Over the past two decades there have been major changes in the management of people with schizophrenia, with a move towards community care. Recently, this has been associated with the use of atypical antipsychotic drugs, which produce fewer extrapyramidal side-effects (Reference Geddes, Freemantle and HarrisonGeddes et al, 2000). Such side-effects are distressing, disfiguring and contribute to the stigma of mental illness. In Nithsdale, in Dumfries and Galloway, south-west Scotland, we have studied the prevalence of movement disorders since 1981 (Reference McCreadie, Barron and WinslowMcCreadie et al, 1982). We report here our most recent findings on: current prevalence (1999/2000); an examination of factors possibly associated with extrapyramidal disorders such as medication and smoking; a comparison with prevalence assessed on six previous occasions (McCreadie et al, Reference McCreadie, Barron and Winslow1982, Reference McCreadie, Robertson and Wiles1992; Reference Kelly, McCreadie and MacEwanKelly et al, 1998); and a longitudinal study of patients assessed in both 1981 and 2000. We hypothesised that the prevalence of movement disorders would be lower than in previous years, and that their prevalence would be lower in patients receiving atypical antipsychotics than in those receiving standard antipsychotics.
METHOD
Setting
Nithsdale is a well-defined area in the Dumfries and Galloway region of southwest Scotland, UK. It has a population of about 57 000. All in-patient services are provided at Crichton Royal Hospital in Dumfries and out-patient care is provided by community mental health teams.
Patient identification
The identification of patients by the ‘key informant’ method has been described previously (Reference McCreadieMcCreadie, 1982; Reference Kelly, McCreadie and MacEwanKelly et al, 1998). There are regular censuses of people with schizophrenia in Nithsdale; patients in the present study were identified in April 1999 and examined over the following 12 months. The census includes all in-patients, day patients and out-patients on the census date with an address in Nithsdale and an ICD—10 (World Health Organization, 1992) clinical diagnosis of schizophrenia. In addition, general practitioners are asked to update the list, as are social workers, community psychiatric nurses and voluntary agencies. Diagnoses using the Operational Checklist for Psychiatric Disorders (OPCRIT; Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991) with a computer-generated ICD—10 diagnosis were generated by J.H.
Assessment
Socio-demographic data were obtained from both case records and interviews. We recorded age, gender, duration of illness (as estimated from the time of the first psychotic episode) and medication both at the time of interview and over the preceding year. Dyskinesia was assessed by four psychiatrists (J.H., S.M., T.M., R.M.) using the Abnormal Involuntary Movements Scale (AIMS; Reference GuyUS Department of Health, Education and Welfare, 1976). Dyskinesia was defined as probably present (Reference Schooler and KaneSchooler & Kane, 1982) if movements were ‘mild’ in at least two of seven body areas or ‘moderate’ in at least one. Parkinsonism was assessed using the Simpson—Angus Rating Scale (Reference Simpson and AngusSimpson & Angus, 1970) and was said to be present if the score was more than 0.3.
Akathisia was measured using the Barnes Akathisia Rating Scale (BARS) and was said to be present if the score was 2 (‘mild’) or more on the global scale (Reference BarnesBarnes, 1989).
Mental state was assessed by the same psychiatrists using the Positive and Negative Syndrome Scale (PANSS) for schizophrenia (Reference Kay, Fishbein and OplerKay et al, 1987); this gives a total score and also scores on positive, negative and general psychopathology sub-scales.
Patients completed a questionnaire about their smoking habits, as in a health and lifestyle survey of the general population in south-west Scotland (Reference Waldron, Chalmers and BoneWaldron et al, 1995).
Training and practice sessions in the assessment of mental state and movement disorders took place before the study began under the supervision of R.M.
Statistical analysis
Differences in proportions were measured using the chi-squared test, with Yate's correction for 2 × 2 tables. Normally distributed data were presented with means and standard deviations, and t-tests were used to measure significance: tests were two-tailed. As there were many comparisons, only differences significant at least at the 1% level are reported. In order to account for the possible correlations between variables, logistic regression was used to identify variables independently associated with tardive dyskinesia. All statistical analyses were performed using Arcus Quickstat Biochemical (Biomedical version 1.2; Longman Software, 1999). Dumfries and Galloway Local Research Ethics Committee approved the study.
RESULTS
Current prevalence
Of the 178 patients identified in April 1999, 136 (76%) were interviewed 73 (54%) were males, 63 (46%) females; mean age was 48 years (s.d.=15); mean duration of illness was 21 years (s.d.=14). An OPCRIT-derived ICD—10 diagnosis of schizophrenia was made in 83% of patients. Probable tardive dyskinesia was found in 43%, parkinsonism in 35% and akathisia in 15% (Table 1). There were no significant gender differences in the prevalence of the three movement disorders. The patients who did not take part in the study did not differ from those who did in age, gender distribution or duration of illness.
Table 1 Prevalence of movement disorders

| Males (n=73) n (%) | Females (n=63) n (%) | Total (n=136) n (%) | |
|---|---|---|---|
| Tardive dyskinesia | 34 (47) | 24 (38) | 58 (43) |
| Parkinsonism | 28 (38) | 19 (30) | 47 (35) |
| Akathisia | 10 (14) | 10 (16) | 20 (15) |
Factors associated with extrapyramidal disorders
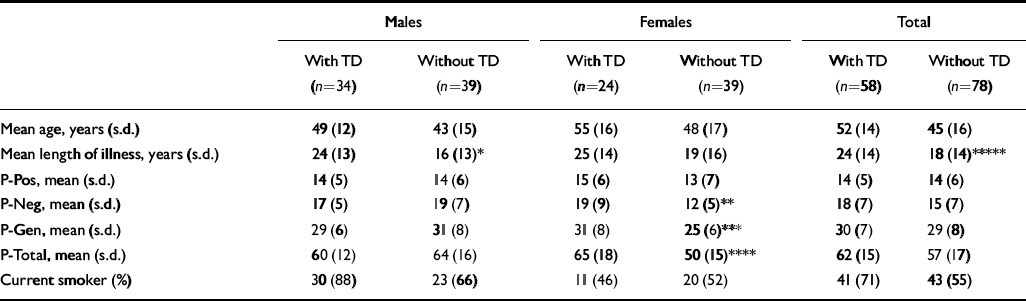
In males there were no significant differences between those with and without tardive dyskinesia in age, mental state and smoking status. Male patients with tardive dyskinesia had a significantly longer duration of illness (Table 2). No other significant differences in males were found.
Table 2 Patients with and without tardive dyskinesia (TD)
| Males | Females | Total | ||||
|---|---|---|---|---|---|---|
| With TD (n=34) | Without TD (n=39) | With TD (n=24) | Without TD (n=39) | With TD (n=58) | Without TD (n=78) | |
| Mean age, years (s.d.) | 49 (12) | 43 (15) | 55 (16) | 48 (17) | 52 (14) | 45 (16) |
| Mean length of illness, years (s.d.) | 24 (13) | 16 (13)* | 25 (14) | 19 (16) | 24 (14) | 18 (14)***** |
| P-Pos, mean (s.d.) | 14 (5) | 14 (6) | 15 (6) | 13 (7) | 14 (5) | 14 (6) |
| P-Neg, mean (s.d.) | 17 (5) | 19 (7) | 19 (9) | 12 (5)** | 18 (7) | 15 (7) |
| P-Gen, mean (s.d.) | 29 (6) | 31 (8) | 31 (8) | 25 (6)*** | 30 (7) | 29 (8) |
| P-Total, mean (s.d.) | 60 (12) | 64 (16) | 65 (18) | 50 (15)**** | 62 (15) | 57 (17) |
| Current smoker (%) | 30 (88) | 23 (66) | 11 (46) | 20 (52) | 41 (71) | 43 (55) |
In females, patients with tardive dyskinesia had significantly higher negative symptom, general psychopathology and total scores on the PANSS (Table 2).
Similar significant differences in PANSS scores were found between females with and without parkinsonism (negative subscale: mean 20 (s.d.=8.8) v. 12.2 (s.d.=5.9); t=4.10, d.f.=61, P=0.0001; general psychopathology subscale: mean 30.5 (s.d.=9.1) v. 25.7 (s.d.=6.4); t=2.37, d.f.=61, P=0.02; total score: mean 66.2 (s.d.=18.7) v. 50.6 (s.d.=14.8); t=3.54, d.f.=61, P=0.0008). Female patients with akathisia had higher positive symptom scores (mean 18.5 (s.d.=6.5) v. 12.6 (s.d.=5.9); t=2.8, d.f.=61, P=0.006).
More female patients with tardive dyskinesia had parkinsonism (54% v. 15%, χ2=8.8, P=0.003) and more had akathisia (33% v. 5%, χ2=6.8, P=0.009).
The variables that were significantly different in female patients with and without tardive dyskinesia on univariate analysis were re-examined using logistic regression. Negative symptoms and akathisia remained independently significantly associated with those females who had tardive dyskinesia (P=0.01, P=0.007, respectively).
Medication
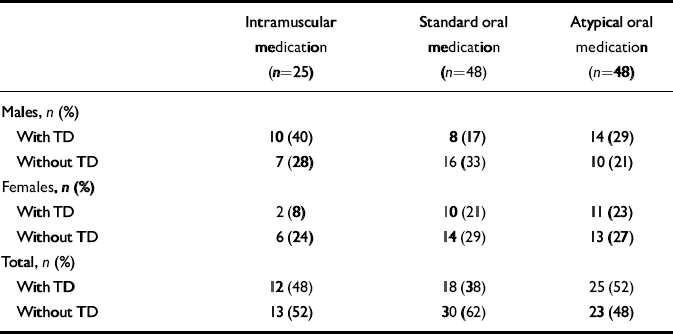
Patients receiving antipsychotic medication were grouped into three categories: those receiving long-acting intramuscular anti-psychotic drugs with or without other anti-psychotics; those receiving standard oral antipsychotic drugs, including sulpiride and thioridazine, with or without oral atypicals; and those receiving atypical oral antipsychotic drugs alone (Table 3). In males and females separately, and in the total group, there were no significant differences between any of the medication categories in the number of patients with and without tardive dyskinesia, either over the past year or at the time of interview (Table 3). At the time of interview, 52% of patients receiving only atypicals had tardive dyskinesia.
Table 3 Current antipsychotic medication of those with and without tardive dyskinesia (TD)1
| Intramuscular medication (n=25) | Standard oral medication (n=48) | Atypical oral medication (n=48) | |
|---|---|---|---|
| Males, n (%) | |||
| With TD | 10 (40) | 8 (17) | 14 (29) |
| Without TD | 7 (28) | 16 (33) | 10 (21) |
| Females, n (%) | |||
| With TD | 2 (8) | 10 (21) | 11 (23) |
| Without TD | 6 (24) | 14 (29) | 13 (27) |
| Total, n (%) | |||
| With TD | 12 (48) | 18 (38) | 25 (52) |
| Without TD | 13 (52) | 30 (62) | 23 (48) |
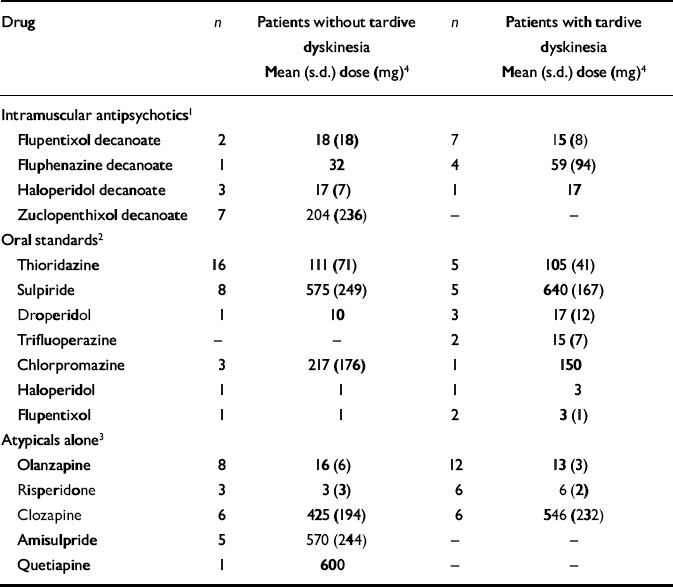
The drugs and their doses are recorded in Table 4. Numbers of patients receiving individual antipsychotic drugs were small and there were no statistically significant differences in the doses of the various drugs in those who did and did not have tardive dyskinesia. Within the group receiving intramuscular antipsychotics, no patient taking zuclopenthixol decanoate had tardive dyskinesia (Fisher's exact test χ2=6.50; d.f.=1, P=0.005).
Table 4 Individual antipsychotic drugs
| Drug | n | Patients without tardive dyskinesia Mean (s.d.) dose (mg)4 | n | Patients with tardive dyskinesia Mean (s.d.) dose (mg)4 |
|---|---|---|---|---|
| Intramuscular antipsychotics1 | ||||
| Flupentixol decanoate | 2 | 18 (18) | 7 | 15 (8) |
| Fluphenazine decanoate | 1 | 32 | 4 | 59 (94) |
| Haloperidol decanoate | 3 | 17 (7) | 1 | 17 |
| Zuclopenthixol decanoate | 7 | 204 (236) | - | - |
| Oral standards2 | ||||
| Thioridazine | 16 | 111 (71) | 5 | 105 (41) |
| Sulpiride | 8 | 575 (249) | 5 | 640 (167) |
| Droperidol | 1 | 10 | 3 | 17 (12) |
| Trifluoperazine | - | - | 2 | 15 (7) |
| Chlorpromazine | 3 | 217 (176) | 1 | 150 |
| Haloperidol | 1 | 1 | 1 | 3 |
| Flupentixol | 1 | 1 | 2 | 3 (1) |
| Atypicals alone3 | ||||
| Olanzapine | 8 | 16 (6) | 12 | 13 (3) |
| Risperidone | 3 | 3 (3) | 6 | 6 (2) |
| Clozapine | 6 | 425 (194) | 6 | 546 (232) |
| Amisulpride | 5 | 570 (244) | - | - |
| Quetiapine | 1 | 600 | - | - |
We examined more closely the patients who were receiving atypicals alone (n=48). The mean length of time for which the 25 who had tardive dyskinesia had been receiving their current drug was 31 months (s.d.=26). The reasons for that drug being started were: fresh episode or worsening of psychotic symptoms (n=16, 64%); unacceptable extrapyramidal side-effects (n=3, 12%); other side-effects (n=4, 16%); not known (n=2, 8%). Of the 23 without tardive dyskinesia, the mean length of time on the current drug was not significantly different (mean 23 months, s.d.=19). The reasons for starting were: fresh episode or worsening of psychotic symptoms (n=13, 57%); unacceptable extrapyramidal side-effects (n=1, 4%); other side-effects (n=5, 22%); not known (n=4, 17%).
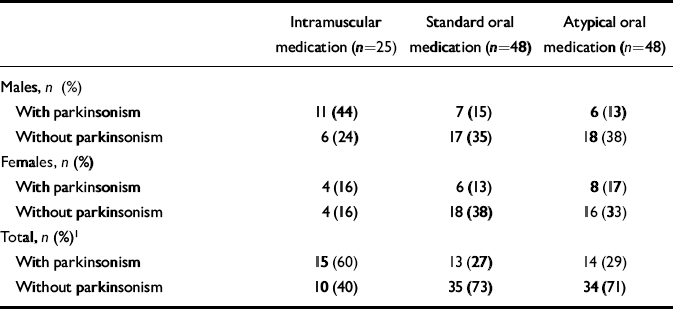
Medication status was also examined in patients with parkinsonism and akathisia over the past year and at the time of interview. When males and females were considered separately, no significant differences between the groups were found. However, in the total group parkinsonism was significantly more common in patients receiving long-acting intramuscular antipsychotic medication (Table 5). Of those receiving atypicals alone, 29% had parkinsonism and 18% had akathisia.
Table 5 Current medication of those with and without parkinsonism
| Intramuscular medication (n=25) | Standard oral medication (n=48) | Atypical oral medication (n=48) | |
|---|---|---|---|
| Males, n (%) | |||
| With parkinsonism | 11 (44) | 7 (15) | 6 (13) |
| Without parkinsonism | 6 (24) | 17 (35) | 18 (38) |
| Females, n (%) | |||
| With parkinsonism | 4 (16) | 6 (13) | 8 (17) |
| Without parkinsonism | 4 (16) | 18 (38) | 16 (33) |
| Total, n (%)1 | |||
| With parkinsonism | 15 (60) | 13 (27) | 14 (29) |
| Without parkinsonism | 10 (40) | 35 (73) | 34 (71) |
With regard to parkinsonism, we examined more closely those patients receiving only standard oral antipsychotic medication (n=34). Of the 25 patients who were receiving sulpiride or thioridazine, 6 (24%) had parkinsonism. Of the 9 on the other standard antipsychotic medication (e.g. trifluoperazine) 6 (67%) had parkinsonism.
In a further comparison of patients with and without movement disorders, there were no significant differences in the numbers of patients receiving either antidepressant or antiparkinsonian medication at the time of interview or over the previous year.
Comparison with previous reviews
The prevalence of tardive dyskinesia has been assessed in Nithsdale using the AIMS scale on seven occasions, including 1999/2000, since 1981 (Table 6). For each assessment, all known patients with schizophrenia are identified. Each cohort contains patients from the previous cohort, still alive and living in Nithsdale, and also new patients who have developed the illness for the first time or who have moved to the area; some from the previous cohort will have moved away or died. Thus, the mean age of the patients over the years remains very similar (Table 6). The prevalence of tardive dyskinesia has more than doubled over 20 years (χ2 for linear trend=16.89, P < 0.0001). The prevalence of parkinsonism and akathisia, assessed less often, has remained broadly the same (Table 6).
Table 6 Prevalence of movement disorders across 20 years
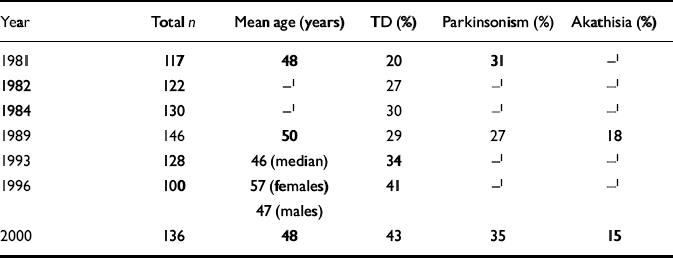
| Year | Total n | Mean age (years) | TD (%) | Parkinsonism (%) | Akathisia (%) |
|---|---|---|---|---|---|
| 1981 | 117 | 48 | 20 | 31 | —1 |
| 1982 | 122 | —1 | 27 | —1 | —1 |
| 1984 | 130 | —1 | 30 | —1 | —1 |
| 1989 | 146 | 50 | 29 | 27 | 18 |
| 1993 | 128 | 46 (median) | 34 | —1 | —1 |
| 1996 | 100 | 57 (females) 47 (males) | 41 | —1 | —1 |
| 2000 | 136 | 48 | 43 | 35 | 15 |
Current medication in 1981 and 1999/2000 was examined. Only four drugs prescribed in 1981 were prescribed to five or more patients in 1999/2000, namely: thioridazine (22 patients in 1981 and 22 in 1991/2000); chlorpromazine (15 and 6 patients, respectively); flupentixol decanoate (29 and 9 patients, respectively); and fluphenazine decanoate (27 and 5 patients, respectively). For the patients receiving thioridazine, the mean dose was significantly lower in 1999/2000 (97 mg (s.d.=48) v. 184 (94); t=3.88, d.f.=42, P=0.0004). There were no differences in the mean doses of the other drugs. There was no statistically significant difference in the numbers of patients in 1981 and 1999/2000 receiving more than one antipsychotic (21% and 16%, respectively). More patients in 1981 were receiving no antipsychotic medication (23/117 v. 10/131; χ2=6.74, d.f.=1, P=0.009).
In the 1981 cohort, 34 patients comprising 19 males and 16 females, mean age 40 years (s.d.=10), mean duration of illness 14 years (s.d.=9), were still alive and living in Nithsdale in 1999/2000, and were re-examined. Of the 34, 23 had been rated on 7 occasions, 9 on 6 occasions, 1 on 5 occasions and 1 on 4 occasions. Seven patients (21%) had never shown tardive dyskinesia compared with 27 (79%) who had tardive dyskinesia on at least one occasion. Of those who had tardive dyskinesia on at least one occasion, 7 (26%) developed the persistent condition (positive rating on the last three assessments); the remaining 20 (74%) had no clear pattern. No patient had persistent tardive dyskinesia (positive on three consecutive occasions) which remitted.
DISCUSSION
Methodological strengths and weaknesses
Of the total number of patients identified by the key informant method, 76% took part in the study. Those who did not take part did not differ in age, gender distribution or duration of illness from those who did. Although not all assessments were carried out by the same psychiatrist, tardive dyskinesia was rated on each occasion over the 20 years using the same scale (AIMS) and one of us (R.M.) was involved in every study and was responsible for training.
Tardive dyskinesia
We found the prevalence of probable tardive dyskinesia to be 43%. This is in keeping with a review (Reference Casey, Hirsch and R.WeinbergerCasey, 1995) that reported mild forms of the condition in approximately 20% of patients, rising to 50% in high-risk groups such as the elderly. Several risk factors for tardive dyskinesia have been identified, such as advanced age and duration of illness (Reference McCreadieMcCreadie, 1982; Reference Kane, Jeste and BarnesKane et al, 1992). Conflicting results have been found with gender (Reference Yassa and JesteYassa & Jeste, 1992) and smoking (Reference Kelly and McCreadieKelly & McCreadie, 1999). The only risk factor in our study significantly associated with tardive dyskinesia was longer duration of illness in male patients. The association of tardive dyskinesia with negative symptoms has been reported elsewhere (Reference Liddle, Barnes and SpellerLiddle et al, 1993). In our study, female patients with tardive dyskinesia were likely to have more negative symptoms and general psychopathology. The finding with regard to negative symptoms remained independently significant on logistic regression. In females, parkinsonism and akathisia were associated with tardive dyskinesia, but following logistic regression only akathisia remained independently significantly associated. We can only speculate why akathisia is associated with tardive dyskinesia in females but not in males. The influence of oestrogen on movement disorders is complex. It has been suggested that females experience a higher frequency of akathisia, possibly because of the additive effects of oestrogen and antipsychotics on dopamine blockade. Also, oestrogen decline in postmenopausal women may precipitate tardive dyskinesia by relieving dopamine blockade, as may withdrawal of antipsychotic medication (Reference Leung and ChueLeung & Chue, 2000). So, the presence of oestrogen in the young and lack of it in the old may be responsible for the association of akathisia with tardive dyskinesia in women.
Parkinsonism and akathisia
Drug-induced parkinsonism occurs especially during the initial period of antipsychotic medication exposure (Reference Casey, Hirsch and R.WeinbergerCasey, 1995). Prevalence rates of 20% (Reference Modestin, Stephan and ErniModestin et al, 2000) and 36% (Reference van Harten, Matroos and Hoekvan Harten et al, 1996) have been reported. This range is in keeping with our study, where the prevalence was 35%.
The prevalence of akathisia, one of the most intolerable extrapyramidal side-effects which often leads to non-compliance, has been reported as 11% (Reference Modestin, Stephan and ErniModestin et al, 2000), much the same as our finding of 15%.
Medication
There was no difference in the prevalence of tardive dyskinesia in the three medication categories; the condition was present in 52% of patients currently receiving atypicals only. There are several possible reasons for the findings in the present study. Tardive dyskinesia secondary to standard antipsychotics may persist even when such drugs are discontinued; the patients with tardive dyskinesia may have been preferentially switched to atypicals; withdrawal dyskinesia may develop after discontinuation of standard antipsychotics; and some patients have spontaneous rather than tardive dyskinesia. With regard to the preferential switch to atypicals, tardive dyskinesia was the reason for the change in only 8% of the 48 patients receiving atypicals.
We must emphasise that our study is one of prevalence, not incidence. The only satisfactory way to assess the incidence of tardive dyskinesia produced by different drugs is to follow prospectively patients suffering their first episode of illness and treated with a single drug.
A recent review has shown that extrapyramidal symptoms, including parkinsonism, are less common with atypical antipsychotic medication than with standard antipsychotics (Reference Geddes, Freemantle and HarrisonGeddes et al, 2000). In our study, parkinsonism was certainly less common than in those receiving long-acting intramuscular antipsychotics; yet the prevalence in those receiving atypicals was 29%. This is not because patients had recently been switched to atypicals and were still experiencing continuing side-effects produced by standard antipsychotics: the mean length of time patients had been receiving atypicals was 27 months. The prevalence of parkinsonism was similar in those receiving oral standards (27%) and atypicals (29%). However, 74% of patients on oral standards were receiving either thioridazine or sulpiride, two drugs which may be ‘older atypicals’; Baldessarini (Reference Baldessarini, Gilman, Goodman and Gilman1980) wrote more than 20 years ago that sulpiride and thioridazine ‘are at least partial exceptions to the formerly almost inevitable association of neurotoxic with antipsychotic effects’.
Movement disorders across 20 years
The prevalence of tardive dyskinesia has doubled over the past 20 years. There are several possible reasons: more patients may be receiving medication; doses may be higher; early intervention may mean patients are starting medication at an earlier age and have been taking drugs longer; and, because more patients are in the community, drug holidays are more likely (Reference van Harten, Hoek and Matroosvan Harten et al, 1998). With regard to the first possibility, more patients indeed were receiving medication in 1999/2000 than in 1981. This is probably the result of more assertive community outreach. With regard to the second possibility, only four drugs were prescribed to substantial numbers of patients in both 1981 and 1999/2000; the mean dose of one of them, thioridazine, was in fact lower in 1999/2000.
Of the 34 patients followed for 20 years, 79% had tardive dyskinesia on at least one occasion. This suggests that in almost all patients with chronic illness, dyskinesia is an inevitable occurrence. We can only speculate how much of this dyskinesia is drug-related and how much spontaneous. A recent review (Reference FentonFenton, 2000) has suggested that 40% of those aged 60 years or over have spontaneous dyskinesia.
Although numbers are small, our results suggest there may be two types of dyskinesia: a milder form which fluctuates over time (the majority) and a more severe form which persists once it develops (the minority). It has long been known that milder forms of tardive dyskinesia may show temporal fluctuations (Reference Bergen, Eyland and CampbellBergen et al, 1989).
Although the number of assessments were fewer, our results suggest that the prevalences of parkinsonism and akathisia have changed little. If some of the reasoning as to why tardive dyskinesia is increasing is correct, then it might be expected that the prevalence of parkinsonism would also rise. However, the switch from long-acting intramuscular antipsychotics and ‘old’ standards to atypicals may be having a protective effect.
We conclude that tardive dyskinesia and parkinsonism remain major problems despite the introduction of atypical antipsychotic medication 10 years ago. Further prospective studies are required to determine whether or not the use of atypicals will impact on the prevalences of these movement disorders.
CLINICAL IMPLICATIONS
-
▪ Tardive dyskinesia remains a common problem; its prevalence is increasing.
-
▪ Over 20 years, most patients have had tardive dyskinesia on at least one occasion.
-
▪ A third of patients receiving only atypicals have parkinsonism.
LIMITATIONS
-
▪ This study has not examined patients who have only ever received atypical antipsychotics.
-
▪ The number of patients receiving individual antipsychotic drugs is small.
-
▪ Only a small number of patients in the original cohort were examined 20 years later.
Acknowledgements
We thank the patients for their continuing cooperation, and Heather Barrington for statistical advice.











eLetters
No eLetters have been published for this article.