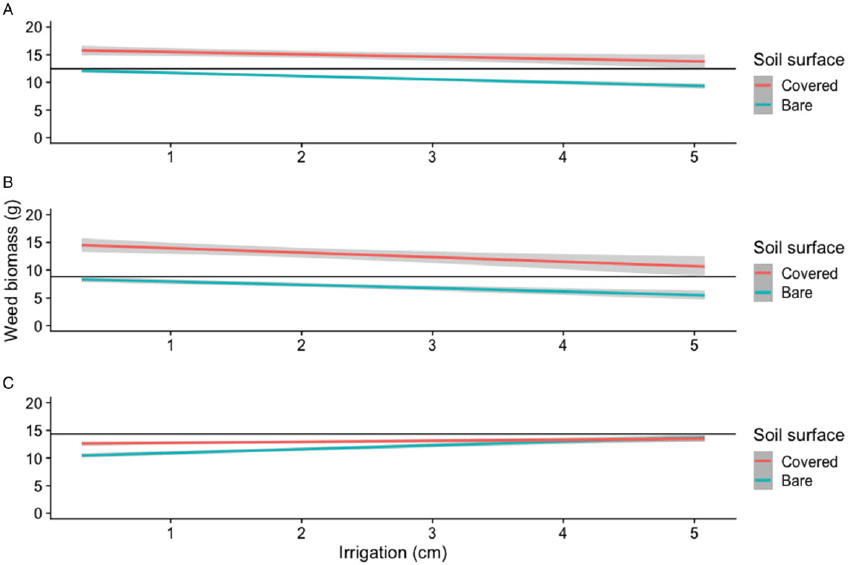
Figure 3. Regression of barnyardgrass biomass 28 DAT as affected by acetochlor (A), pyroxasulfone (B), or pendimethalin (C) applied to bare soil or wheat straw–covered soil and simulated overhead irrigation amount in greenhouse experiments conducted in Mississippi in 2018. (A) Gray bands represent 95% confidence intervals. (B) Horizontal black lines represent the upper limit of the 95% confidence interval for the maximum of bare soil applications. (C) Mean biomass of bare and wheat residue–covered soil nontreated control was 23 and 19 g, respectively.
The negative relationship between pendimethalin efficacy and increasing amounts of simulated overhead irrigation observed in the current study is unusual, as the other tested herbicides exhibited the inverse. High levels of rainfall or irrigation after applications of highly water-soluble herbicides, such as dicamba, have been shown to cause herbicide leaching below the weed seed germination layer (Friesen Reference Friesen1965). Furthermore, Banks and Robinson (Reference Banks and Robinson1984) reported that applications of oryzalin, a dinitroaniline herbicide similar to pendimethalin, were more influenced by the amount of rainfall after application than by the level of wheat residue present, suggesting that herbicide release from wheat residue was alleviated by rainfall. Pendimethalin adsorbs to plant residue and organic matter much more strongly than acetochlor or pyroxasulfone and is highly water insoluble (0.275 mg L−1) compared to acetochlor (223 mg L−1) and pyroxasulfone (3.49 mg L−1) (Shaner Reference Shaner2014). Consequently, based on pendimethalin’s water solubility and Koc of 17,200 mL g−1, it is highly unlikely that increased simulated rainfall caused pendimethalin to leach in the soil, resulting in reduced efficacy (Shaner Reference Shaner2014). However, acetochlor and pyroxasulfone are nonvolatile, whereas pendimethalin is moderately volatile, especially when exposed above the soil (Cooper et al. Reference Cooper, Jenkins and Curtis1990; Shaner Reference Shaner2014). In fact, the negative relationship between simulated rainfall and pendimethalin in our study agrees with previous research indicating that increased moisture conditions promote dinitroaniline herbicide volatility losses (Bardsley et al. Reference Bardsley, Savage and Walker1968; Ketcherside et al. Reference Ketcherside, Bovey and Merkle1969; Parochetti et al. Reference Parochetti, Dec and Burt1976; Parochetti and Hein Reference Parochetti and Hein1973). This phenomenon could also explain why trends across simulated rainfall amounts between bare soil and wheat straw–covered soil pendimethalin applications were so diverse (Figure 1). In addition, pendimethalin applied to bare soil likely moved deeper into the soil, avoiding further losses to volatility.
Findings suggest that improvement in weed control from applications of acetochlor or pyroxasulfone to wheat straw–covered soil can be achieved with optimal levels of overhead irrigation; however, as Banks and Robinson (Reference Banks and Robinson1982) also suggested, there is a limit to how much herbicide can be released from wheat straw once it is intercepted. Furthermore, some herbicides, such as pendimethalin, may be adversely affected by increased amounts of irrigation (and rainfall) due to the promotion of volatility or other means of herbicide loss. Residual herbicides are an important component of integrated weed management in conservation tillage. Future research should evaluate whether increased overhead irrigation amounts impact weed control by other commonly used residual herbicides with varying adsorption, solubility, and volatility characteristics applied to crop residue–covered soil. Additionally, the acetochlor formulation used in these studies is microencapsulated, which may differ from the emulsifiable concentrate formulation.
Acknowledgments
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station. Material is based on work partially supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project, under accession number 230090. The Mississippi Soybean Promotion Board also provided partial funding for this research through the Hartwig Endowed Chair of Agronomy. The authors thank all the undergraduate and graduate research assistants at Mississippi State University for their assistance in conducting this research. No conflicts of interest have been declared.









