Birth weight is an important indicator of early childhood survival and health. Low-birth-weight babies are at an increased risk of stunting, infant mortality and morbidity, poor cognitive development, and chronic diseases such as diabetes and CVD later in life( Reference Black, Victora and Walker 1 ). In low- and middle-income countries, maternal undernutrition contributes to intra-uterine growth restriction, which results in low birth weight( Reference Black, Victora and Walker 1 , Reference Kelly, Kevany and de Onis 2 ). Good nutrition during pregnancy is essential for ensuring fetal growth and development and subsequent childhood health and survival. While studies have identified effective supplementation interventions to address maternal undernutrition in low-income countries, the causes of maternal undernutrition in different contexts remain relatively unexplored( Reference Ota, Tobe-Gai and Mori 3 , Reference Stevens, Buettner and Watt 4 ). To design programmes that address maternal undernutrition, organisations often assess food security and dietary diversity using tools that provide a snapshot of the situation at one point in time. Dietary diversity over a reference period acts as a proxy indicator of dietary quality and is associated with nutrient adequacy( Reference Moursi, Arimond and Dewey 5 , Reference Ruel 6 ). Household food security status reflects availability, accessibility and utilisation of food at a household level. Both dietary diversity and household food security are associated with access to and availability of foods, and seasonality is recognised as a key element of food availability in many low-income countries. While it may appear logical for seasonality to be associated with dietary diversity and household food security, programme decision makers rarely consider seasonality when designing new programmes to address maternal undernutrition.
Bangladesh has among the highest rates of maternal and child undernutrition globally. One in three pregnant women is undernourished (BMI <18·5 kg/m2), one in five babies is born with low birth weight, and one in three children aged 6–59 months is stunted( 7 , 8 ). In Bangladesh, the times of the year when there are seasonal fluctuations in income and employment are referred to as ‘monga’. Monga occurs twice annually: the main monga occurs from 15 September to 14 November (Bangladeshi months of Ashbin and Kartik), prior to the main rice harvest, and the lesser monga occurs from 15 March to 14 April (Bangladeshi month of Choitro)( Reference Zug 9 ). During monga Bangladeshis face seasonal food shortages that often result in household food shortages, particularly among those in rural areas who rely on subsistence farming. The documented consequences of seasonal food shortages on the nutritional status of women in low-income countries include reduced energy expenditure, weight loss, insufficient weight gain and subsequent low birth weight( Reference Rayco-Solon, Fulford and Prentice 10 – Reference Roberts, Paul and Cole 12 ). Despite a number of studies exploring the role of seasonality on dietary diversity and household food security status at specific time points (often pre and post the lean or monsoon season), the role of annual seasonal variations on dietary diversity and household food security, especially among pregnant women, remains largely unknown( Reference Savy, Martin-Prével and Traissac 13 , Reference Hillbruner and Egan 14 ). By understanding seasonal fluctuations in dietary diversity and household food security among pregnant women, programme decision makers can better design interventions to address maternal undernutrition.
The aim of the present study was to investigate the role of seasonality on dietary diversity and household food security for pregnant women living in rural Bangladesh. We also explored relationships of seasonality with maternal nutritional status. We anticipate that these findings will highlight the importance of understanding seasonal variations in maternal dietary diversity and household food security and inform the design of future nutrition interventions in Bangladesh and other low-income contexts.
Methods
Study design
A cross-sectional study was conducted. Recruitment occurred over the period February 2013 to February 2015. The sample consisted of individuals from all villages previously selected for a cluster randomised controlled trial which aimed to measure the effect of a locally produced food-based supplement on reducing intra-uterine growth restriction in undernourished pregnant women. While the sample for the cluster randomised controlled trial consisted of undernourished pregnant women only, the current study looked at all pregnant women from these villages, regardless of their nutritional status. Therefore, participants for the current sample were from two purposively selected unions in Pirganj sub-district, one selected as the intervention and the other matched as the control. From the intervention union, eight villages were randomly selected using computer-generated random numbers. From the control union, four villages were purposively matched. The number of villages was determined based on average population number, prevalence and estimated incidence of pregnant women across the study period, and the required sample size. The data used for the current study come from surveys completed by consenting women confirmed to be pregnant within the twelve villages of interest.
Setting
The study was conducted in twelve rural villages of Pirganj sub-district of Rangpur District, located in northern Bangladesh. According to the 2011 Population and Housing Census, Pirganj sub-district covers an area of 411·34 km2, consists of 332 villages and has 385 499 inhabitants( 15 ). The majority of the population is Muslim, with a minority of people belonging to the Santal ethnic group who are predominantly Christian. The average household size is 3·78 and the literacy rateFootnote * is 45·4 %( 15 ). The area has a tropical monsoon climate, and experiences high temperatures and humidity and heavy seasonal rainfall from June to November. Rangpur is commonly referred to as ‘monga prone’ and reported as more vulnerable to seasonal food insecurity than other areas of Bangladesh( 16 ). Rangpur’s main employment source is agricultural labour, although wages are very low compared with neighbouring districts( Reference Zug 9 ). The villages are typical of villages in northern Bangladesh and have dirt road access that is often inaccessible during the wet season. The communities are largely dependent on subsistence farming and have limited experience with non-Bangladeshi foods.
Participants and recruitment
In each of the selected villages, all women were invited to participate in the study if they: (i) were suffering no illness requiring medical referral; and (ii) were confirmed to be pregnant by a midwife, skilled community health volunteer or other health professional. Prior to the commencement of the study, eight female community nutrition volunteers and two (one male, one female) supervisors from the selected villages were trained on the basics of nutrition, study purpose and design. The community nutrition volunteers had at least a primary-school education, spoke the local dialect, and were aged between 21 and 49 years. Community nutrition volunteers compiled lists of all pregnant women in the twelve villages and produced village maps that determined the location of each woman’s household. These women were identified through community discussions, door-knocking and snowballing. If the woman was interested, the community nutrition volunteer then verified that she met the inclusion criteria and referred the woman to a skilled health worker if the pregnancy was not yet confirmed. Women were given a brief overview of the project and invited to participate. Written or verbal (with thumbprint) consent to participate was obtained after participants heard the project information sheet read aloud. A copy of the information sheet in the local language was provided to participants for their further reference. Verbal consent was also obtained from the leaders of each village for inclusion of their village in the study.
The current project had human research ethical approval from the James Cook University (Australia) Ethics Committee (H4498) and the Bangladesh Medical Research Council (BMRC/NREC/2010-2013/58). The research was registered with the ISRCTN registry (ISRCTN97447076).
Data collection
The community nutrition volunteers assisted participants to complete a survey comprising: (i) background demographics; (ii) household food security; (iii) dietary diversity; and (iv) anthropometry. We used the validated Food and Nutrition Technical Assistance Project’s Household Food Insecurity Access Scale (HFIAS) questionnaire and the validated FAO dietary diversity questionnaire to explore food security and dietary diversity, respectively( Reference Arimond, Wiesmann and Becquey 17 – Reference Coates, Swindale and Bilinsky 19 ). The HFIAS questionnaire consisted of nine occurrence questions (conditions) and nine frequency-of-occurrence questions. With a recall period of 30 d, each occurrence question reflected a condition that represented an increasing level of severity of food insecurity (access) and the frequency-of-occurrence questions determined how often the condition occurred. The nine questions were grouped to form three domains to provide additional information on anxiety and uncertainty about household food supplies, insufficient quality and insufficient food intake and its physical consequences. The frequency-of-occurrence questions were also used to calculate scores, which were then grouped into two categories: food secure and food insecure( Reference Coates, Swindale and Bilinsky 19 ). The dietary diversity questionnaire consisted of thirteen questions that were later aggregated to form nine food groups. With a recall period of 24 h, this questionnaire allowed us to calculate the woman’s dietary diversity score (WDDS), identify individual food groups consumed and calculate specific indicators of interest for micronutrient-rich food groups( Reference Arimond, Wiesmann and Becquey 20 ). The study lead author adapted the HFIAS and dietary diversity questionnaires through community dialogue( Reference Stevens, Watt and Clough 21 ). The HFIAS questionnaire was adapted to ensure a common understanding of certain words (e.g. a locally appropriate definition of ‘household’ and ‘meal’). The dietary diversity questionnaire was adapted to reflect locally available foods. Questionnaires were translated into local terminology, back-translated and field-tested prior to use. In addition, an interviewer’s guide was developed to provide additional information on each question, as well as examples to ensure that questions were understood appropriately.
Mid-upper arm circumference (MUAC) measurements were performed using the standardised procedures recommended by the WHO( 22 ). The community nutrition volunteer took duplicate measurements of the left arm measured to the nearest millimetre with adult MUAC tapes. Triplicate measurements were taken if a variation occurred between the two measurements. Maternal undernutrition was defined as MUAC ≤22·1 cm. MUAC was the preferred indicator to identify maternal undernutrition based on its association with low birth weight( Reference Ververs, Antierens and Sackl 23 , 24 ). The cut-off was determined after a review of the evidence and a discussion with organisations conducting nutrition programmes and research in Bangladesh( 24 ).
Statistical methods
The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) Checklist for cross-sectional studies was used to ensure comprehensive reporting of the present study. Data management was performed using the statistical software package IBM SPSS Statistics, Version 23.0©. Data quality was ensured by quality checks associated with the data-entry process, double entry and data cleaning.
Food insecurity
Household food insecurity was determined using the HFIAS for Measurement of Food Access: Indicator Guide, Version 3. Dichotomous occurrence and ordinal frequency categorical variables were created for each condition; frequencies for each food insecurity access domain (anxiety, food quality and food quantity) and HFIAS scores were used to identify the prevalence of different levels of household food insecurity( Reference Coates, Swindale and Bilinsky 19 ).
Dietary diversity
Women’s dietary diversity was determined using the FAO guidelines to measure household and individual dietary diversity( Reference Kennedy, Ballard and Dop 18 ). Dietary diversity was summarised to create dichotomous occurrence variables for each food group and indicators of specific interest, and aggregated to create the WDDS( Reference Kennedy, Ballard and Dop 18 ). The nine food groups were: (i) starchy staples; (ii) dark green leafy vegetables; (iii) vitamin A-rich fruits/vegetables; (iv) other fruits/vegetables; (v) organ meats; (vi) meat/poultry/fish; (vii) eggs; (viii) legumes/nuts/seeds; and (ix) milk/milk products. The WDDS were used as discrete quantitative variables and divided into tertiles to distinguish diets of high, medium and low diversity. As there are no universally agreed cut-offs to define WDDS tertiles, we created tertiles based on recommendations in current literature( Reference Ruel 6 ). After aggregating household food security and dietary diversity according to the respective guidelines, we analysed the means of the variables, with a higher HFIAS indicating more food insecure and a lower WDDS indicating lower dietary diversity. To explore consumption of vitamin A- and Fe-rich foods, indicators from specific related food groups were created( Reference Kennedy, Ballard and Dop 18 ).
Household food security, dietary diversity and nutritional status were used as dependent variables and differences in these variables were examined based on the season variable. Grouping questionnaires by the six Bangladeshi seasons (summer=15 April–14 June; monsoon=15 June–14 August; autumn=15 August–14 October; late autumn=15 October–14 December; winter=15 December–14 February; spring=15 February–14 April) created the season variable. We used inferential statistics to test for relationships and differences between the variables of interest.
Data were not normally distributed, so non-parametric tests were used. Questions related to food security, dietary diversity and anthropometry based on season were analysed using the Kruskal–Wallis one-way ANOVA by ranks test followed by the post hoc Mann–Whitney U test. Comparisons between frequencies of participants’ responses on the dietary diversity and food security variables were analysed using the χ 2 test. Statistical significance was accepted at P<0·05.
Results
Participants
From February 2013 to February 2015, 289 pregnant women were identified as potentially eligible. One woman refused participation for unknown reasons. Thus, a total of 288 women were enrolled in the study.
Descriptive data
Background characteristics of the 288 women surveyed are presented in Table 1. The mean age of women was 25·3 (sd 5·7) years. Most women had attended school, with the majority reaching either primary or lower secondary education (42·0 and 42·4 %, respectively). The mean height of women was 148·5 (sd 7·7) cm, with almost half of women below 148 cm (47·4 %). The mean MUAC of women was 23·73 (sd 2·27) cm, with 29·7 % of women below 22·1 cm. Most women were in their second trimester (54·9 %) of their pregnancy. Of women in the first trimester (n 85), the mean BMI was 20·4 (sd 3·4) kg/m2. One in three women reported that she was experiencing pregnancy for the first time (36·5 %).
Table 1 Background characteristics of participating pregnant women (n 288) from twelve villages in rural northern Bangladesh, February 2013–February 2015
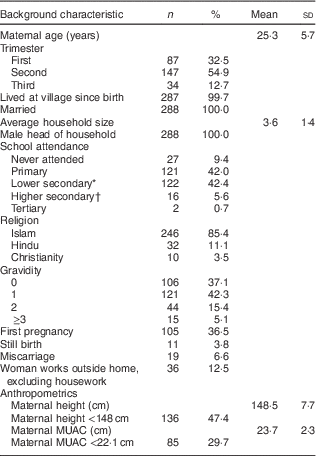
MUAC, mid-upper arm circumference.
* Grade 7–10.
† Grade 11–12.
Main results
Food security
Food security characteristics are presented in Table 2. The mean HFIAS score was 4·06 (sd 2·86; range 0–13 of a possible 27). Of the women, most were identified as food insecure (87·6 %), with 7·7 % identified as severely food insecure.
Table 2 Food security characteristics of pregnant women (n 288) from twelve villages in rural northern Bangladesh, February 2013–February 2015
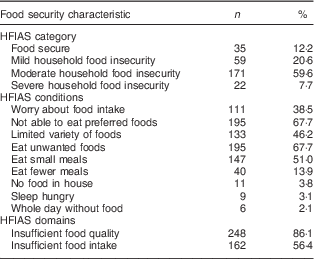
HFIAS, Household Food Insecurity Access Scale.
Dietary diversity
Over nine possible food groups, women’s dietary diversity ranged from two to eight food groups. The mean number of food groups consumed was at 4·8 (sd 1·1), indicating low probability of adequate dietary intake( Reference Kennedy, Fanou-Fogny and Seghieri 25 ). Figures 1 and 2 illustrate the food groups consumed by pregnant women. To further explore the frequency of food groups consumed, Table 3 illustrates the food groups consumed by more than 50 % of women by WDDS tertile.
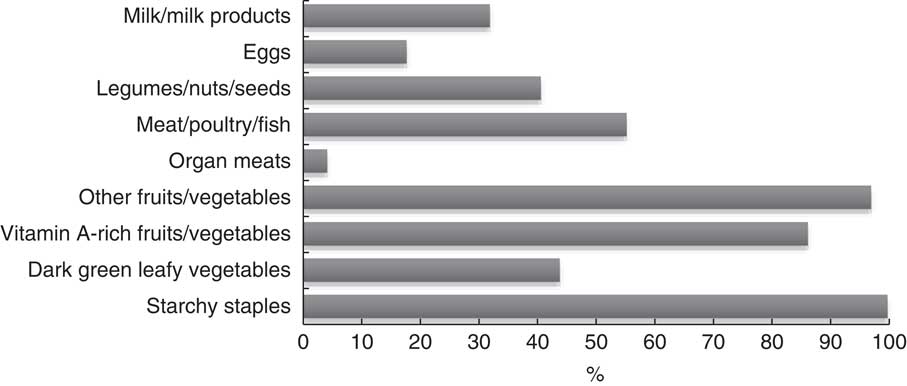
Fig. 1 Food groups consumed over a 24 h period by pregnant women (n 288) from twelve villages in rural northern Bangladesh, February 2013–February 2015
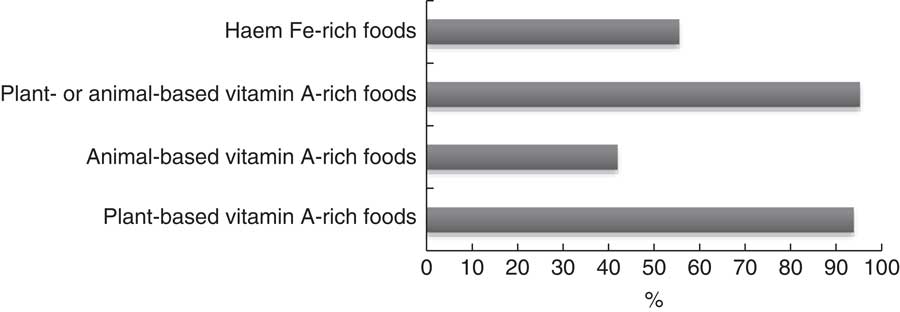
Fig. 2 Micronutrient-rich food groups consumed over a 24 h period by pregnant women (n 288) from twelve villages in rural northern Bangladesh, February 2013–February 2015
Table 3 Food groups, according to tertile of dietary diversity, consumed by ≥50 % of pregnant women (n 288) from twelve villages in rural northern Bangladesh, February 2013–February 2015

Seasonal variations in dietary diversity and household food security status
Table 4 presents the percentage of participants reporting consuming each food group (dietary diversity) and their agreement with various statements in relation to household food security, based on season. The relevant medians and interquartile ranges for these analyses can be seen in Table 5. No significant differences in MUAC based on season were found (P=0·130). The median WDDS varied as a function of season (H(5)=12·97, P=0·026). Mann–Whitney U tests revealed that the median value for dietary diversity was significantly lower in summer than in late autumn (U=2202, r=2·19, P=0·029) and winter (U=802·5, r=2·21, P=0·027) and significantly lower in spring than in late autumn (U=1286·5, r=2·08, P=0·038) and winter (U=463·5, r=2·23, P=0·026). Chi-square analyses revealed that the consumption of meat/poultry/fish varied as a function of season (χ 2 =11·74, P=0·039). Based on the percentages presented in Table 4, it appears as though a greater proportion of women consumed meat/poultry/fish in late autumn and winter compared with other seasons. This may have contributed to the significantly higher number of women eating food groups rich in haem Fe during late autumn and winter compared with other months (see Table 4; χ 2 =12·9, P=0·034).
Table 4 Percentage response to dietary diversity, food security and nutritional status questions as a function of season among pregnant women (n 288) from twelve villages in rural northern Bangladesh, February 2013–February 2015
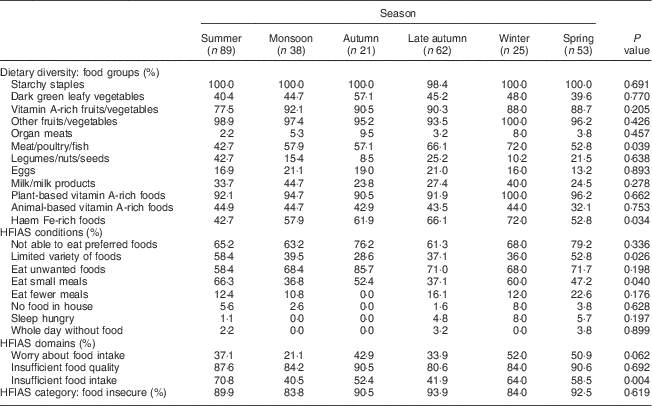
HFIAS, Household Food Insecurity Access Scale.
Table 5 Median (and interquartile range (IQR)) dietary diversity score, food security score and mid-upper arm circumference (MUAC) as a function of season among pregnant women (n 288) from twelve villages in rural northern Bangladesh, February 2013–February 2015

WDDS, woman’s dietary diversity score; HFIAS, Household Food Insecurity Access Scale.
A Kruskal–Wallis test determined that the median HFIAS score varied as a function of season (H(5)=11·68, P=0·039). Mann–Whitney U tests revealed that the median HFIAS score was significantly higher in spring than in the monsoon season (U=649·5, r=−2·73, P=0·006) and late autumn (U=1181, r=−2·61, P=0·009). Further analyses of the HFIAS conditions based on season revealed a significant difference in the proportion of participants who reported having to eat a limited variety of foods due to a lack of resources (χ 2 =12·72, P=0·026). The percentages reported in Table 4 suggest that more participants reported having to eat a limited variety of foods due to a lack of resources in the summer and spring seasons than all other seasons. The proportion of participants who reported having to eat a smaller meal than they felt they needed to because there was not enough food also differed significantly based on season (χ 2 =17·312, P=0·04). Based on the percentages in Table 4, it appears as though participants reported having to eat a smaller meal less often in the monsoon and late autumn seasons than in all other seasons.
Further analysis of the HFIAS domains revealed a significant difference in the proportion of participants who reported having insufficient food intake across the seasons (χ 2 =46·57, P=0·004). From the percentages presented in Table 4, it appears as though participants are most concerned about not getting sufficient food intake in the summer and winter seasons.
Seasonal variations in anthropometry
A Kruskal–Wallis test revealed no significant differences in MUAC based on season (H(5)=8·53, P>0·05; see Table 5 for more detail).
Discussion
In northern Bangladesh, we found that dietary diversity and household food security were sensitive to seasonal variations, a finding which has been observed in similar studies( Reference Savy, Martin-Prével and Traissac 13 , Reference Hillbruner and Egan 14 ). Seasonality was significantly associated with dietary diversity and food security status of pregnant women. A common limitation in the use of the HFIAS and women’s dietary diversity tools is that they capture a ‘snapshot’ of the situation and do not reflect seasonal variances. While a few studies have attempted to address this limitation, studies have traditionally been cross-sectional at two points in time( Reference Savy, Martin-Prével and Traissac 13 , Reference Hillbruner and Egan 14 ). Our study is unique as it recruited women over a 24-month period, taking into consideration seasonal variances across the year. A 2-year period was necessary to reach the required sample size to maximise the chance of finding a significant result. Contrary to findings reported elsewhere, we did not identify significant differences in maternal nutritional status based on season( Reference Hillbruner and Egan 14 ).
Women had higher dietary diversity in autumn and winter, corresponding with the first month of monga and the month between the main monga and lesser monga, respectively. The higher dietary diversity in autumn corresponded with a higher consumption of dark green leafy vegetables, vitamin A-rich fruits/vegetables and organ meats; while the higher dietary diversity in winter corresponded with a higher consumption of other fruits/vegetables, organ meats, meat/poultry/fish and milk/milk products. Late autumn and winter corresponded with a higher consumption of Fe-rich foods. Interestingly, these two seasons had the lowest consumption of legumes/nuts/seeds. This may be due to the higher consumption of fresh produce such as fruits, vegetables and meat. During these months, legumes, nuts and seeds may be stored for periods when fresh produce is no longer available or accessible. Women had lower dietary diversity in summer and spring, corresponding with the first month of the lesser monga and the month directly after the lesser monga. This may be due to households depleting food supplies during the main monga and having no reserves for the lesser monga. While the first monga may be considered the ‘main’ monga due to its longer duration, the effects of the lesser monga may be more detrimental on the nutritional status of pregnant women in the household.
We identified that household food insecurity peaked during monga (autumn) and lesser monga (spring). A similar study conducted in northern Bangladesh, but with a different target group, identified that the prevalence of both food insecurity and undernutrition was higher during the monsoon season compared with the dry season (winter)( Reference Hillbruner and Egan 14 ). While our findings may differ, this is likely due to the study methodology; the other study conducted a survey at two points in time only (monsoon and winter) and therefore did not analyse the differences between other seasons. Our findings clearly illustrate fluctuations in dietary diversity and household food security across the seasons. Spring, which corresponds with the lesser monga, had high proportions of participants reporting both low variety of foods and food insecurity. By summer, we continued to see a high proportion of participants reporting low variety of foods, coupled with insufficient food intake, and consumption of smaller sized meals. By autumn, while dietary diversity was higher than in the previous season, household food insecurity remained high. Late autumn, which corresponds to the end of the main monga, appeared to be the most food-secure season where dietary diversity was at its highest and where food insecurity affected the lowest proportion of households for the year.
We did not identify a relationship between seasonality and maternal nutritional status (as measured by MUAC). Recent evidence from a similar study in a neighbouring district of Bangladesh contradicts this( Reference Hillbruner and Egan 14 ). In that study, seasonality was associated with nutritional status. However, the target group in Hillbruner and Egan’s study was children aged 6–72 months( Reference Hillbruner and Egan 14 ) and the target group in the present study was pregnant women. Hence, the conflicting findings may be due to differences in target group. The reason for seasonality not being associated with maternal nutritional status in the present study may be that the negative outcomes of seasonality on dietary diversity and household food security do not last long enough to be associated with maternal nutritional status in the Bangladeshi context. Alternatively, it may be that women are able to mitigate potential associations between seasonal variation and maternal nutritional status by adapting their diet accordingly. The WDDS and HFIAS tools are limited to identifying food groups consumed and classification of household food security status. By using a 24 h dietary recall tool, further analyses may have explored whether seasonal declines in maternal nutritional status were prevented through changes in the quantity of food consumed.
Study limitations
Our study had a number of limitations. First, the month of Ramadan resulted in difficulties finding an ‘average’ day for the dietary diversity and household food insecurity questionnaires. To address Ramadan, participants observing Ramadan were rescheduled to complete the interview process on a ‘normal’ day, even if this meant waiting until after Ramadan. Second, we were unable to control for potential differences in seasonal impacts and differences in women across the 2-year enrolment period due to the small sample size. It is important to note, however, that while Bangladesh is disaster-prone, no natural disasters occurred in the study areas across the study period. Third, both the dietary diversity and household food insecurity questionnaires have a recall period (24 h and 30 d, respectively) which may have resulted in a recall bias. Participants may forget items, and particularly for the dietary diversity questionnaire, the time period may be insufficient to capture the typical food groups consumed by the participant and may capture episodic foods that are not typically consumed. Fourth, respondent bias may also be an issue. In population groups where food assistance or developmental aid assistance is frequent, participants may over-report food insecurity and under-report dietary diversity with the expectation of receiving assistance. Conversely, participants may modify their responses based on social desirability. Lastly, despite our active home visits by the community nutrition volunteers, the proportion of women included early in pregnancy was lower than desired, a limitation experienced by others( Reference Huybregts, Roberfroid and Lanou 26 ).
Conclusion
In conclusion, the present study identified that seasonality was significantly associated with dietary diversity and household food security of pregnant women in Pirganj, Bangladesh. Women’s household food security status was significantly worse during the two mongas and dietary diversity was significantly lower during the lesser monga and the month immediately after the lesser monga. While the highest annual levels of food security and dietary diversity occurred during the season after the main monga, indicating a quick recovery from the main monga, we identified a high proportion of food insecurity during the lesser monga and an even higher proportion of food insecurity in the season immediately after the lesser monga, potentially indicating that households struggled to recover from the lesser monga. While economic and nutritional support is required during monga, continued support is also required for the period between the lesser monga and the main monga. This support could be through behavioural change strategies, food banks and diversification of household food production. Support during this time may lessen the shocks of the two mongas on household food security and maternal dietary diversity.
Our study highlights the importance of measuring WDDS and HFIAS across the year in order to identify seasonal variations. By recognising these seasonal variations, policy makers and programme decision makers can design context-specific interventions to improve the diet of pregnant women while incorporating initiatives to prevent negative seasonal declines in food security and dietary diversity, thus contributing to better development outcomes for the mother and child.
Acknowledgements
Acknowledgements: The authors thank the study participants, local communities and World Vision Bangladesh (non-governmental organisation) for their support and for making this research possible. They particularly thank Francis P. Nath and Nomita Sarker from World Vision for supporting community relationships, logistics, translation and back-translation, data storage and data entry. They also thank the community nutrition volunteers for their invaluable enthusiasm to support the study, especially for training participation, assisting with study recruitment and data collection, and for building strong relationships with and within the communities. Funding: This work was supported by World Vision New Zealand. World Vision New Zealand had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: B.S. oversaw conception and design, with contributions from J.B., A.C. and J.J. B.S. coordinated the collection of data. B.S. and D.L. performed the data analyses and interpretation with assistance from K.W. B.S. drafted the article. J.J., J.B., A.C., K.W. and D.L. revised the article for important intellectual content. Ethics of human subject participation: This project had human research ethical approval from the James Cook University (Australia) Ethics Committee (H4498) and the Bangladesh Medical Research Council (BMRC/NREC/2010-2013/58). This research was registered with the ISRCTN registry (ISRCTN97447076).












