Premorbid developmental and social impairments have been well documented in adult schizophrenia (Reference Done, Crow and JohnstoneDone et al, 1994; Reference Jones, Rogers and MurrayJones et al, 1994; Reference Malmberg, Lewis and DavidMalmberg et al, 1998). Studies of child- and adolescent-onset schizophrenia suggest that premorbid impairments might be more common and severe than in the adult-onset disorder (Reference Alaghband-Rad, McKenna and GordonAlaghband-Rad et al, 1995; Reference HollisHollis, 1995; Reference Nicholson, Lenane and SingaracharluNicholson et al, 2000). However, several important questions remain unresolved regarding the significance of premorbid impairment in psychosis. First, it is unclear whether premorbid impairments are specific to child- and adolescent-onset schizophrenia, or whether they also occur in other psychotic disorders. There are reports of premorbid impairment associated with affective disorders (Reference Cannon, Jones and GilvarryCannon et al, 1997; Reference van Os, Jones and Lewisvan Os et al, 1997; Reference Malmberg, Lewis and DavidMalmberg et al, 1998; Reference Jones and TarrantJones & Tarrant, 1999) and affective psychoses in adolescence (Reference Sigurdsson, Fombonne and SayalSigurdsson et al, 1999). However, no study has compared premorbid impairment in child- and adolescent-onset schizophrenia with other early-onset psychoses. Second, it is unclear whether an association with psychotic symptom dimensions rather than diagnostic categories better explains the link between premorbid impairment and psychosis. This study addresses these questions by examining the relationship between premorbid functioning, psychotic symptoms and diagnosis in consecutive series of patients with first-episode child- and adolescent-onset psychosis.
METHOD
Sample
The sample was obtained using a two-stage retrospective survey of consecutive hospital contacts. Details of the initial screening and final sample selection procedure are described elsewhere (Reference HollisHollis, 2000). In summary, an initial retrospective psychosis screen was applied to all patients under 18 years of age who had attended the Maudsley Hospital in south London between 1973 and 1991. The Maudsley Hospital Children's Department clinical data summaries (‘item sheets’) were screened for psychotic symptoms (hallucinations, delusions or ideas of reference) and/or an ICD-9 psychotic diagnosis (World Health Organization, 1978). In addition, patients attending the Maudsley Hospital Adult Department were included in the ‘screen-positive’ sample if they were under the age of 18 years at the time of baseline assessment and had an ICD-9 psychotic diagnosis (ICD-8 codes were used from 1973 to 1977). A total of 196 screen-positive psychosis cases were identified.
The second stage involved a detailed chart review of the 196 screen-positive cases. The selection criterion was the unequivocal evidence of at least one psychotic symptom according to the Research Diagnostic Criteria (RDC) (Reference Spitzer, Endicott and RobbinsSpitzer et al, 1978). Of these 196 cases, 23 had missing case notes or insufficient clinical detail to determine with confidence the presence or absence of psychotic symptoms; 58 were confirmed as ‘non-psychotic’ after examination of the case records; and 5 had a diagnosis of autism in the absence of an RDC psychotic symptom. The remaining 110 cases constituted the child- and adolescent-onset psychosis sample for this study.
Measures
Clinical and demographic information was extracted from the patients' medical records using a structured coding sheet specifically designed for the study. The quality of case-note information recorded by Maudsley Hospital psychiatry trainees was uniformly high and followed the guidelines on obtaining and recording clinical information produced by the Maudsley Hospital and Institute of Psychiatry (Reference GoldbergGoldberg, 2002). To minimise potential bias and to avoid inferential impressions, items were rated only if the case notes contained explicit positive statements concerning the patient's status.
Rating of psychopathology
Psychopathological characteristics were rated from medical records using the Operational Criteria (OPCRIT) checklist for psychotic illness, version 3.31 (Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991). This comprises a checklist of 90 items constructed from operational criteria for the major psychiatric classifications and a suite of computer programs which allow psychopathological data to be entered, edited and diagnoses to be generated according to each set of diagnostic criteria. The OPCRIT system has been shown to have good reliability for DSM-III-R diagnoses (American Psychiatric Association, 1987) using the 90-item checklist (κ=0.73) (Reference Williams, McGuffin and AckenheilWilliams et al, 1996). The concurrent validity of OPCRIT DSM-III-R diagnoses has been established with good to excellent agreement with consensus best-estimate diagnoses (Reference Craddock, Asherson and OwenCraddock et al, 1996).
Other ratings during the first psychotic episode
Data were collected on psychotropic medication exposure and the occurrence of urinary incontinence during the first psychotic episode.
Obstetric complications
Obstetric complications were recorded on the Lewis—Murray scale (Reference Lewis, Owen, Murray, Schultz and TammingaLewis et al, 1989) using a summary score of 0, absent; 1, equivocal; 2, definite.
Premorbid behaviour and development
Premorbid behaviour and development were recorded using three scales: the General Developmental Scale, the Childhood Behaviour Scale and the Premorbid Adjustment Scale. Ratings were made from patient case-note information. Ratings required that clear behavioural descriptions or developmental data existed in the records. In the case of discrepancies, ‘positive’ clear symptoms took precedence over negative statements, and symptoms recorded at the time they were observed took precedence over those recollected. Not all items could be completed for every patient. A decision was taken not to prorate scores but to record data as missing if less than half of the items in the scale were completed. Where doubt remained concerning the onset of symptoms, ratings were always made for the ‘highest’ level of premorbid functioning.
General Developmental Scale. The General Developmental Scale (GDS) is a composite scale constructed specifically for this study, to record early childhood developmental delays and neurodevelopmental problems. Seven areas are assessed: motor milestones, language milestones, impaired social development, reading problems, neurodevelopmental problems, enuresis and encopresis (see Appendix for details of items and scoring).
Childhood Behaviour Scale. The Childhood Behaviour Scale (CBS) is a modified form of the Premorbid Schizoid and Schizotypal Scale described by Foerster et al (Reference Foerster, Lewis and Owen1991). It contains ten items covering the following areas: social isolation, social aloofness, separation or social anxiety, unusual stereotyped interests and preoccupations, deviant social communication or comprehension, quality of affect, suspiciousness and sensitivity, thought content and beliefs, deviant speech, and antisocial behaviour. In order to avoid rating prodromal symptoms, the premorbid period was defined as ending 1 year before the onset of psychotic symptoms. Where doubt remained about the onset of prodromal symptoms, the highest level of premorbid functioning was recorded (see Appendix for details of items and scoring).
Premorbid Adjustment Scale. In the Premorbid Adjustment Scale (PAS; Reference Cannon-Spoor, Potkin and WyattCannon-Spoor et al, 1982) ‘premorbid’ was defined as the period ending 1 year before the onset of overt psychosis. In this study, the ‘childhood to 11 years’ section of the PAS was completed. The original PAS uses a seven-point scale (0, normal; 6, severely impaired). In this study, scores were collapsed into three categories (0, normal/above average; 1, mild impairment; 2, severe impairment). Individual items were social withdrawal (as defined by avoidance of social interaction and social contexts), peer relationships, scholastic performance, social and behavioural adaptation to school, and interests or hobbies.
IQ measures
Scores of IQ based on the Wechsler Intelligence Scale for Children — Revised (WISC—R; Reference WechslerWechsler, 1974) were available for 64 out of 110 (58%) of the baseline sample.
Reliability of premorbid data
The premorbid measures (GDS, CBS and PAS) were constructed or modified specifically for this study and were of unknown reliability. In a random sample of 25 cases, information on premorbid development and behaviour were extracted from the case notes and ratings made by a second experienced child psychiatrist (Karmen Slaveska), who remained blind to psychopathological data and diagnoses. For the three scales, the intraclass correlations (r) were uniformly high: GDS, r=0.91 (95% CI 0.81-0.96); CBS, r=0.91 (95% CI 0.81-0.96); PAS, r=0.97 (95% CI 0.94-0.99). For all three measures, random and observer error accounted for less than 10% of the observed variance in scores.
Analyses
Factor analysis
Twenty items were selected from the OPCRIT checklist, reflecting the main psychotic and affective symptoms and signs (see Table 2). Manic and depressive symptoms were each entered as the sum of the individual items for mania and depression. Of the items included, the median number of non-zero (0 indicating absence of symptom or sign) items was 32.5% (range 12-84%). Initial unrotated factors were extracted by principal components analysis. Factors with an eigenvalue greater than 1 were then subjected to a varimax rotation. Finally, regression factor scores were produced for each case and saved for further analyses.
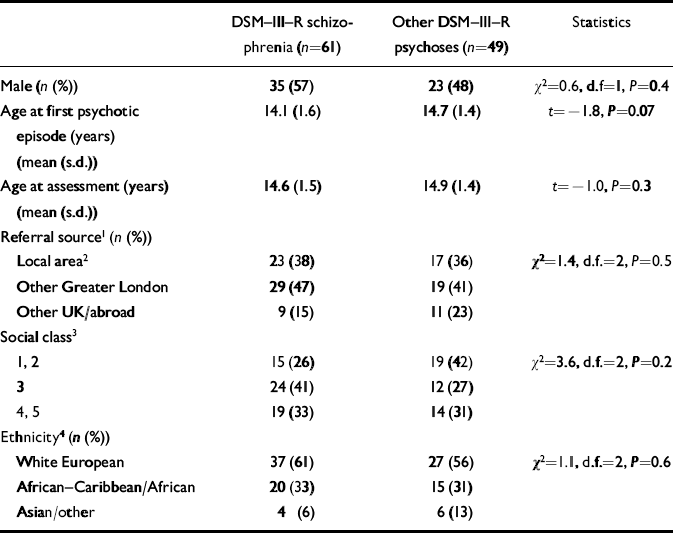
Table 1 Demographic characteristics according to diagnosis
| DSM-III-R schizophrenia (n=61) | Other DSM-III-R psychoses (n=49) | Statistics | |
|---|---|---|---|
| Male (n(%)) | 35 (57) | 23 (48) | χ2=0.6, d.f=1, P=0.4 |
| Age at first psychotic episode (years) (mean (s.d.)) | 14.1 (1.6) | 14.7 (1.4) | t=-1.8, P=0.07 |
| Age at assessment (years) (mean (s.d.)) | 14.6 (1.5) | 14.9 (1.4) | t=-1.0, P=0.3 |
| Referral source1 (n (%)) | |||
| Local area2 | 23 (38) | 17 (36) | χ2=1.4, d.f.=2, P=0.5 |
| Other Greater London | 29 (47) | 19 (41) | |
| Other UK/abroad | 9 (15) | 11 (23) | |
| Social class3 | |||
| 1, 2 | 15 (26) | 19 (42) | χ2=3.6, d.f.=2, P=0.2 |
| 3 | 24 (41) | 12 (27) | |
| 4, 5 | 19 (33) | 14 (31) | |
| Ethnicity4 (n (%)) | |||
| White European | 37 (61) | 27 (56) | χ2=1.1, d.f.=2, P=0.6 |
| African—Caribbean/African | 20 (33) | 15 (31) | |
| Asian/other | 4 (6) | 6 (13) |
Table 2 Factor analysis of Operational Criteria (OPCRIT) checklist items

| OPCRIT items1 | Factors following varimax rotation and % variance explained | ||||||
|---|---|---|---|---|---|---|---|
| Frequency item present n (%) | Factor 1 ‘negative syndrome’ (23.2%)3 | Factor 2 ‘depression’ (9.6%)3 | Factor 3 ‘disorganisation’ (8.9%)3 | Factor 4 ‘positive I’2 (7.0%)2 | Factor 5 ‘positive II’2 (6.4%)2 | Factor 6 ‘mania’ (5.2%)2 | |
| Poor rapport | 60 (54) | 0.81 | |||||
| Restricted affect | 53 (48) | 0.77 | |||||
| Negative thought disorder | 25 (23) | 0.67 | |||||
| Blunted affect | 21 (19) | 0.57 | |||||
| Inappropriate affect | 63 (57) | 0.50 | |||||
| Insidious onset | 51 (46) | 0.50 | |||||
| Depression score | - | 0.85 | |||||
| Depressive delusions | 13 (12) | 0.74 | |||||
| Positive thought disorder | 30 (27) | 0.75 | |||||
| Speech difficult to understand | 36 (33) | 0.63 | |||||
| Bizarre behaviour | 80 (73) | 0.52 | |||||
| Passivity phenomena | 26 (24) | 0.80 | |||||
| Thought interference | 13 (12) | 0.68 | |||||
| Bizarre delusions | 30 (27) | 0.60 | |||||
| Hallucinations | 76 (69) | 0.74 | |||||
| Delusions | 92 (84) | 0.69 | |||||
| Lack of insight | 87 (79) | 0.48 | |||||
| Grandiose delusions | 14 (13) | 0.77 | |||||
| Mania score | - | 0.54 | |||||
Univariate analyses
Comparisons were made between schizophrenia and other psychoses. Categorical data were analysed using a chi-squared test of significance and a continuity (Yates') correction. Categorical r×2 tables with ordered categories were analysed using the χ2 test for linear trend. Fisher's exact test was used when expected cell numbers were less than 5. For continuous variables, Student's t-test was used when assumptions of normality and homogeneity were met; when these assumptions were violated we used non-parametric tests such as the Mann—Whitney U test (corrected for ties). All reported tests of significance are two-sided.
Multivariate analyses
The strength of association between individual premorbid variables and diagnostic status was assessed using logistic regression. Odds ratios were adjusted for gender, social class, ethnicity and catchment-area status. The strength of association between premorbid functioning and symptom dimensions was assessed using multiple regression analysis. Continuous premorbid variables (GDS, CBS, PAS items) were treated as dependent variables and regressed onto the six symptom dimensions (regression factor scores) entered simultaneously into the regression model. Premorbid variables (GDS and CBS) were log transformed to remove skewness. Logistic regression was used to assess the association between symptom dimensions and dichotomous developmental variables found in Table 4, plus the variable ‘urinary incontinence while psychotic’. Standardised regression coefficients (β) and odds ratios (OR) were adjusted for gender, social class and catchment-area status.
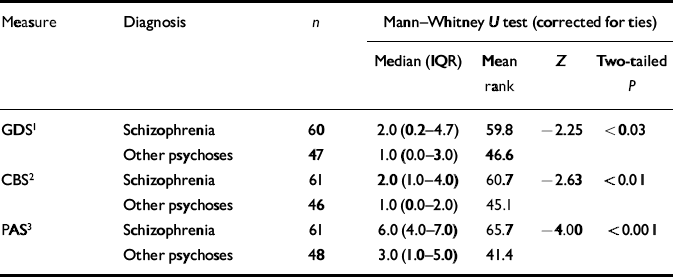
Table 3 Premorbid functioning according to DSM-III-R diagnosis
| Measure | Diagnosis | n | Mann—Whitney U test (corrected for ties) | |||
|---|---|---|---|---|---|---|
| Median (IQR) | Mean rank | Z | Two-tailed P | |||
| GDS1 | Schizophrenia | 60 | 2.0 (0.2-4.7) | 59.8 | -2.25 | <0.03 |
| Other psychoses | 47 | 1.0 (0.0-3.0) | 46.6 | |||
| CBS2 | Schizophrenia | 61 | 2.0 (1.0-4.0) | 60.7 | -2.63 | <0.01 |
| Other psychoses | 46 | 1.0 (0.0-2.0) | 45.1 | |||
| PAS3 | Schizophrenia | 61 | 6.0 (4.0-7.0) | 65.7 | -4.00 | <0.001 |
| Other psychoses | 48 | 3.0 (1.0-5.0) | 41.4 | |||
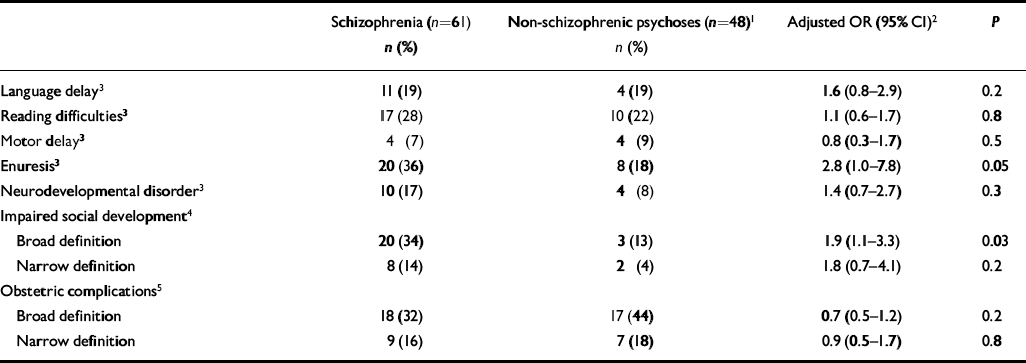
Table 4 Perinatal and developmental problems according DSM-III-R diagnosis
| Schizophrenia (n=61) n (%) | Non-schizophrenic psychoses (n=48)1 n (%) | Adjusted OR (95% CI)2 | P | |
|---|---|---|---|---|
| Language delay3 | 11 (19) | 4 (19) | 1.6 (0.8-2.9) | 0.2 |
| Reading difficulties3 | 17 (28) | 10 (22) | 1.1 (0.6-1.7) | 0.8 |
| Motor delay3 | 4 (7) | 4 (9) | 0.8 (0.3-1.7) | 0.5 |
| Enuresis3 | 20 (36) | 8 (18) | 2.8 (1.0-7.8) | 0.05 |
| Neurodevelopmental disorder3 | 10 (17) | 4 (8) | 1.4 (0.7-2.7) | 0.3 |
| Impaired social development4 | ||||
| Broad definition | 20 (34) | 3 (13) | 1.9 (1.1-3.3) | 0.03 |
| Narrow definition | 8 (14) | 2 (4) | 1.8 (0.7-4.1) | 0.2 |
| Obstetric complications5 | ||||
| Broad definition | 18 (32) | 17 (44) | 0.7 (0.5-1.2) | 0.2 |
| Narrow definition | 9 (16) | 7 (18) | 0.9 (0.5-1.7) | 0.8 |
RESULTS
Sample characteristics
Of the 110 patients in the sample, 58 (53%) were male, and the mean age of onset of psychosis was 14.4 years (range 10-17, s.d. 1.5). The mean duration from onset of psychotic symptoms to baseline assessment was 5.2 months (range 0-36, s.d. 6.9). At the baseline assessment 61 patients (55%) had an OPCRIT DSM-III-R diagnosis of schizophrenia, 15 (14%) had a schizoaffective psychosis, 26 (24%) had an affective psychosis (unipolar major depressive or bipolar psychoses) and 8 (7%) had an atypical psychosis (unspecified functional psychoses). All non-schizophrenic psychoses (n=49) were combined for further analysis. Table 1 describes the characteristics of the 61 patients with schizophrenia and the 49 patients with other non-schizophrenic psychoses. Both diagnostic groups were similar in terms of age at onset, duration of follow-up, gender ratio, catchment area (local area v. elsewhere), social class and ethnicity. Urinary incontinence during the first psychotic episode was more common in schizophrenia (n=21; 34%) than in other psychoses (n=7; 14%); χ2=5.0, d.f.=1, P<0.02.
Psychotic symptom dimensions: factor analysis of OPCRIT items
Table 2 shows the frequency and factor analysis of the 20 main OPCRIT psychopathology items. Six factors had eigenvalues greater than 1, accounting for 60.3% of the total variance. Regression factor scores for each dimension were approximately normally distributed (mean of zero with unit standard deviation).
Premorbid functioning and diagnosis
Table 3 shows that DSM-III-R schizophrenia was associated with higher (more deviant) scores on each of the three premorbid scales.
Table 4 presents the frequency of perinatal and developmental problems for schizophrenia and non-schizophrenic psychoses. Delays in the onset of urinary continence and broadly defined premorbid social impairments were significantly more common in DSM-III-R adolescent-onset schizophrenia. Delays in language milestones, reading and neurodevelopmental disorders were also more common in those with a diagnosis of schizophrenia, although none of these associations reached statistical significance at the 5% level. There was no difference between the diagnostic groups in the rates of obstetric complications, encopresis or delays in motor development.
IQ measures
Full-scale IQ was measured during the index assessment using the WISC—R on 37 out of 61 (61%) of the schizophrenia group and 27 out of 48 (56%) of those with non-schizophrenic psychoses. Full-scale IQ was significantly lower in the schizophrenia group (mean 79.5, s.d. 14.6) v. the non-schizophrenia group (mean 90.4, s.d. 17.9; t=2.7, P=0.009). The IQ scores for both groups were distributed normally, with no evidence for a low-IQ subgroup. For those with a diagnosis of schizophrenia, 26 (70%) had IQ scores within the normal range (70-130); the remainder fell into the category of mild ‘mental retardation’ or learning disability (50-69). Of those with non-schizophrenic psychosis, 25 (93%) fell within the normal 70-130 IQ range, with only 2 cases (7%) falling into the category of mild learning disability (Fisher's exact test, P=0.03).
Symptom dimensions and premorbid functioning
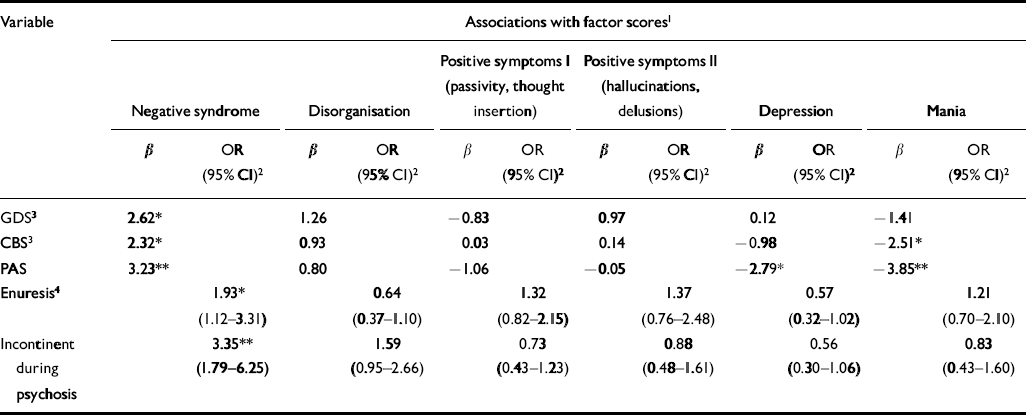
Table 5 shows the associations (standardised regression coefficients and odds ratios) between premorbid variables and symptom dimensions. The ‘negative syndrome’ was specifically associated with impaired premorbid functioning (measured on the GDS, CBS and PAS), premorbid enuresis and urinary incontinence during the psychotic episode. In contrast, both the ‘depression’ and ‘mania’ symptom dimensions were associated with relatively better premorbid functioning within the sample.
Table 5 Associations between premorbid development and symptom dimensions
| Variable | Associations with factor scores1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative syndrome | Disorganisation | Positive symptoms I (passivity, thought insertion) | Positive symptoms II (hallucinations, delusions) | Depression | Mania | |||||||
| β | OR (95% CI)2 | β | OR (95% CI)2 | β | OR (95% CI)2 | β | OR (95% CI)2 | β | OR (95% CI)2 | β | OR (95% CI)2 | |
| GDS3 | 2.62* | 1.26 | -0.83 | 0.97 | 0.12 | -1.41 | ||||||
| CBS3 | 2.32* | 0.93 | 0.03 | 0.14 | -0.98 | -2.51* | ||||||
| PAS | 3.23** | 0.80 | -1.06 | -0.05 | -2.79* | -3.85** | ||||||
| Enuresis4 | 1.93* | 0.64 | 1.32 | 1.37 | 0.57 | 1.21 | ||||||
| (1.12-3.31) | (0.37-1.10) | (0.82-2.15) | (0.76-2.48) | (0.32-1.02) | (0.70-2.10) | |||||||
| Incontinent | 3.35** | 1.59 | 0.73 | 0.88 | 0.56 | 0.83 | ||||||
| during psychosis | (1.79-6.25) | (0.95-2.66) | (0.43-1.23) | (0.48-1.61) | (0.30-1.06) | (0.43-1.60) | ||||||
DISCUSSION
Findings
Child- and adolescent-onset schizophrenia was associated with a greater premorbid impairment than other child- and adolescent-onset non-schizophrenic psychoses. Comparing specific domains of development, those with schizophrenia were more likely to have experienced premorbid social impairments and enuresis (late onset of urinary continence). There was a trend for those with schizophrenia to have experienced more difficulties in language development and reading. No diagnostic difference was found in the frequency of obstetric complications or motor delays. The IQ measured at the first psychotic episode was significantly lower in schizophrenia (mean 79.5), with 30% of cases in the mild learning disability range (50-69).
Factor analysis revealed six psychotic symptom dimensions: negative symptoms; disorganisation; two positive symptom factors; mania; and depression. The negative symptom dimension was specifically associated with premorbid impairment. Both the manic and depressive symptom dimensions were associated with better premorbid functioning. Negative symptoms were specifically associated with enuresis (late onset of urinary continence) and the occurrence of urinary incontinence during the first psychotic episode.
Strengths and limitations of the methodology
The study is based on a large consecutive series of first-episode child- and adolescent-onset psychoses collected over an 18-year period. The sample design allows premorbid functioning to be contrasted between schizophrenia and other early-onset psychotic disorders. It also allows symptom dimensions to be examined across a broad range of psychoses rather than within a single diagnostic group. The choice of a first-episode sample means that associations between premorbid functioning and psychopathology are not confounded by outcome. The quality of the case notes was high, with the majority containing contemporaneous descriptions of child development and behaviour (e.g. school and health reports) in addition to retrospective parental accounts obtained at the index episode. The OPCRIT method of rating psychopathology is well suited to case-note ratings and has been demonstrated to have good reliability and validity. Data on premorbid functioning was collected blind to OPCRIT diagnostic status, with high interrater reliability, suggesting that a surprisingly high degree of precision was possible when rating these high-quality case notes. Premorbid developmental and social functioning was recorded and analysed as both composite scores and individual items, to reduce the possibility of spurious chance associations with multiple comparisons.
There are several limitations in the study design. First, a single person made the case-note ratings of both premorbid functioning and psychopathology. This introduces the possibility of information bias — i.e. premorbid ratings could have been influenced by knowledge of diagnosis and symptoms, or diagnostic ratings could have been influenced by premorbid data. The first possibility seems unlikely, as a second, independent, rater achieved a high level of agreement with the main rater when assessing premorbid functioning blind to both symptoms and diagnosis. Although the main rater was clearly aware of symptoms recorded in the case notes, neither rater knew the OPCRIT-derived DSM-III-R diagnosis when rating premorbid data. There was no difference between the diagnostic groups in the amount of case-note information available on premorbid development. Although the Maudsley case records were extremely detailed, the secondary rating of chart data collected by a large number of different examining psychiatrists is likely to have introduced considerable random error into the ratings. Given this caveat, the observed association between premorbid impairments and schizophrenia and the specific continuity with negative symptoms was impressive, and may in fact underestimate true effects. Second, the low incidence of child- and adolescent-onset psychoses necessitated retrospective case ascertainment and limited the available sample size. The sample size necessitated the grouping together of non-schizophrenic psychoses and provided limited power to detect small effects associated with individual developmental variables. Birth cohort studies identifying adult-onset psychoses have larger control groups and greater power to detect small effects of individual developmental variables (Reference Jones, Rogers and MurrayJones et al, 1994; Reference Cannon, Caspi and MoffittCannon et al, 2002). Power was also reduced by the necessity of using categorical ratings of what are in reality continuous developmental variables. Finally, it seems unlikely that premorbid impairments identified in this study simply represent prodromal psychotic symptoms: first, the rating of the ‘premorbid’ period was based on the highest level of functioning from early childhood, and second, the ‘premorbid’ period excluded the 12 months prior to the onset of psychosis.
What do the results mean?
The results of this study suggest that the premorbid phenotype of child- and adolescent-onset schizophrenia can be distinguished from other early-onset psychoses by a higher rate of premorbid impairments, particularly affecting the domains of social development and the onset of urinary continence. However, in this study premorbid motor impairments and obstetric complications fail to distinguish between schizophrenia and other early-onset psychoses. In other words, impaired ‘sociability’ (similar to concepts of schizoid personality and ‘schizotypy’) may provide the clearest distinction between the developmental phenotype of schizophrenia and precursors of other psychoses. The occurrence of social and language impairments in non-schizophrenic psychoses indicates that they are not diagnosis-specific — although the magnitude of the association seems to be greater in schizophrenia. The evidence of a specific continuity between premorbid impairments and negative symptoms suggests possible developmental continuity at the level of symptom dimensions.
Several rather different mechanisms may underlie the association between developmental impairment and psychosis. First, general developmental delay, reflected in late milestones, low premorbid IQ and broad cognitive impairments, could reduce the threshold for the expression of all forms of psychosis in a non-specific way, with only the magnitude of effect being greater for schizophrenia. Hence, non-specific developmental delay could act as a continuous independent risk factor for a broad range of psychopathological outcomes, including psychosis. Second, impaired premorbid sociability may be a more direct expression of genetic vulnerability to schizophrenia. However, premorbid social impairment could be a developmental precursor of the negative symptom dimension rather than of schizophrenia per se. The links between negative symptoms, enuresis and urinary incontinence during psychotic episodes suggest that these symptoms might result from a common neural mechanism, possibly involving aspects of prefrontal cortical function.
The results in context
Previous studies have reported separately on the increased risks of premorbid impairment in child- and adolescent-onset schizophrenia (Reference Asarnow, Tompson and GoldsteinAsarnow et al, 1994; Reference HollisHollis, 1995; Reference Nicholson, Lenane and SingaracharluNicholson et al, 2000) and adolescent affective psychoses (Reference Sigurdsson, Fombonne and SayalSigurdsson et al, 1999). However, to date, no study has compared premorbid functioning in different child- and adolescent-onset psychoses. This study extends the findings of previous investigations with adult patients that describe more marked premorbid social impairments in schizophrenia compared with affective psychoses (Reference Foerster, Lewis and OwenFoerster et al, 1991; Reference Cannon, Jones and GilvarryCannon et al, 1997). However, unlike the reports of Foerster et al (Reference Foerster, Lewis and Owen1991) and Done et al (Reference Done, Crow and Johnstone1994), in this study the precursors of psychosis were independent of gender. These findings concur with Nicholson et al (Reference Nicholson, Lenane and Singaracharlu2000), who reported that premorbid impairments in childhood-onset schizophrenia are independent of gender. The association described here between childhood-onset schizophrenia and primary enuresis supports the findings of Done et al (Reference Done, Johnstone and Firth1991) from the 1958 British birth cohort and Isohanni et al (Reference Isohanni, Rantakallio and Jones1998) from the North Finland birth cohort, both studies finding an association between delayed onset of urinary continence and later schizophrenia. The present study also found, in agreement with Done et al (Reference Done, Johnstone and Firth1991), that the degree of cognitive impairment was significantly greater in schizophrenia than in other psychoses.
The underlying symptom dimensions reported in this study are similar to the pattern described by van Os et al (Reference van Os, Fahy and Jones1996) in adult-onset first-episode psychosis. Few studies have examined symptom dimensions in child- and adolescent-onset psychoses (Reference Maziade, Bouchard and GingrasMaziade et al, 1996; Reference Bunk, Eggers and KlapalBunk et al, 1999). Unlike the study by Maziade et al (Reference Maziade, Bouchard and Gingras1996), the present study found a significant association between premorbid functioning and negative symptoms.
Clinical and research implications
The concept of a premorbid or longitudinal phenotype of schizophrenia raises important questions about developmental mechanisms, as well as the tantalising possibility of early detection and prevention of psychosis. First, the premorbid phenotype of schizophrenia as currently conceived in terms of impaired sociability and developmental impairments lacks both precision and specificity. Not only does it overlap with premorbid impairments reported in other psychoses, but also with the clinical features of childhood developmental disorders such as developmental language disorder, attention-deficit hyper-activity disorder and autistic spectrum disorders (Reference Hollis, Taylor, Keshervan and MurrayHollis & Taylor, 1997). Comparisons at the behavioural and neurocognitive levels between children at ‘high risk’ of schizophrenia and other developmental disorders will be needed to identify more specific behavioural or neurocognitive precursors of schizophrenia. Second, the viability of early detection and screening depends crucially on whether treatment of the ‘pre-schizophrenic state’ can improve outcome. Clearly, much more fine-grained behavioural and neurocognitive characterisation is required of the prepsychotic developmental phenotype before screening or early detection is feasible. It may be more fruitful to look for developmental and neurocognitive continuities and prediction at the level of symptom dimensions rather than diagnostic categories.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Premorbid developmental and social impairments are more common in child- and adolescent-onset schizophrenia than in other child- and adolescent-onset psychoses. However, these premorbid impairments are not specific to schizophrenia.
-
▪ There appears to be a specific developmental continuity from premorbid impairment to negative psychotic symptoms. This may represent a longitudinal syndrome of social impairment.
-
▪ Negative symptoms are associated with delayed onset of urinary continence and with urinary incontinence during psychotic episodes. These symptoms may be common manifestations of underlying prefrontal cortical dysfunction.
LIMITATIONS
-
▪ The results apply to an adolescent-onset psychosis sample; they may not apply to samples with earlier or later onset of psychosis.
-
▪ Both premorbid and psychopathological data were obtained from case notes.
-
▪ The study sample was recruited from a tertiary referral centre. Replication is required in a population-based sample.
APPENDIX
General Developmental Scale
-
1. Delayed motor development (e.g. first sat unsupported >8 months and/or walked >18 months).
-
2. Delayed speech/language development (e.g. first word other than ‘mama/dada’ >24 months, first meaningful two- or three-word phrases >36 months).
-
3. Impaired social development aged 0-6 years. This requires a definite history of at least one of the following: lack of gesture to communicate, lack of reciprocal social communication, stereotyped or idiosyncratic use of language, abnormal prosody, lack of imaginative/imitative play, failure to regulate gaze/facial expression/posture in social communication, failure to make friends and share interests, failure to seek comfort or share pleasure.
-
4. Reading difficulties (confirmed by school report or reading tests).
-
5. Any neurodevelopmental disorder (e.g. hyper-kinesis, tics, autism, learning disabilities, i.e. IQ <70).
-
6. Enuresis (wetting at least once a week beyond age 5 years).
-
7. Encopresis (soiling at least once a week over age 4 years, for a minimum of 6 months).
Scoring
Items 1-5: 0, no/absent; 1, equivocal; 2, definite; 9, not known/missing data.
Items 6-7: 0, no/absent; 1, present; 9, not known/missing data.
The total GDS score has a range from 0 to 12.
Childhood Behaviour Scale
This scale is a modified form of the Premorbid Schizoid and Schizotypal Scale described by Foerster et al (Reference Foerster, Lewis and Owen1991). Ratings are made for the premorbid period, age 6-11 years. The premorbid period is defined as ending 12 months before the onset of the first psychotic symptom. The scale consists of the following ten items:
-
1. Social isolation (0, none; 1, mild; 2, marked; 9, not known/missing data).
-
2. Social aloofness (0, none; 1, mild; 2, marked; 9, not known/missing data).
-
3. Separation anxiety/social anxiety (0, none; 1, mild; 2, marked; 9, not known/missing data).
-
4. Unusual stereotyped interests and preoccupations (0, none; 1, mild; 2, marked; 9, not known/missing data).
-
5. Deviant social communication/comprehension (0, none; 1, mild; 2, marked; 9, not known/missing data).
-
6. Affect (0, warm/spontaneous; 1, rare displays of affection; 2, cold, restricted affect; 9, not known/missing data).
-
7. Suspiciousness/sensitivity (0, none; 1, mild; 2, marked; 9, not known/missing data).
-
8. Thought content/beliefs (0, no abnormality; 1, occasional odd ideas/ideas of reference; 2, marked/persistent abnormality; 9, not known/missing data).
-
9. Deviant speech (0, no abnormality; 1, mildly deviant, i.e. digressive, over elaborate; 2, marked abnormality; 9, not known/missing data).
-
10. Antisocial behaviour (0, none; 1, mild; 2, marked; 9, not known/missing data).
The total CBS score has a range from 0 to 20.
Acknowledgements
The author thanks the UK Medical Research Council, who supported this study through the award of a Research Training Fellowship, and Professor Eric Taylor, Dr Karmen Slaveska, Kate Garrett and Pam Remon for their help with the study.










eLetters
No eLetters have been published for this article.