Introduction
Grain sorghum is the fifth most important cereal crop grown in the world and the third most common cereal crop grown in the United States following corn (Zea mays L.) and wheat (Triticum aestivum L.) (Defelice Reference Defelice2006; Pandian et al. Reference Pandian, Sexton-Bowser, Prasad and Jugulam2021). The United States ranked first (9.47 × 106 kg) in grain sorghum production globally (62.25 × 106 kg) in 2020 (USDA-FSA 2021; USDA-NASS 2021). According to a recent survey, Kansas was a leading state in grain sorghum production (1.33 million ha) followed by Texas (0.78 million ha; USDA-NASS 2023). Major grain sorghum production within Kansas occurs in the western parts of the state (part of central Great Plains) under semiarid conditions where no-tillage fallow-based production systems are predominant (Nielsen Reference Nielsen2018; Peterson and Westfall Reference Peterson and Westfall2004). Sorghum is mainly grown for food, feed, fodder, syrup, and biofuel production worldwide, whereas it is mainly used for livestock feed and ethanol production in the United States (Paterson Reference Paterson2008). Due to a wider adaptability and tolerance to various biotic (diseases and insects) and abiotic (heat, drought, high salinity, and low nutrition) stresses and a relatively higher water use efficiency, grain sorghum is an important climate-resilient crop (Stamenković et al. Reference Stamenković, Siliveru, Veljković, Bankovic-Ilic, Tasic, Ciampitti, Đalovi, Mitrovi, Sikora and Prasad2020; Taylor et al. Reference Taylor, Schober and Bean2006). The area under grain sorghum production has declined in many parts of the United States in the recent years in spite of its gluten-free food value (Werle et al. Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017). However, sorghum has gained considerable attention as an alternative, low-input biofuel crop, especially under drought conditions and on marginal lands of the midwestern United States (Maw et al. Reference Maw, Houx and Fritschi2017)
Weeds pose a serious challenge for successful production of grain sorghum and are probably one of the major limitations for an increase in acreage and production (USDA-NASS 2021). Lack of weed control in grain sorghum can reduce grain yields by 47% in the United States, valued approximately at US$953 million (Dille et al. Reference Dille, Stahlman, Thompson, Bean, Soltani and Sikkema2020). Herbicide options for weed control in grain sorghum are relatively limited when compared to those for other crops such as corn and soybean (Glycine max L.). More specifically, no effective postemergence herbicide is labeled for grass weed control in grain sorghum (Thompson et al. Reference Thompson, Dille and Peterson2019). Consequently, grain sorghum producers primarily rely on preemergence (PRE) herbicides for annual grass weed control (Hennigh et al. Reference Hennigh, Al-Khatib and Tuinstra2010; Werle et al. Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017). However, dry environments and lack of adequate soil moisture at the time of PRE applications reduce the activation and effectiveness of these herbicides in grain sorghum (Hennigh et al. Reference Hennigh, Al-Khatib and Tuinstra2010; Werle et al. Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017).
More recently, grain sorghum hybrids (igrowth® from Advanta Alta Seeds, Amarillo, TX; and Inzen™ from Corteva Agriscience, Indianapolis, IN) with resistance to ALS inhibitors have been developed. The Inzen sorghum will allow producers to use postemergence applications of nicosulfuron (Zest™ WDG herbicide; Corteva Agriscience), whereas igrowth sorghum will allow PRE and postemergence applications of imazamox (IMIFLEX™ herbicide; UPL Company, King of Prussia, PA) for grass weed control. In addition, sorghum hybrids (Double Team™ from S&W Sorghum Partners, Longmont, CO) with resistance to acetyl-CoA-carboxylase (ACCase) inhibitors, such as quizalofop-p-ethyl (FirstAct™ herbicide, Adama Agricultural Solutions, Ashdod City, Israel) have also been developed. All three (igrowth, Inzen, and Double Team) technologies were soft-launched in 2021 and then commercially launched in 2022 growing season and can potentially improve the grass weed control options in grain sorghum (Hennigh et al. Reference Hennigh, Al-Khatib and Tuinstra2010).
Shattercane is one of the most troublesome summer annual grass weed species in corn and grain sorghum production in the central Great Plains and Midwest (Hans and Johnson Reference Hans and Johnson2002; Kegode and Pearce Reference Kegode and Pearce1998; Pandian et al. Reference Pandian, Sexton-Bowser, Prasad and Jugulam2021; Werle et al. Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017). A season-long interference of shattercane can reduce corn yields by 85% (Hans and Johnson Reference Hans and Johnson2002). Shattercane interference in grain sorghum is a major concern, and up to 95% of sorghum grain yield reductions have been reported (Dille et al. Reference Dille, Stahlman, Thompson, Bean, Soltani and Sikkema2020; Werle et al. Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017).
Cultivated sorghum (grain, forage, sweet, sudangrass) is a member of the Poaceae family, with many similarities to the weedy relative shattercane and both species are diploid (2n = 2x = 20) in nature and has tendency to produce fertile hybrids (Defelice Reference Defelice2006; Sahoo et al. Reference Sahoo, Schmidt, Pedersen, Lee and Lindquist2010; Werle et al. Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017). Hybrids of shattercane and grain sorghum have similar ecological fitness (i.e., biomass and seed production) to the wild-type parents (Sahoo et al. Reference Sahoo, Schmidt, Pedersen, Lee and Lindquist2010). Shattercane hybridizes easily with grain sorghum cultivars, resulting in gene transfer (∼5%) and ultimately reducing the quality and value of harvested seed (Defelice Reference Defelice2006; Werle et al. Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017). This clearly indicates that any neutral or beneficial trait(s) may possibly persist in shattercane, even without any additional selection pressure (Arriola and Ellstrand Reference Arriola and Ellstrand1997; Sahoo et al. Reference Sahoo, Schmidt, Pedersen, Lee and Lindquist2010; Werle et al. Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017).
Two major mechanisms, target-site resistance (TSR) and nontarget-site resistance (NTSR), have been reported in several ALS-inhibitor-resistant weed species (Murphy and Tranel Reference Murphy and Tranel2019; Soni et al. Reference Soni, Westra, Allegretta, Araujo, de Pinho, Morran, Lerchl, Dayan, Westra and Gaines2022). The TSR mechanisms involve single or multiple point mutations that lead to a change in the binding affinity between the ALS inhibitor and its target enzyme (Murphy and Tranel Reference Murphy and Tranel2019), while NTSR mechanisms involve pathways that reduce the amount of the herbicide reaching the target enzyme, including limited or reduced absorption and cellular transport, organelle sequestration, or detoxification by an enhanced metabolism (Jugulam and Shyam Reference Jugulam and Shyam2019; Soni et al. Reference Soni, Westra, Allegretta, Araujo, de Pinho, Morran, Lerchl, Dayan, Westra and Gaines2022; Yu and Powles Reference Yu and Powles2014). The NTSR mechanisms for ALS herbicides are primarily related to metabolic detoxification pathways (Jugulam and Shyam Reference Jugulam and Shyam2019; Soni et al. Reference Soni, Westra, Allegretta, Araujo, de Pinho, Morran, Lerchl, Dayan, Westra and Gaines2022). These pathways are regulated by enzymes that include cytochrome P450 (CYP450) monooxygenase, glycosyl transferase, and glutathione S-transferase enzymes (Yu and Powles Reference Yu and Powles2014). The role of CYP450 monooxygenase enzymes in metabolic detoxification of ALS-inhibiting herbicides have been reported (Jugulam and Shyam Reference Jugulam and Shyam2019; Soni et al. Reference Soni, Westra, Allegretta, Araujo, de Pinho, Morran, Lerchl, Dayan, Westra and Gaines2022). Malathion is an organophosphate insecticide that inhibits the activity of CYP450 monooxygenases and can indirectly elucidate the role of these enzymes in metabolic herbicide resistance (Yu and Powles Reference Yu and Powles2014).
Shattercane populations with resistance to imazamox, nicosulfuron, and primisulfuron-methyl were first reported from corn and sorghum fields in southwestern Kansas in 1996 (Heap Reference Heap2023). However, the level of resistance and possible mechanisms conferring this cross-resistance to ALS inhibiting herbicides were not quantified. Furthermore, the ALS-inhibitor-resistant shattercane populations have also been reported in seven other U.S. states (Illinois, Indiana, Iowa, Nebraska, Ohio, Pennsylvania, and Virginia; Heap Reference Heap2023). Considering the potential adoption of three newly developed herbicide-resistant grain sorghum technologies, a field survey was initiated in fall 2019 to determine the response of shattercane and Johnsongrass (Sorghum halepense L.) populations from the central Great Plains region to ALS-inhibiting and ACCase-inhibiting herbicides. The main objectives of this research were to 1) confirm and characterize the level of resistance to imazamox in three putative imazamox-resistant (IMI-R) shattercane populations, 2) investigate the underlying mechanism of resistance, and 3) determine the effectiveness of postemergence herbicides for controlling IMI-R shattercane populations.
Materials and Methods
Seed Source
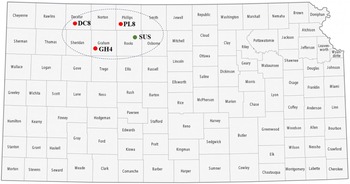
Matured seeds of shattercane and Johnsongrass were collected during a field survey that was initiated in fall 2019 in the major grain sorghum producing areas of western Kansas, western Oklahoma, and northern Texas. Whether the shattercane or Johnsongrass plants had been treated with an herbicide or emerged after an herbicide application was unknown. Historically, the majority of these fields may have been treated with ALS-inhibiting herbicides to control winter annual weeds during wheat growing seasons. Seeds of shattercane and Johnsongrass (about 50 field populations for each species) were collected from grain sorghum fields during 2019 and 2020 growing seasons. A total of 40 to 60 seed heads for each population were collected and combined to create a composite sample, air dried for a week, and manually threshed. In a preliminary discriminate-dose experiments at Kansas State University Agricultural Research Center in Hays, Kansas (KSU-ARCH), three shattercane populations from Decatur (DC8), Graham (GH4), and Phillips (PL8) counties in northwestern Kansas survived (≤65% control at 21 d after treatment [DAT]) the field-use rate (52 g ai ha-1) of imazamox. Survivors from each population were allowed to grow for seed production in a greenhouse and used in subsequent experiments. In addition to these three putative IMI-R populations, a shattercane population with known susceptibility to imazamox (SUS) was identified from Rooks County, KS (Figure 1). Seeds of all IMI-R populations were collected from sorghum fields, and these field sites were historically under a typical 3-yr crop rotation (wheat-summer crop-fallow) for >10 yr with an unknown herbicide use history.

Figure 1. A Kansas map highlighting counties where seeds of imazamox-resistant [DC8 (39.629167°N, 100.535278°W), GH4 (39.305833°N, 99.996944°W), and PL8 (39.756389°N, 99.283889°W)] and imazamox-susceptible (39.509722°N, 99.150556°W) shattercane populations were collected. Map was adapted from GIS Geography (https://gisgeography.com/kansas-county-map/). Abbreviations for shattercane populations: DC8, Decatur county; GH4, Graham county; PL8, Phillips county; SUS, imazamox-susceptible, from Rooks county.
Imazamox Dose-Response
Seeds from each selected shattercane population (DC8, GH4, PL8, and SUS) were separately planted in plastic pots (10 ×10 ×10 cm) with a commercial potting mixture (Miracle-Gro Moisture Control Potting Mix; Scotts MiracleGro Company, Marysville, OH) in the greenhouse at KSU-ARCH. The greenhouse was maintained at 26/24 ± 3 C day/night temperatures and 16/8-h day/night photoperiods. Seedlings were watered daily to avoid moisture stress and maintain good plant growth. At the 3- to 4-leaf stage (8- to 12-cm-tall plants), seedlings from each IMI-R population were separately treated with imazamox at 0, 13, 26, 52, 78, 104, 156, and 208 g ai ha−1; and seedlings from the SUS population were treated with imazamox at 0, 3, 7, 13, 26, 52, 78, 104, 156 and 208 g ai ha−1. All imazamox treatments included 1% (v/v) methylated seed oil. Treatments were arranged in a randomized complete block (blocked by population) design with 12 replications (one plant/pot/replication). All experiments were repeated at least once. All selected doses of imazamox were applied using a stationery cabinet spray chamber (Research Track Sprayer; De Vries Manufacturing, Hollandale, MN) equipped with an 8001 even flat-fan nozzle tip (TeeJet 8001EXR, Spraying Systems Company, Glendale Heights, IL) calibrated to deliver 132 L ha−1 of spray solution at 240 kPa. For each population by herbicide dose, shattercane plants were cut at the soil surface at 21 DAT, dried at 65 C for 96 h, and weighed to obtain shoot dry weight. Shoot dry weights of treated plants were expressed as a percentage reduction relative to the nontreated control.
Mechanism(s) of Imazamox Resistance
ALS Gene Sequencing
The ALS gene was sequenced from each IMI-R (DC8, PL8, GH4) and SUS shattercane population to identify any known target-site mutations conferring resistance to imazamox. Young leaf tissue samples (200 mg) from three surviving plants per population were collected and shipped to the weed research laboratory at Colorado State University in Fort Collins. Genomic DNA (gDNA) was extracted using the DNeasy Plant Mini kit (Qiagen, Valencia, CA) and quantified using a NanoDrop UV-Vis spectrophotometer (ThermoFisher Scientific, Waltham, MA). A 1,691 base pair (bp) section of the ALS gene from each IMI-R and SUS shattercane individuals covering all known target site mutations was amplified using a forward primer (5′-TCGTCGAGGCTCTTGAGC-3) and a reverse primer (5′-GCCATCACCATCCAGGATCA-3′). A polymerase chain reaction (PCR) assay was performed in a T100 thermal cycler (Bio-Rad, Hercules, CA) using EconoTaq PLUS 2XPCR master mix (Lucigen, Middleton, WI). Each reaction contained 10 μL of Master Mix, 2.5 μL each of the forward and reverse primers (5 μM), 5 μL of gDNA template (10 ng μL−1), and 5 μL of nuclease-free water. The following thermal cycling conditions were used for PCR amplification: 95 C for 3 min, 30 cycles at 95 C for 30 s, 58 C for 30 s and 72 C for 90 s, followed by 72 C for 8 min. The PCR products were examined on a 1.0% agarose gel stained with GelRed™ nucleic acid gel stain (Biotium, Fremont, CA) to confirm the amplicon size. Sanger sequencing (Genewiz, South Plainfield, NJ) with the same primers used for amplification sequenced the purified PCR products. The sequence reads of the ALS genes were aligned to a reference ALS sequence (accession NC012873.2 from the National Center for Biotechnology Information [NCBI] GenBank) using Geneious Prime (https://wwwgeneious.com) for any known target-site mutations that confer resistance to ALS-inhibiting herbicides.
Effect of Malathion on Imazamox Resistance
Shattercane seedlings from PL8 and SUS populations (240 seedlings per population) were grown in plastic pots in a greenhouse at the KSU-ARCH as described in the dose-response study. Half of those seedlings from each population (120 seedlings) were treated with malathion (Malathion 5EC; Drexel Chemical Company, Memphis, TN) at 1,000 g ha−1 when seedlings were at the 3- to 4-leaf stage and other half were left untreated. About 4 h later, seedlings of both malathion treated and untreated from PL8 and SUS populations were sprayed separately with imazamox doses at 0, 3, 7, 13, 26, 53, 105, 211, and 421 g ha−1 along with methylated seed oil at 1% (v/v). Malathion and imazamox treatments were applied by using a cabinet spray chamber as previously described. Experiments were conducted in a randomized complete block design, with a factorial arrangement of treatments (factor A = PL8 and SUS populations; factor B = malathion treated or nontreated; factor C = imazamox doses), six replications (one plant/pot/replication), and repeated in time. Shoot dry weights of all treated PL8 and SUS seedlings (expressed as % of nontreated) were determined at 21 DAT as previously described.
Effectiveness of Postemergence Herbicides
A greenhouse study was conducted at the KSU-ARCH to determine the efficacy of alternative postemergence herbicides for controlling IMI-R shattercane populations. Shattercane plants from IMI-R (DC8, GH4, PL8) and SUS populations were grown in plastic pots (10 × 10 × 10 cm) containing the commercial potting mixture as previously described. Seedlings from each population were treated at the 3- to 4-leaf stage using the stationary spray chamber with field-use rates of postemergence herbicides (listed in Table 1). Experiments were conducted in a randomized complete block design with 12 replications. Similar greenhouse conditions were maintained throughout the study period as detailed in dose-response assays. Data on percent visible injury estimates (on a scale of 0% to 100%, where 0% = no control, and 100% = total plant death) were recorded at 21 DAT.
Table 1. Postemergence herbicides used for controlling imazamox-resistant and imazamox-susceptible shattercane populations in a greenhouse study. a, b

a Abbreviations: ACCase, acetyl-CoA carboxylase; ALS, acetolactate synthase; EPSPS, 5-enolpyruvylshikimate-3-phosphate synthase.
b Greenhouse studies were carried out at Kansas State University Agricultural Research Center near Hays, Kansas.
c Nonionic surfactant at 0.25% (v/v) was included.
d Crop oil concentrate at 0.5% (v/v) was included.
e Ammonium sulfate at 2% (wt/v) was included.
Statistical Analyses
Data were checked for ANOVA assumptions (normality of residuals and homogeneity of variance) using the Shapiro-Wilk (P value = 0.3241) and Levene (P value = 0.734) tests with the UNIVARIATE and GLM procedures, respectively, with SAS software (version 9.3 SAS Institute, Cary, NC). All data met both assumptions except for the alternative postemergence herbicide study. Data on percent visual injury estimates from the alternative postemergence herbicides study were square root transformed before analysis to improve the normality of residuals and homogeneity of variance. Nontransformed means were presented based on the interpretation from the transformed data. Data from dose-response assay, malathion-based assay, and alternative postemergence herbicide efficacy were subjected to ANOVA using the MIXED procedure with SAS software (version 9.3) to test the significance of fixed effects (i.e., population, chemical [imazamox doses in dose-response, malathion treatment, or alternative postemergence treatments], and their interactions). Random effects in the model were run as an experiment and replications were nested within experimental runs (SAS software, version 9.3). Data were combined across runs for each experiment due to a nonsignificant interaction (P = 0.125 for imazamox dose-response experiment; P = 0.328 for malathion experiment; P = 0.623 for postemergence herbicide experiment) of treatment by experimental run. Shoot dry weights (% of nontreated) of each population from the dose-response study and malathion assay were regressed against imazamox doses using a three-parameter log-logistic model (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015):
Where y is the shoot dry weight reduction (% of nontreated), d is the maximum shoot dry weight, e is the imazamox dose needed for 50% reduction in shoot dry weight (referred to as GR50 values, respectively), x is the imazamox dose, and b represents the slope of each curve. The Akaike information criterion was used to select the nonlinear three-parameter model. A lack-of-fit test (P > 0.05) was used to confirm that the selected model described the shoot dry weights of each shattercane population (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015). All nonlinear regression parameters were estimated using the drc package in R software (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015). The resistance index (R/S ratio) was estimated by dividing the GR50 value for each IMI-R population by the GR50 value of the SUS population. For alternative postemergence herbicide efficacy study, treatment means were separated using the Fisher’s protected LSD test at P ≤ 0.05.
Results and Discussion
Imazamox Dose Response
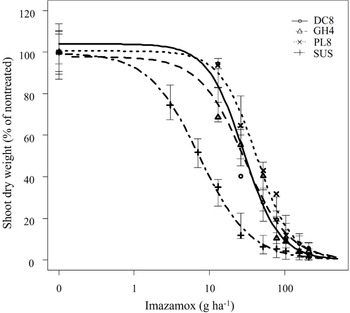
The nontreated mean shoot dry weight of DC8, GH4, PL8, and SUS populations was 2.51, 3.72, 3.99, and 2.83 g plant−1, respectively. Results from whole-plant dose-response studies indicated that the three putative shattercane IMI-R (DC8, GH4, and PL8) populations from northwestern Kansas were resistant to imazamox (Table 2). The imazamox dose causing 50% shoot dry weight reduction (GR50 values) of these three populations ranged from 29 to 42 g ha−1, which was greater than that of the SUS population (7 g ha−1). Similarly, the estimated imazamox dose causing 90% shoot dry weight reduction (GR50 values) of the three IMI-R populations ranged from 96 to 139 g ha−1, which was greater than that of the SUS population (40 g ha−1) and the field-use rate (52 g ha−1). Based on GR50 values, these three IMI-R populations had 4.1-fold to 6.0-fold resistance to imazamox, compared with the SUS population (Table 2; Figure 2). In contrast to our results, Zelaya and Owen (Reference Zelaya and Owen2004) previously reported a shattercane population from Malvern County, Iowa, with a 29-fold resistance to imazethapyr. Similarly, Lee et al. (Reference Lee, Martin, Roeth, Johnson and Lee1999) reported two shattercane populations from central and south-central Nebraska with 1,200-fold to 1,260-fold resistance to primisulfuron-methyl and 6-fold resistance to nicosulfuron. In the same study, another shattercane population with 10-fold resistance to imazethapyr was confirmed. Werle et al. (Reference Werle, Jhala, Yerka, Dille and Lindquist2016) also documented five different shattercane populations from Nebraska with resistance to imazethapyr and four populations with resistance to nicosulfuron, although the levels of resistance in those populations were not characterized. Compared with all previous reports on shattercane populations with resistance to ALS-inhibiting herbicides in the United States, shattercane populations in northwestern Kansas have evolved low-level resistance to imazamox.
Table 2. Regression parameter estimates of the 3-parameter log-logistic equation fitted to shoot dry weight (% of nontreated) of shattercane populations at 21 d after treatment with various imazamox doses in a greenhouse study. a, b

a Abbreviations: DC8, GH4, PL8, and SUS (susceptible) are shattercane populations collected from sorghum fields in fall of 2020 from Decatur, Graham, Phillips, and Rooks Counties, respectively; d is the upper limit, b is the slope of each dose-response curve, and GR50 is the effective dose (g h−1) of imazamox herbicide needed for 50% shoot dry weight reduction (% of nontreated) for each shattercane population; CI, confidence interval; R/S is the ratio of GR50 values of each suspected resistant population relative to that of GR50 value of susceptible population; SE, standard error.
b Greenhouse studies were carried out at Kansas State University Agricultural Research Center near Hays, Kansas.

Figure 2. Shoot dry weight response (% of nontreated) of imazamox-resistant and imazamox-susceptible shattercane populations treated with various doses of imazamox at 21 d after treatment. Symbols indicate actual values of shoot dry weights (% of nontreated), and lines indicate predicted values of shoot dry weights (% of nontreated) obtained from the three-parameter log-logistic model. Vertical bars indicate model-based standard errors (plus and minus) of predicted mean. Abbreviations for shattercane populations: DC8, Decatur county; GH4, Graham county; PL8, Phillips county; SUS, imazamox-susceptible, from Rooks county.
Mechanisms of Imazamox Resistance
ALS Gene Sequencing
The ALS gene sequences (1691 bp) obtained from all three IMI-R shattercane populations (DC8, GH4, PL8) did not reveal any known target-site mutations at amino acid positions Ala122, Pro197, Ala205, Asp376, Arg377, Val560, Trp574, or Ser653 (sequences available at NCBI GenBank, accessions OM315265 to OM315273 for three IMI-R populations and OM334809 to OM334811 for the SUS population). Werle et al. (Reference Werle, Begcy, Yerka, Mower, Dweikat, Jhala and Lindquist2017) reported a Val560 mutation in three imazethapyr/nicosulfuron-resistant shattercane populations from Nebraska. Imazamox resistance due to point mutations in the ALS gene has been reported in various grass and broadleaf weeds, with the most common mutation at Ser653 (Kumar and Jha Reference Kumar and Jha2017; Park and Mallory-Smith Reference Park and Mallory-Smith2004; Tranel and Wright Reference Tranel and Wright2002). Target-site mutations of Pro197 and Ser653 conferring imazamox resistance in downy brome (Bromus tectorum L.) populations from Oregon and Montana, respectively, have previously been documented (Kumar et al. Reference Kumar and Jha2017; Park and Mallory-Smith Reference Park and Mallory-Smith2004). Similarly, an Ala122Thr point mutation in the ALS gene has been reported to confer a high-level resistance to imazamox in a jointed goatgrass (Aegilops cylindrical L.) population from Washington (Rodriguez et al. Reference Rodriguez, Hauvermale, Carter, Zuger and Burke2021). The absence of any known target-site mutations in the ALS genes of IMI-R shattercane from northwestern Kansas warrants further investigation on the possibility of non-target site resistance mechanisms.
Effect of Malathion on Imazamox Resistance
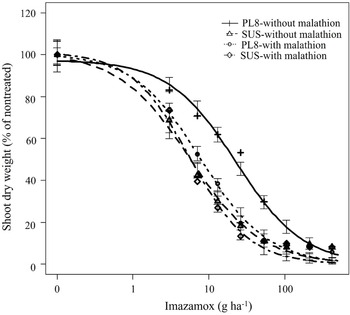
Compared to no malathion treatment, a pretreatment with malathion at 1,000 g ha−1 followed by imazamox applied at various doses significantly reduced the GR50 value (22 vs. 8 g ha−1) in the PL8 population leading to a drastic change in the R/S ratio from 4.4-fold to 1.3-fold (Table 3; Figure 3). These results indicate that the pretreatment of malathion reversed the imazamox resistance phenotype of the PL8 shattercane population, suggesting that an enhanced metabolism of imazamox by CYP450 monooxygenases might be involved in conferring the low-level resistance to imazamox. Enhanced metabolism of ALS-inhibiting herbicides via CYP450 enzymes has been previously reported in various grass weed species, including annual ryegrass (Lolium rigidum Gaudin), barnyard grass (Echinochloa crus-galli), downy brome (Bomu tectorum L.), and smallflower umbrella-sedge (Cyperus difformis) (Heap Reference Heap2023; Park et al. Reference Park, Fandrich and Mallory-Smith2004; Torra et al. Reference Torra, Montull, Taberner, Onkokesung, Boonham and Edwards2021; Yu and Powles Reference Yu and Powles2014). Imazamox resistance due to enhanced metabolism was recently reported in feral rye (Secale cereale L.) (Soni et al. Reference Soni, Westra, Allegretta, Araujo, de Pinho, Morran, Lerchl, Dayan, Westra and Gaines2022). Nevertheless, these findings can play a crucial role in developing effective, alternative management strategies, to control IMI-R populations and prevent the further evolution of imazamox resistance in shattercane populations in the region.
Table 3. Effect of malathion on regression parameter estimates based on shoot dry weights (% of nontreated) of PL8 and SUS shattercane populations treated with various doses of imazamox in a greenhouse study. a, b, c

a Abbreviations: PL8, and SUS (susceptible) are shattercane populations collected from sorghum fields in fall of 2020 from Phillips, and Rooks Counties, respectively; d is the upper limit, b is the slope of each dose-response curve, and GR50 is the effective dose (g h−1) of imazamox herbicide needed for 50% shoot dry weight reduction (% of nontreated) for each shattercane population; CI, confidence interval; R/S is the ratio of GR50 values of each suspected resistant population relative to that of GR50 value of susceptible population; SE, standard error.
b Greenhouse studies were carried out at Kansas State University Agricultural Research Center near Hays, Kansas.
c Malathion at 1,000 g ai ha-1 was applied to shattercane plants followed by imazamox 4 h later.

Figure 3. Shoot dry weight response (% of nontreated) of PL8 and SUS shattercane populations with no pretreatment or with pretreatment of malathion (1,000 g ha−1) followed by various doses on imazamox at 21 d after treatment. Symbols indicate actual values of shoot dry weights (% of nontreated), and lines indicate predicted values of shoot dry weights (% of nontreated) obtained from the three-parameter log-logistic model. Vertical bars indicate model-based standard errors (plus and minus) of predicted mean. Abbreviations: PL8, shattercane population from Phillips county; SUS, imazamox-susceptible population from Rooks county.
Effectiveness of Postemergence Herbicides
There was no significant interaction (P = 0.131) between postemergence herbicides and shattercane populations for percent visible injury at 21 DAT, indicating that all three IMI-R populations and the SUS population responded similarly to postemergence herbicides tested (Table 4). All tested postemergence herbicides including nicosulfuron, quizalofop, clethodim, and glyphosate provided >96% control of all three IMI-R populations at 21 DAT (Table 4). In contrast, King et al. (Reference King, Ritter, Hagood and Menbere2007) reported 71% to 98% control of imazamox-resistant shattercane 4 mo after glyphosate treatment in corn. Rosales-Robles (Reference Rosales-Robles1993) reported >90% control of shattercane at 35 d after treatment with a postemergence application of nicosulfuron.
Table 4. Visual injury response of three imazamox-resistant and one imazamox-susceptible shattercane populations at 21 d after treatment with a herbicide at recommended field-use rates.a,b

a Abbreviations: DC8, Decatur county shattercane population; GH4, Graham county shattercane population; PL8, Phillips county shattercane population; SUS, imazamox-susceptible shattercane population, from Rooks county. DC8, GH4, PL8, and SUS populations were collected from sorghum fields in fall 2020.
b Treatments were carried out in a greenhouse study at Kansas State University Agricultural Research Center near Hays, KS.
c Treatments were applied at the two- to three-leaf stage of shattercane plants. Means for a shattercane population within a column followed by similar lowercase letters are not significantly different based on Fisher’s protected LSD test at P < 0.05; means for a herbicide within a row followed by similar uppercase letters are not significantly different based on Fisher’s protected LSD test at P < 0.05.
d Nonionic surfactant at 0.25% (v/v) was included.
e Crop oil concentrate at 0.5% (v/v) was included.
f Ammonium sulfate at 2% (wt/v) was included.
Practical Implications
Results from the dose-response study confirmed the evolution of low-level resistance to imazamox in three IMI-R shattercane populations (DC8, GH4, and PL8) collected from northwestern Kansas. Sequence analysis of the ALS genes in IMI-R shattercane plants showed the absence of any known target-site mutations. However, reversal of imazamox resistance by a pretreatment of malathion followed by postemergence applications of imazamox indicated the possible existence of a CYP450 monooxygenase–mediated metabolism-based mechanism in the PL8 population. It is important to note that other physiological mechanisms, such as alteration in absorption and translocations of imazamox or glutathione S-transferase–based metabolic resistance, were not explored in this study and warrant future research. To our knowledge, this study reports the first case of evolution of imazamox-resistant shattercane with enhanced metabolism via CYP450 as a possible mechanism that confers resistance. Previous studies have reported a lack of fitness penalty associated with target-site and nontarget site–based mechanisms, especially metabolism-based, in ALS-resistant weed species (Park and Mallory-Smith Reference Park and Mallory-Smith2005; Vila-Aiub et al. Reference Vila-Aiub, Neve and Powles2009). However, the growth and reproductive fitness of these IMI-R shattercane populations is unknown. Controlling these IMI-R shattercane populations would be a challenging task for sorghum producers in the region. Occurrence of IMI-R shattercane populations would also pose a threat to the newly commercialized igrowth sorghum technology. Therefore, it is advisable to adopt proper imazamox (IMIFLEX) use stewardship guidelines if sorghum producers are planning to plant igrowth sorghum technology. It is also important to note that both shattercane and Johnsongrass are not included on IMIFLEX (a herbicide registered to be used on igrowth sorghum), Zest (a herbicide registered to be used with Inzen sorghum), or FirstAct (a herbicide registered to be used with Double Team sorghum) labels. Nevertheless, the postemergence herbicides tested in this study, including nicosulfuron, quizalofop, clethodim, and glyphosate would provide effective control of those IMI-R shattercane populations in various summer crops (corn, soybean, cotton, sunflower, canola, etc.) or fallow phase of a 3-yr crop rotation in the region. Cultural or mechanical practices should also be used to prevent further spread of these IMI-R shattercane in production fields.
A multistate field survey for monitoring the status of herbicide resistance in shattercane and johnsongrass populations across the south-central Great Plains is currently underway. Future studies will assess whole genome sequencing of these IMI-R populations to identify mutations affecting amino acid biosynthetic pathways and/or others such as CYP450 monooxygenase pathway. Multi-state and multi-location field studies will also investigate the integrated effect of crop competition, newly developed herbicide-resistant sorghum technologies, cover crops, harvest weed seed control, and targeted tillage on soil seedbank depletion of IMI-R shattercane populations in grain sorghum-based cropping systems.
Acknowledgments
No conflicts of interest have been declared. The United Sorghum Checkoff Program supported this work.