Schizophrenia is treatment resistant in about one in three patients. Reference Mortimer, Singh, Shepherd and Puthiryackal1–Reference Taylor, Paton and Kapur4 This has been defined as an inadequate response to sequential treatment with two different antipsychotics at adequate dose, duration and adherence. 3,Reference Taylor, Paton and Kapur4 Clozapine is the only drug treatment currently licensed for patients with treatment-resistant schizophrenia, and is associated with lower rates of re-admittance to hospital compared with other antipsychotics. Reference Taylor, Paton and Kapur4,Reference Tiihonen, Haukka, Taylor, Haddad, Patel and Korhonen5 However, long delays in initiating clozapine in routine clinical practice have been reported. Reference Harrison, Janlov and Wheeler6–Reference Wheeler9 Since our previous study, Reference Taylor, Young and Paton8 clinical guidelines from the National Institute for Health and Clinical Excellence (NICE) and other organisations have been published recommending that clozapine be offered at the earliest opportunity for patients with treatment-resistant schizophrenia. Reference Barnes10,11 Although there is little clear benefit of clozapine as a first-line treatment, Reference Agid, Remington, Kapur, Arenovich and Zipursky12 a study of patients presenting to services for the first time found that 75% of patients who failed to respond to sequential 4-week treatment trials with two different antipsychotics then responded to clozapine. Reference Agid, Arenovich, Sajeev, Zipursky, Kapur and Foussias13
Guidelines issued by NICE and other organisations also state that there is little evidence to support the use of antipsychotic doses above the licensed maximum dose or for antipsychotic polypharmacy (other than for short periods during cross-tapering) and recommend that these strategies should be used only in exceptional cases. Reference Barnes10,11 The aim of our current study was to determine whether prescribing practice follows NICE clinical guidelines. Specifically, we sought to determine the time taken to initiate clozapine after a patient had completed adequate treatment with two different antipsychotic drugs, and the frequency of antipsychotic polypharmacy and high-dose antipsychotic treatment prior to clozapine initiation.
Method
The study included all patients identified using the clozapine monitoring system who commenced clozapine for the first time from 1 January 2006 to 15 April 2010 in the hospital or out-patient services, including tertiary services (<50 patients with schizophrenia), at the South London and Maudsley NHS Foundation Trust. The study was approved by the Trust's Drugs and Therapeutics Committee.
The following data were extracted from the clinical records: the primary diagnosis (according to ICD-10 criteria), 14 self-reported ethnicity (categorised as White British/other, Black British/other, Asian British/other, or mixed); duration of illness (defined as the time from the first recording of the diagnosis of a psychotic illness by a clinician to the present); and treatment history. An ‘antipsychotic treatment episode’ was defined as the prescription of a regular daily dose of an antipsychotic at any dose for at least 24 h. Antipsychotic drugs prescribed ‘as required’ were not counted. An adequate treatment episode was defined as an antipsychotic prescribed regularly (defined as above) at or above the minimum therapeutic dose (according to the British National Formulary (BNF)) 15 given the patient's age and dosing schedule for at least 6 weeks in line with the NICE guidelines. Adequate use of the same drug at different times was recorded as multiple episodes. Where treatment adherence was noted to be poor, the episode was counted as inadequate.
Information on antipsychotic treatment was extracted as follows:
-
(a) the total number of antipsychotic treatment episodes prior to the first use of clozapine (where the same antipsychotic was given on more than one occasion separated by at least 6 weeks, and each occasion met the treatment episode criteria, these were counted as separate episodes);
-
(b) the number of different antipsychotic drugs used (as treatment episodes) before the first use of clozapine;
-
(c) the number of adequate antipsychotic treatment episodes before first clozapine use;
-
(d) the number of adequate treatment episodes of different antipsychotics before the first use of clozapine using the same definition of adequate treatment as above and with no double counting of drugs;
-
(e) the number of antipsychotic treatment episodes with different second-generation drugs before first starting clozapine. Second-generation antipsychotic drugs were defined as amisulpride, aripiprazole, olanzapine, quetiapine, remoxipride (now withdrawn in Europe), risperidone, sertindole, ziprasidone and zotepine, and each drug was only counted once (even if a drug had been used for more than one treatment episode).
Antipsychotic polypharmacy was defined as the regular co-prescription of two or more antipsychotics each meeting the criteria for a treatment episode as above (drugs prescribed ‘as required’ or given irregularly were not included). Treatment episodes wherethe antipsychotic dosewas abovethe maximumtherapeutic dose or below the minimum therapeutic (subtherapeutic) dose given in the BNF were recorded separately (in the case of polypharmacy this was determined from the combined total dose, calculated as described in the Maudsley Prescribing Guidelines). Reference Taylor, Paton and Kapur4,15
The primary outcome measure was the maximum theoretical delay in clozapine initiation. This was determined in patients who had received adequate antipsychotic treatment episodes (as defined above) with at least two different antipsychotic drugs (the point at which their illness could first meet NICE criteria for treatment resistance) and was defined as the time from the end of the second adequate antipsychotic treatment episode to the first clozapine use. Thus, a patient who completed the second adequate antipsychotic treatment episode in January 2005 and who started clozapine in January 2006 would have a theoretical delay of 1 year. The period before January 1990 was excluded as clozapine was not available in the UK before then. In cases where a patient's first documented antipsychotic prescription involved polypharmacy, each additional adequate antipsychotic trial was counted as an additional treatment episode. Where the adequacy of a treatment episode could not be determined because information on dose or duration was missing, this episode was excluded from the analyses of adequate trials but it was counted for the purposes of total number of antipsychotic treatment episodes.
TABLE 1 Comparison between patients included in and excluded from the study
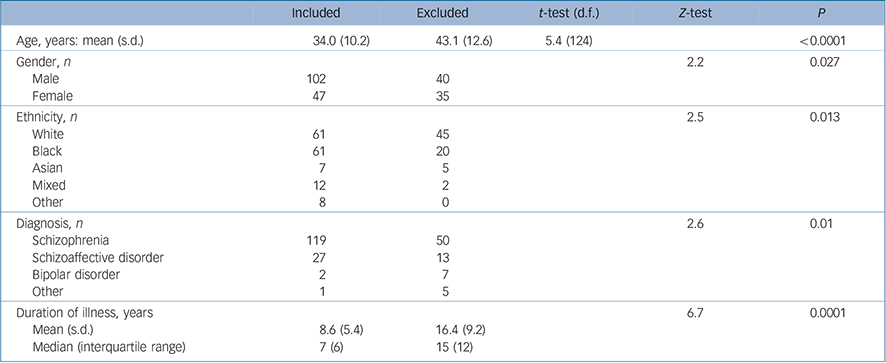
| Included | Excluded | t-test (d.f.) | Z-test | P | |
|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 34.0 (10.2) | 43.1 (12.6) | 5.4 (124) | <0.0001 | |
| Gender, n | 2.2 | 0.027 | |||
| Male | 102 | 40 | |||
| Female | 47 | 35 | |||
| Ethnicity, n | 2.5 | 0.013 | |||
| White | 61 | 45 | |||
| Black | 61 | 20 | |||
| Asian | 7 | 5 | |||
| Mixed | 12 | 2 | |||
| Other | 8 | 0 | |||
| Diagnosis, n | 2.6 | 0.01 | |||
| Schizophrenia | 119 | 50 | |||
| Schizoaffective disorder | 27 | 13 | |||
| Bipolar disorder | 2 | 7 | |||
| Other | 1 | 5 | |||
| Duration of illness, years | 6.7 | 0.0001 | |||
| Mean (s.d.) | 8.6 (5.4) | 16.4 (9.2) | |||
| Median (interquartile range) | 7 (6) | 15 (12) |
Statistical analysis
After determining whether variances were equal and conformed to a normal distribution using Levene's test and the Kolmorogov–Smirnov Z-test respectively, two-tailed Mann–Witney U- or Kruskal–Wallis tests were used as appropriate to test whether there were differences in theoretical delay by gender, diagnostic or ethnic group. In an exploratory analysis, the relationship between theoretical delay (dependent variable) and age and illness duration (independent variables) was determined using regression.
Results
We identified 227 patients who commenced clozapine for the first time between 1 January 2006 and 15 April 2010. Of these, 78 (34.4%) were excluded from subsequent analyses because notes were missing, and so a complete prescribing history was not available. The demographic and clinical characteristics of the included and excluded individuals are shown in Table 1. There were significant differences in age, gender, ethnicity, diagnosis and duration of illness between the two groups, with the included participants tending to be younger, to have shorter duration of illness, more likely to be male, diagnosed with schizophrenia and be from a Black, Asian or mixed ethnic group (Table 1).
Of the 149 individuals included in the study, 68.5% were male, 79.9% were diagnosed with schizophrenia, 18.1% with schizoaffective disorder, 1.3% with bipolar disorder and 0.7% with psychotic depression. In total, 132 participants (88.6%) were started on clozapine for the first time as in-patients and 17 (11.4%) started it in the community. The range of duration of illness was 1.8–30 years.
There were a total of 825 antipsychotic treatment episodes recorded for the participants included in the study. A total of 91 (11.0%) episodes could not be evaluated due to incomplete information (either the dose or duration was missing for some time points). Of the remaining 734 episodes, 189 (25.7%) were inadequate, either because they used a subtherapeutic dose (n =83, 11.3%) or were too short in duration (n = 88, 12.0%) or were both at a subtherapeutic dose and too short in duration (n =18, 2.5%).
The antipsychotic treatment history preceding clozapine is summarised in Table 2. Before commencing clozapine, 143 patients (96.0%) received at least one adequate trial of a second-generation antipsychotic, and 85 patients (57%) had received a depot antipsychotic. Polypharmacy was evident in 54 patients (36.2%). A total of 51 patients (34.2%) had received antipsychotic treatment above the maximum licensed dose (45.9% during monotherapy, 54.1% during polypharmacy).
In total 95 patients (63.8%) were switched directly from an oral antipsychotic medication to clozapine. Of these, 86 (90.5%) were switched from a second-generation drug, 8 (8.4%) from a first-generation drug and 1 (1.1%) from a combination of a first- and a second-generation drug. Also, 34 patients (22.8%) were switched directly from depot medication, 16 (10.7%) from a combination of a depot and oral medication, and 4 (2.7%) had received no treatment for at least 1 month prior to commencing clozapine.
Before commencing clozapine, 129 patients (86.6%) had received at least two adequate trials of different antipsychotics. The remaining 20 (13.4%) had not received two adequate antipsychotic treatment episodes (18 (12.1%) had received one and 2 (1.3%) had not received any adequate treatment trials) before commencing clozapine. The mean theoretical delay in the 129 individuals who had received two adequate treatment trials was 47.7 (s.d. = 49.7) months (median 32 (interquartile range, IQR = 58), range 0–219).
There was a significant relationship between theoretical delay and age (β = 0.3, t = 3.5, P = 0.001), and between delay and illness duration (β = 0.7, t = 12.5, P<0.001, Fig. 1, where theoretical delay (months) was 16 + 0.7 × illness duration (months)). However, the relationship between age and delay was not significant after adjusting for illness duration (β = 0.02, t = 0.3, P = 0.8). There was no significant difference in theoretical delay between female and male patients (female: mean 64.8 months (s.d. = 64), median 46.5 (IQR = 93); male: mean 39.5 months (s.d. = 39), median 29 (IQR = 43); Z = 1.5, P = 0.13) or between diagnostic groups (schizophrenia: mean 47.5 months (s.d. = 52.4), median 29 (IQR = 57); any other diagnosis: mean 48.6 months (s.d. = 39.5), median 41 (IQR = 70); Z = 0.8, P = 0.4), or ethnicity (White: mean 48.3 months (s.d. = 53), median 31.5 (IQR = 57); other: mean 47.4 months (s.d. = 47.9), median 32 (IQR = 59); Z = 0.2, P = 0.9).
Discussion
This study had two main findings. First, that in routine clinical practice, clozapine initiation was delayed by a mean of 4 years, and second, that other antipsychotics were often used instead, in ways not recommended by therapeutic guidelines. Thus, prior to commencing clozapine, over a third of patients had received antipsychotic polypharmacy, and over a third had received antipsychotic treatment above the licensed maximum dose, even though there is little evidence to support either strategy, and both are associated with increased risks of side-effects and complications. Reference Taylor, Paton and Kapur4,Reference Barnes10,Reference Barnes and Paton16,Reference Howes, Egerton, Allan, McGuire, Stokes and Kapur17 Prior to commencing clozapine, patients received, on average, more than five different antipsychotic treatment episodes. About 50 patients started clozapine each year. Given that there are over 5000 patients with schizophrenia in the Trust and about 350 new patients present each year (details available from the authors on request), this suggests that clozapine is underused.
TABLE 2 Antipsychotic treatment prior to commencing clozapine
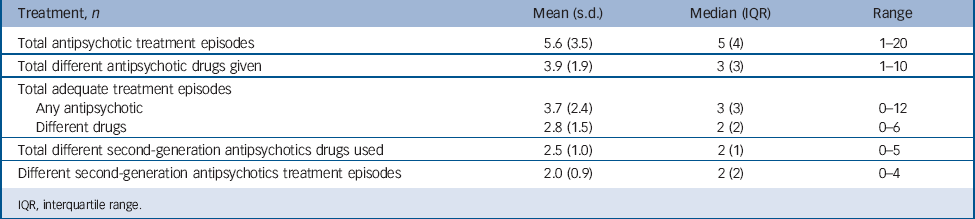
| Treatment, n | Mean (s.d.) | Median (IQR) | Range |
|---|---|---|---|
| Total antipsychotic treatment episodes | 5.6 (3.5) | 5 (4) | 1–20 |
| Total different antipsychotic drugs given | 3.9 (1.9) | 3 (3) | 1–10 |
| Total adequate treatment episodes | |||
| Any antipsychotic | 3.7 (2.4) | 3 (3) | 0–12 |
| Different drugs | 2.8 (1.5) | 2 (2) | 0–6 |
| Total different second-generation antipsychotics drugs used | 2.5 (1.0) | 2 (1) | 0–5 |
| Different second-generation antipsychotics treatment episodes | 2.0 (0.9) | 2 (2) | 0–4 |
IQR, interquartile range.
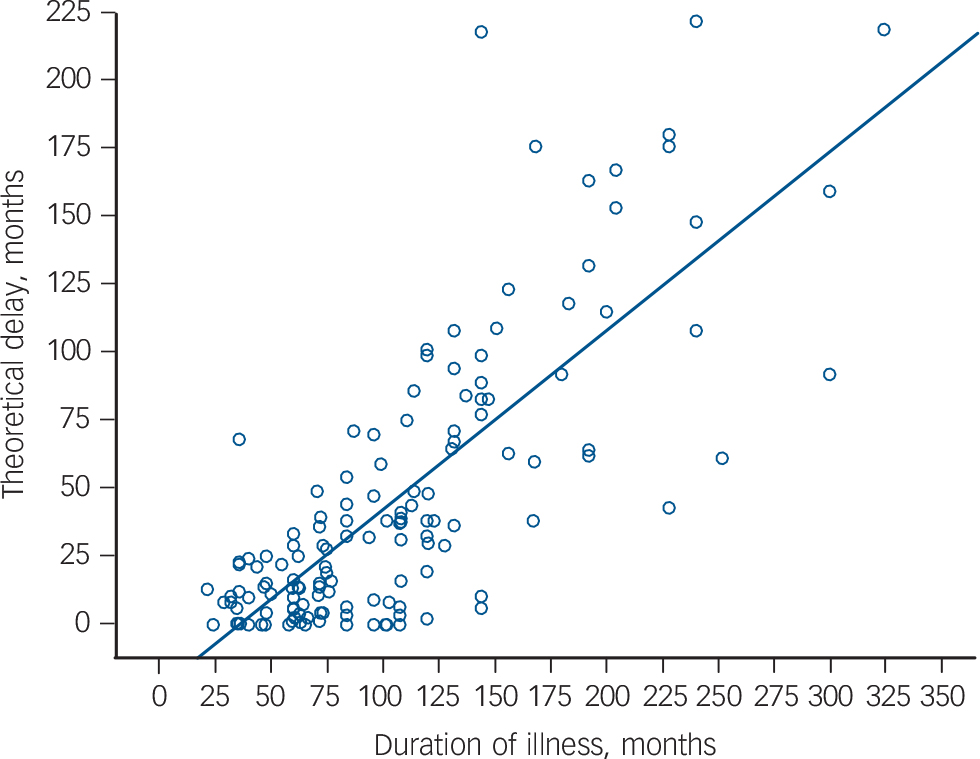
FIG. 1 The relationship between theoretical delay to clozapine initiation and illness duration (β = 0.7, P<0.001).
Methodological considerations
One potential limitation is that we did not determine the reason for initiating clozapine. It is possible that some of the patients commencing clozapine were doing so for treatment intolerance rather than treatment resistance. However, a number of second-generation antipsychotics have been available since the mid-1990s as alternatives to clozapine for treatment intolerance. Reference Taylor, Paton and Kapur4,Reference Howes, Bhatnagar, Gaughran, Amiel, Murray and Pilowsky18–Reference Howes, Wheeler, Pilowsky, Landau, Murray and Smith20 As 96% of the patients had received at least one second-generation antipsychotic, it is thus unlikely that antipsychotic intolerance was the reason for many, if any, of the patients commencing clozapine. A further potential limitation is that we did not determine the response to prior antipsychotic treatment. It is thus possible that some patients initially showed a good response but developed treatment resistance later. Although prospective studies are needed to definitively test this, for it to account for the long time to initiate clozapine, patients would have been treated on one drug for a long time, and then, as treatment resistance developed, switched to another drug before starting clozapine within a relatively short period of time. However, this pattern of treatment was not evident –instead they received multiple treatment episodes and different antipsychotic drugs. Although we excluded treatment episodes where adherence was noted to be poor, another limitation of the study is that some adequate trials may in fact have been inadequate due to covert non-adherence.
Our data on antipsychotic high doses and polypharmacy were restricted to regular prescriptions: the rates of both would have been higher if we had included ‘as required’ prescriptions as well. A number of patients had to be excluded from the study because complete treatment histories were not available and this may limit the generalisability of our findings. As the excluded patients had significantly longer illness durations than the included patients, and greater illness duration was associated with greater initiation delay, the delay in initiating clozapine may be longer if we had been able to include all patients. A total of 91 (11%) treatment episodes were not included because of missing data and this means there is some imprecision in our data. For example, if all of these episodes had been inadequate this would have increased the percentage of inadequate episodes by 8.2% (from 25.7 to 33.9%), and reduced the percentage of adequate episodes by the same amount. Finally, our study is limited to patients who have started clozapine –the treatment of refractory patients who have not started clozapine remains to be determined.
Implications of our findings
In comparison with the mean delay of 5 years in our 2002 study in the same setting, Reference Taylor, Young and Paton8 the delay to clozapine initiation in our current study was shorter but remained substantial despite the publication of the NICE guidelines in 2002. 11 As the guidelines were disseminated to all clinicians, unlike in 2002, it is not possible to argue that lack of familiarity with the recommendations or the evidence underlying them, underlies these findings. Furthermore, it is also clear from guidelines published since then that the consensus on these issues did not change during our study period. 3,Reference Barnes10,Reference Buchanan, Kreyenbuhl, Kelly, Noel, Boggs and Fischer21 Our current findings, thus, suggest that schizophrenia treatment guidelines have had little impact on the time taken to initiate clozapine, or on the use of high-dose antipsychotics or polypharmacy prior to clozapine in this setting, although it could be argued that we would see greater effects if the study was restricted to patients whose first antipsychotic treatment began after the publication of the guidelines. The mean number of different adequate antipsychotic treatment episodes was 2.8, lower than in our previous study (four drugs) Reference Taylor, Young and Paton8 – suggesting that this aspect of prescribing is closer to guidelines now.
Coupled with evidence of the underuse of clozapine in many settings, Reference Kelly, Dixon, Kreyenbuhl, Medoff, Lehman and Love22 the long delay in initiating clozapine and also the common use of treatments that guidelines recommend are restricted to exceptional cases, our study suggests that there are major barriers to clinicians initiating clozapine. Patient-related factors, such as refusing blood monitoring, are likely to contribute to clozapine's underuse and delays in initiation, although it is noteworthy that once patients are on clozapine the vast majority report its advantages outweigh its disadvantages. Reference Taylor, Shapland, Laverick, Bond and Munro23,Reference Angermeyer, Loffler, Muller, Schulze and Priebe24 Furthermore, although patient factors are unlikely to vary markedly between settings, the use of clozapine varies greatly across settings and countries Reference Wheeler9,Reference Downs and Zinkler25,Reference Tang, Mao, Jiang, Chen, Wang and Cai26 and organisational changes have been associated with a 2.9-year reduction in the mean delay to initiate clozapine. Reference Harrison, Janlov and Wheeler6 This suggests that organisational- and clinician-related factors have a major impact and are potentially modifiable contributors to the delay we observed.
Clozapine has a complex dose titration regime and requires daily monitoring during the initiation phase and careful management of treatment emergent side-effects. Many clinicians have few patients on clozapine under their care, and are less familiar with managing the initiation of clozapine than other antipsychotic drugs. Reference Nielsen, Dahm, Lublin and Taylor27 These factors may contribute to the delay we observed. However, it is possible to initiate clozapine quickly for individuals whose condition is treatment resistant where resources are available, Reference Agid, Arenovich, Sajeev, Zipursky, Kapur and Foussias13 suggesting that the availability of additional resources and expertise might reduce delays in clozapine initiation.
In summary, in routine practice there is often a long delay before the use of clozapine. Moreover, during this period, other antipsychotics are commonly used in higher than licensed doses, or in combination, despite the lack of evidence to support these strategies.
Funding
This study was funded by an MRC, UK grant to O.D.H. (grant code MC-A656-5QD30) and the National Institute of Health Research Biomedical Research Centre.








eLetters
No eLetters have been published for this article.