National and international health authorities recommend infants breastfeed to the exclusion of all other foods and fluids for the first 6 months of life and continue breast-feeding as part of a mixed diet well into early childhood(1,2) . This remarkable unanimity recognises breast-feeding as necessary for physiological growth and development and immune protection during the amount of time that the infant is immune incompetent(Reference Brandtzaeg3). Increasingly, it is recognised that the immunological resilience conferred through breast-feeding has lasting effects throughout life. Nevertheless, it is estimated that worldwide less than 40 % of infants under 6 months of age are exclusively breastfed(Reference Victora, Bahl and Barros4). In Australia, although 96 % of mothers initiate breast-feeding, by 3 months only 39 % are still breast-feeding and only 15 % continue to 5 months(5). Powdered infant formula (PIF) is commonly used as a replacement for breast-feeding. The normalisation of its use has seen its change from a last resort intervention for infants who cannot be breastfed or fed with donor milk to a routine infant feeding practice(Reference Berry and Gribble6,Reference Victora, Morris and Barros7) . However, there is an unavoidable risk of intrinsic and extrinsic bacterial contamination with PIF use, in addition to the risks associated with the removal of the protective factors in breast milk that should be taken into consideration when deciding upon infant feeding mode.
Many structural and societal factors contribute to low breast-feeding rates. These include insufficient awareness of breast milk as a complex functional food; the lack of availability of donor milk, including pasteurised donor human milk (PDHM); little information about the comparative risks across all infant feeding modes; a poor appreciation of the intrinsic and extrinsic pathogen risks of PIF use and, no less important, gender discriminatory work and employment practices. Information on pathogen risk and morbidity associated with PIF use and recalls of PIF products due to microbial contamination are not always made available or easily accessible to the public. As a result, it is challenging for mothers to make a fully informed choice between the different infant feeding methods. Our systematic analysis of the scientific literature compares the reported pathogen risks of breast-feeding, PDHM, expressed breast milk (unpasteurised) and PIF use by collating available infant morbidity and mortality data. This review is intended as a comparative overview of the reported microbial risks involved across all the main feeding modes for infants, rather than an in-depth analysis of any particular risk. Human milk fortifiers (derived from sterilised human milk, where processing practices are not fully disclosed(Reference Czank, Simmer and Hartmann8) and bovine milk) were not included in this review.
Factors affecting pathogen risk in infant feeding
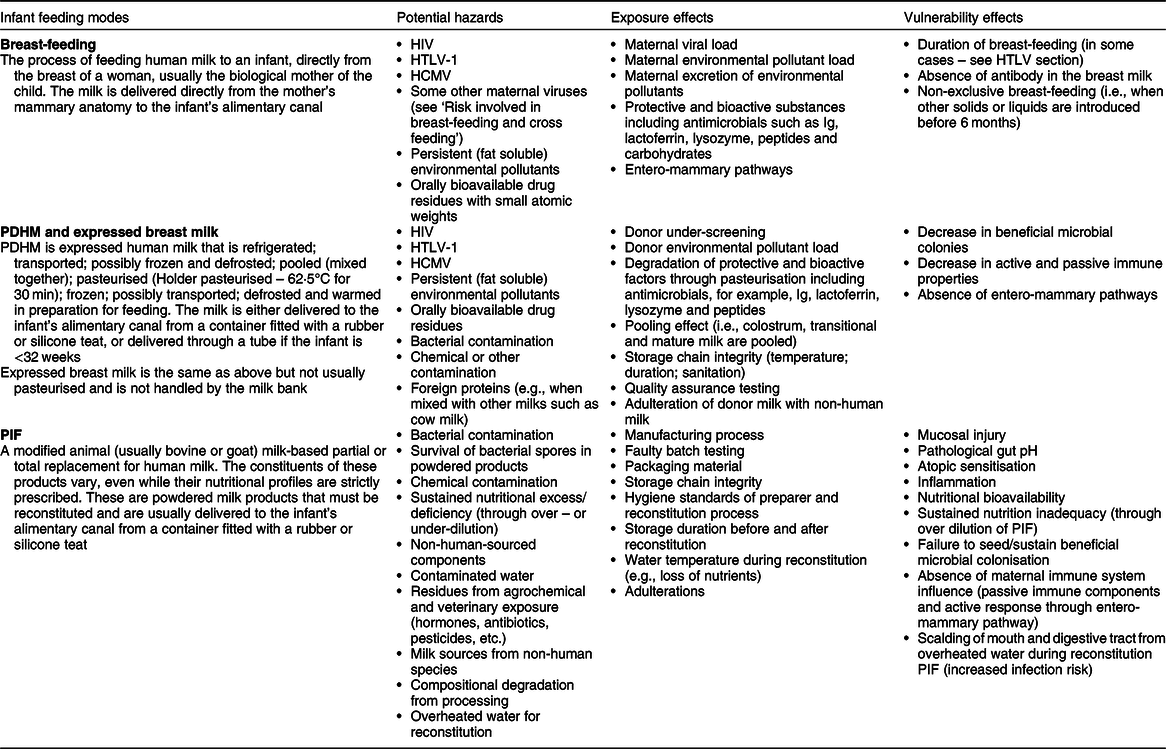
A hazard is any source or cause of potential damage. In regard to PIF use and breast milk feeding, the main hazard is the possible presence of pathogens and/or a reduced resilience against pathogens. Risk can be expressed as a product of hazard, exposure and vulnerability (Fig. 1). A hazard only becomes a risk to a target if it is exposed to that hazard and it is vulnerable. Risk may be mitigated by minimising exposure to the hazard or by reducing the target’s vulnerability. By including the latter, the assessment of risk also considers factors inherent to the milk, the processes required to produce and deliver it (Fig. 2Reference Hartmann, Pang and Keil9–13) and factors inseparable from the feeding mode that mitigate hazards (e.g., antimicrobial properties of human milk) or reduce the infant’s vulnerability (e.g., immunological properties of human milk) (Table 1). Table 1 attempts to list the known hazards and mitigation factors. Non-biological hazards are mentioned for the sake of completeness (Table 1) but are beyond the scope of this review that focuses on infectious pathogens.
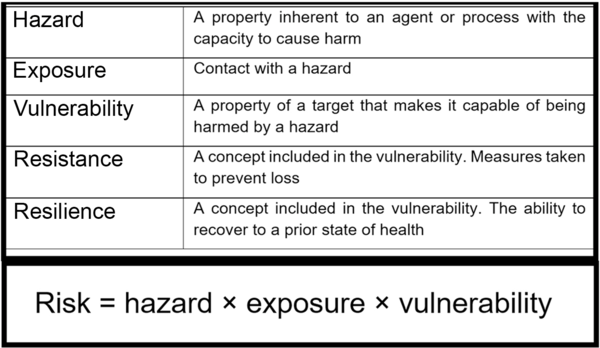
Fig. 1 Risk equation and definitions of terms. Risk is an expression of the probability that exposure to a hazard will cause harm to a vulnerable organism or group. Resistance and resilience are both factors included in the broader definition of vulnerability
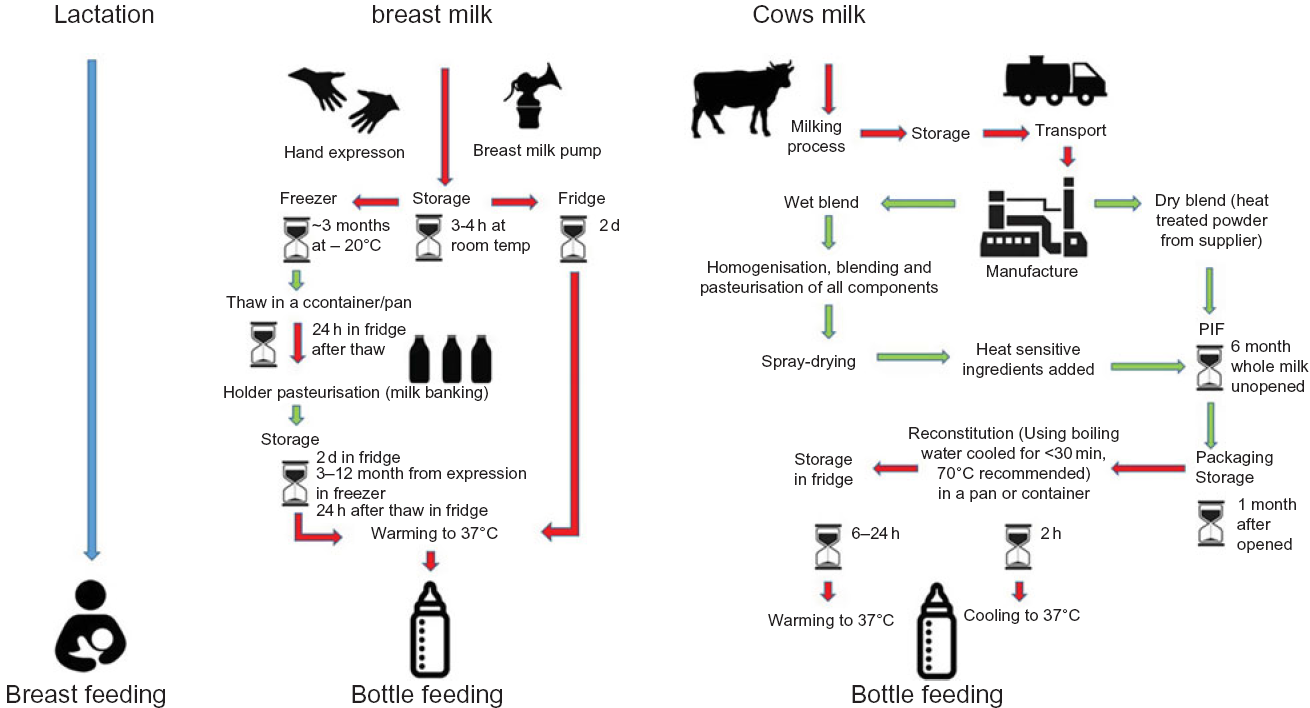
Fig. 2 Flow diagram of infant feeding pathways. While in breast-feeding (blue) there is minimal external entry of pathogens other than from the maternal skin, all other modes of infant feeding have numerous entry points for pathogens from the environment(Reference Hartmann, Pang and Keil9–Reference Gibson, Sahanggamu and Fatmaningrum11). Red indicates those steps in which the food preparation and feeding processes are particularly prone to entry and/or growth of pathogens. Recommended safety steps for handling powdered infant formula (PIF) as set out by WHO(12) (not included in this diagram) should also be taken into consideration. The schematic is based on information taken from FAO 2004(13)
Table 1 Hazards and mitigation factors related to breast-feeding, donated breast milk (PDHM) and PIF
Literature review methodology
Search criteria
The databases Scopus and Web of Science were selected to retrieve peer-reviewed scientific publications. The search through the Web of Science databases comprised the Web of Science Core Collection, BIOSIS previews, CABI: CAB Abstracts®, Chinese Science Citation DatabaseSM, Current Contents Connect, KCI-Korean Journal Database, MEDLINE®, Russian Science Citation Index, SciELO Citation Index and Zoological Record. Initial search terms included (‘HIV’ or ‘Human immunodeficiency virus’) and (‘Human milk’ or ‘breastmilk’ or ‘breast milk’) and ‘Transmission’; (‘HTLV’ or ‘human T-lymphotropic virus’ or ‘human T-cell lymphotropic virus’ or ‘human T-cell leukemia-lymphoma virus’) and (‘Human milk’ or ‘breastmilk’ or ‘breast milk’) and ‘Transmission’; (‘Cronobacter sakazakii’ or ‘Enterobacter sakazakii’ or ‘salmonella’ or ‘salmonellosis’) and (‘PIF’ or ‘powdered infant formula’ or ‘infant formula’ or ‘milk substitute’ or ‘replacement formula’); and (‘donated milk’ and pathogen or morbidity).
For each topic, papers from all sources were integrated with duplicates removed and the final list curated by the lead author removing irrelevant publications based on title, abstract and finally full paper text in a three-step process (online Supplemental Fig. 1). After the initial search was completed, the reference list was expanded by including the term ‘donor milk’ as a more common term. Additional literature was extracted from the reference lists of publications retrieved through the search terms of the initial search.
Inclusion and exclusion criteria
Publications were only included in the review if they provided quantitative data of morbidity or mortality and specifically traced the source of the pathogen. Publications on pathogens known to be associated with PIF but without an examination or comment on the association with PIF in the reported specific case were excluded. Likewise, publications that did not specify the feeding mode were excluded as were publications on potential disease transmission through breast-feeding if the infant feeding mode was insufficiently specified, for example, breastfed exclusively, or mixed.
Pathogen risks associated with breast-feeding
Exclusive breast-feeding is not normally associated with any risk, even in the case of maternal infection (online Supplemental Table 1). The main hazards of breast-feeding are exposure to the maternal viral pathogens HIV and human T-cell lymphotropic virus-1 (HTLV-1). However, for both of these pathogens the actual transmission risk is not fully understood(Reference Gribble and Hausman14). Other viruses or pathogens may be present in breast milk but are rarely associated with subsequent infection in the infant (online Supplemental Table 1). This may be due to the concurrent presence of specific antibodies and antimicrobial factors in the breast milk(Reference Workowski and Bolan15). Breast-feeding has been described as a form of natural, broad-spectrum immunisation as it can, apart from passive immune transfer, elicit antigen-specific immune responses in the infant, boosting immunity to infectious diseases long-term(Reference Verhasselt16). Consequently, the WHO recommends exclusive breast-feeding, except in the presence of a very limited number of pathogens as listed in online Supplemental Table 1.
As an example, human cytomegalovirus may also be transmitted via breast-feeding and infants can test positive. However, the vast majority of infants with cytomegalovirus remain asymptomatic. Symptomatic cytomegalovirus infection is usually only a concern for preterm infants (<32 weeks), and the approach to management remains controversial. One group has suggested that preterm infants receive pasteurised mothers milk(Reference Picaud, Buffin and Gremmo-Feger17), while others note that post-partum cytomegalovirus produces negligible severe outcomes and that the benefits of pasteurisation may not be justified by the risks or cost(Reference Jim, Chiu and Ho18).
Hazard: HIV in breast milk
It is increasingly recognised that when infants are exclusively breastfed by an HIV positive mother, the infant usually remains HIV negative. However, an accurate transmission risk is difficult to obtain as studies designed to investigate this risk often either do not control for mixed feeding (combinations of breast-feeding and PIF and/or solid foods) or allow for variable amounts of mixed feeding even in the designated exclusive breast-feeding groups, which is a potential compounding hazard in its own right(Reference Coutsoudis, Pillay and Spooner19–Reference Nlend, Motaze and Sandie22).
HIV transmission through breast-feeding is mainly seen in endemic areas, such as sub-Saharan Africa(Reference Kalu, Reynolds and Petra23) with its high background prevalence of infant mortality and maternal under-nutrition.
In areas where HIV is endemic, the risk of HIV transmission through breast-feeding must be compared with the risks associated with PIF feeding, which include malnutrition, diarrhoea or pneumonia(24). This is illustrated well by an outbreak in Botswana in 2006. In an area where PIF use was high due to endemic HIV infection, heavy rains caused an outbreak of diarrhoea that disproportionally affected PIF-fed infants, regardless of HIV status. Of the 153 children hospitalised with diarrhoea, 88 % were not breastfed; of the thirty-three infants who died, twenty-seven were not breastfed. Non-breastfed infants were often undersupplied with PIF and therefore malnourished, an additional contextual determinant of the overall health outcome(Reference Creek, Kim and Lu25). Where PIF feeding cannot be done safely, breast-feeding is critical to infant survival, regardless of HIV status(Reference Creek, Kim and Lu25,Reference Rollins, Ndirangu and Bland26) . Where HIV is endemic, lifelong maternal antiretroviral therapy is recommended for HIV positive mothers, and exclusive breast-feeding to 6 months and continued breast-feeding to at least 12 months is recommended as these measures are associated with a lower risk of infant morbidity and mortality then mixed or PIF feeding(24,27) . Despite this recommendation, PIF use is common in heavily HIV-affected areas.
A meta-analysis was performed in all publications specifically reporting transmission rates for mother-to-child transmission rate of HIV. Across those sixteen studies, estimates of transmission were 6·2 % for exclusively breastfed infants, 15·3 % for mixed breastfed/PIF-fed infants and 12·7 % for exclusively PIF-fed infants (Table 2). In some reports, the definition of exclusive breast-feeding allowed for some solids or PIF to be used for short periods of time(Reference Coovadia, Rollins and Bland21,Reference Magoni, Bassani and Okong28,Reference Natchu, Liu and Duggan34) , which confounds interpretation. Additionally, while most reports had a study duration starting at 6 weeks of life, others started the study duration from birth, which may not clearly delineate between congenital and postnatal transmission (Table 2). The presence of HIV transmission in exclusively PIF-fed infants may indicate unsuccessful HIV testing for congenital transmission, or undisclosed occasional breast-feeding, which was noted in at least one of the studies, but is likely common(Reference Magoni, Bassani and Okong28).
Table 2 Vertical postnatal HIV transmission data (2000–2018) separated by the infant feeding modes (breastfed, mixed-method and PIF-fed infants)*
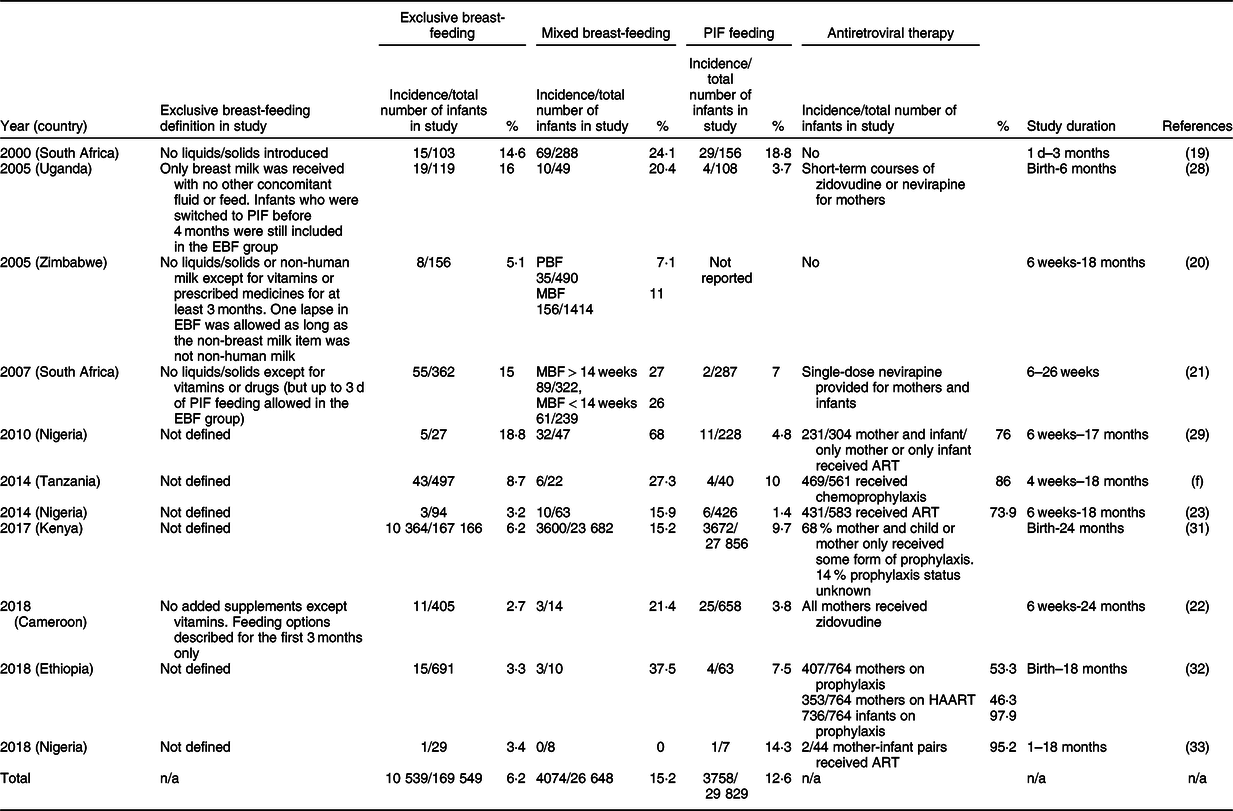
PIF, powdered infant formula; MBF, mixed breast-feeding; PBF, predominantly breast-feeding; EBF, exclusively breast-feeding; EFF, exclusive formula feeding; ART, antiretroviral therapy; HAART, highly active antiretroviral therapy.
* This review records the incidence in each study of vertical HIV transmission, the total number of infants in the study and the percentage of the transmission rate. It should be noted that some of the included studies began their study from birth, which may not differentiate accurately between congenital and postnatal transmission.
Exposure effects
Exposure to antiretroviral therapy for both the infant and mother during pregnancy and breast-feeding has been shown to reduce the risk of transmission(Reference Flynn, Taha and Cababasay35), as this reduces the viral load. Mixed feeding is also associated with higher rates of HIV transmission (Table 2Reference Coutsoudis, Pillay and Spooner19–Reference Kalu, Reynolds and Petra23,Reference Magoni, Bassani and Okong28–Reference Afolabi, Bakarey and Kolawole33 ).
Vulnerability effects
Breast milk contains antimicrobial substances that support host defence(Reference Lönnerdal36) and protects against infections through entero-mammary pathways(Reference Rodríguez37). Secretory IgA, an anti-inflammatory antibody, is the first line of defence against pathogens gaining entry through the mucosal lining of the gut(Reference Brandtzaeg3). During the first few months of life where the infant’s secretory immune system (production of antibodies) is deficient, and the mucosal gut barrier is not established adequately(Reference Brandtzaeg38), the infant is reliant on the supply of antibodies from breast milk(Reference Brandtzaeg, Nilssen and Rognum39). Therefore, the absence of breast milk can disrupt the mucosal barrier. Breast milk colonises the gut with Bifidobacteria for which breast milk oligosaccharides provide the favourable growth conditions(Reference Cacho and Lawrence40). In contrast, infants fed with reconstituted PIF or prematurely with solid foods show an increased gut pH and altered microbiota(Reference Lee, Lim and Kim41,Reference Walker42) . This dysbiosis, along with increased disruption of the mucosal barrier(Reference Brandtzaeg3) and increased gut permeability, may result in translocation of luminal contents(Reference Mai, Torrazza and Ukhanova43) and make the infant more vulnerable to pathogens(Reference Bezirtzoglou, Tsiotsias and Welling44). Altered gut mucosal lining and permeability may be particularly important in HIV transmission(Reference Kutty45), which could explain in part the increased HIV transmission under mixed feeding conditions.
Hazard: HTLV-1 in breast milk
HTLV-1 is a retrovirus that causes life-long infection. It is transmitted sexually or through an iatrogenic route in adults or through breast milk. While infants remain largely asymptomatic, about 10 % of adult HTLV-1 carriers develop adult T-cell leukaemia(Reference Rosadas and Taylor46). Adult T-cell leukaemia has a high mortality rate and is linked to mother-to-child transmission of HTLV-1 (rather than adult infection of HTLV-1)(Reference Rosadas and Taylor46). At least 5–10 million people are living with HTLV-1. Areas of high prevalence are found in parts of Africa, Peru, Iran, Brazil, Japan(Reference Rosadas and Taylor46) and central Australia(Reference Einsiedel, Pham and Woodman47), although the true prevalence of HTLV-1 is unknown(Reference Zihlmann, Mazzaia and Alvarenga48). The exact portion of infection globally that comes from mother-to-child transmission is not known and varies regionally(Reference Rosadas and Taylor46). Our meta-analysis included all publications reporting morbidity and mortality thought to be due to mother-to-child transmission of HTLV-1. Across eleven retrieved studies, the overall transmission rates were 5·9, 14·1, 23·7 and 4·3 % for infants breastfed for <6, <12, >12 months and PIF-fed infants, respectively (Table 3Reference Nakano, Ando and Saito49–Reference Paiva, Assone and Haziot59). Two reports were excluded in these overall rates due to incidence rates being reported without sufficient information about the duration of breast-feeding(Reference Nakano, Ando and Saito49,Reference Ando, Nakano and Saito50) .
Table 3 Vertical postnatal HTLV-1 transmission data (1984–2018) separated by the infant feeding modes and duration of breast-feeding (breastfed for <6 months, >6 months, mixed-method and PIF-fed infants)
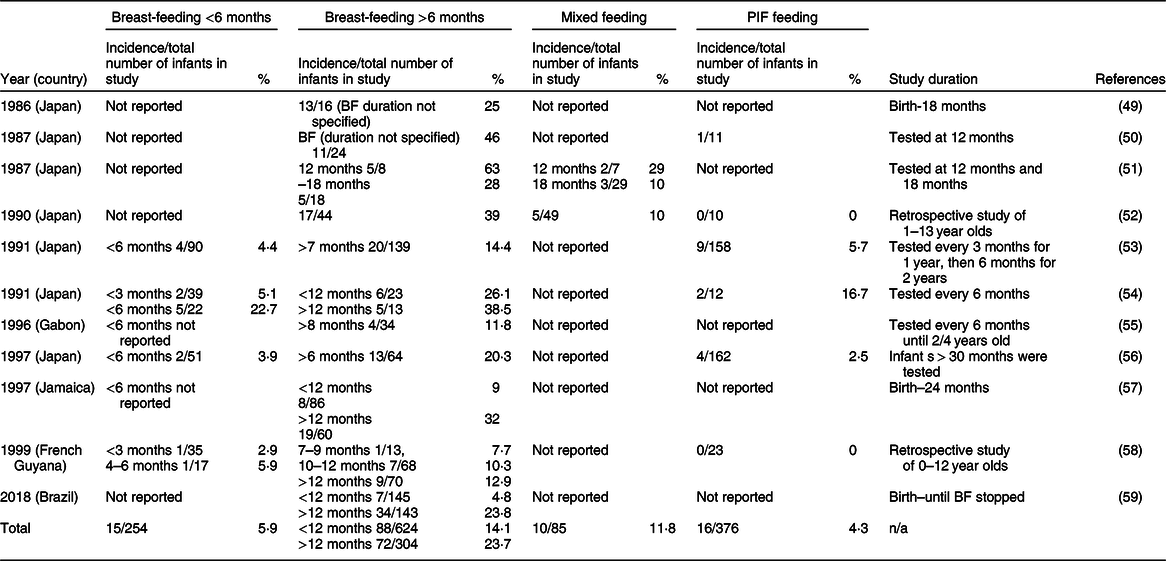
* This review records the incidence in each study of vertical HTLV-1 transmission, the total number of infants in the study and the percentage of the transmission rate (incidence/total number of infants in study (%)). It should be noted that no definitions were found for feeding modes; therefore, it is not known if breast-feeding was strictly exclusive or if other solids and/or liquids were allowed. Therefore, all HTLV-1 reports must be viewed as potentially having some PIF exposure.
Exposure effects
The mechanism of transmission during breast-feeding is not well understood(Reference Rosadas and Taylor46). Mechanisms for both transmission through disrupted and intact epithelium have been reported(Reference Pique and Jones60). The transmission rate is lower when the duration of breast-feeding is shorter than 6–12 months(Reference Rosadas and Taylor46,Reference Paiva, Assone and Haziot59) . Since these studies do not specify whether ‘breast-feeding’ refers to exclusive or mixed modes, it is not known whether and how much a ‘mixed feeding mode’ might increase the risk of HTLV-1 transmission. In some regions of high prevalence, breast-feeding mothers lacking screening and education are unaware of their HTLV-1 status and may transmit the virus unknowingly(Reference Einsiedel, Pham and Woodman47). Increased pro-viral load(Reference Paiva, Assone and Haziot59,Reference Li, Biggar and Miley61) increases transmission, irrespective of breast-feeding duration(Reference Li, Biggar and Miley61). Additional independent variables for increased transmission also include more than one child with HTLV-1 and mothers who are >26 years old(Reference Paiva, Assone and Haziot59). No clinical trials have been undertaken to test the efficacy of antiretroviral therapy for HTLV-1 positive mothers, which may help to reduce viral loads and transmission(Reference Rosadas and Taylor46).
Vulnerability effects
The major factor in the infants’ vulnerability to HTLV-1 seems to be the protective presence of the maternal HTLV-1 antibody in the milk for the first 6–12 months(Reference Rosadas and Taylor46,Reference Takahashi, Takezaki and Oki53,Reference Hirata, Hayashi and Noguchi54) . Although there has been a strong focus on the effects of mixed feeding on HIV transmission, no such studies have been conducted for HTLV-1. The integrity of the infant gut may be a factor in transmission of HTLV-1. However, no reports were found where mixed feeding was included as a variable.
Pasteurised donor human milk and expressed breast milk
In this review, PDHM refers to donor milk that is handled by staff in milk banks following prescribed processes, that is, low temperature long time heat treated (Holder pasteurisation). Expressed milk refers to milk that is expressed for either the mother’s own child or unofficially shared with other mothers and handled in an unregulated manner and is not pasteurised(Reference Picaud, Buffin and Gremmo-Feger17). Holder pasteurisation is generally recognised as a necessary step for donor milk to ensure compliance with microbiological safety standards(Reference Moro, Billeaud and Rachel62). The current pasteurisation standards are based on the destruction in cows’ milk of Coxiella burnetii, the cause of Q fever(Reference Enright, Sadler and Thomas63). The temperature and time established for the pathogen control C. burnetii, however, significantly reduces the protein, lipid and lactose content compared with unpasteurised breast milk(Reference Piemontese, Mallardi and Liotto64), as well as reducing protein function, antibody content, antioxidants, vitamin C, B12, growth factors(Reference Wesolowska, Sinkiewicz-Darol and Barbarska65) and the natural microbiota present in milk(Reference Fernandez, Ruiz and Jara66). For this reason, milk banks in Norway rely on donor screening rather than pasteurisation, and the provision of unpasteurised human milk has not been associated with any increase in infant morbidity(Reference Grøvslien and Grønn67). Limited supply of donated milk in milk banks usually limits PDHM to premature infants in hospitals(Reference Hartmann, Pang and Keil9). Despite the prevalence of bacterial contamination present in expressed breast milk and PDHM before and after pasteurisation (up to 25 % being discarded as a result)(Reference Mullié, Obin and Outurquin68,Reference Boo, Nordiah and Alfizah69) clinically significant infection events associated with either of these feeding modes are rare (online Supplemental Table 2).
Hazards: transmissible maternal viral pathogens and opportunistic pathogens
The main hazards include maternal pathogens present in the breast milk and contamination with a pathogen after expression. In PDHM, the main isolates before and after pasteurisation are Staphylococci and the spore-forming, heat resistant Bacillus cereus, respectively(Reference Mullié, Obin and Outurquin68,Reference Lewin, Delage and Bernier70) . The presence of Staphylococcus aureus pre-pasteurisation may produce heat resistant enterotoxins, and these may still be present after pasteurisation(Reference Almutawif, Hartmann and Lloyd71). The presence of heat stable enterotoxins or bacteria (Bacillus cereus) emphasises the importance of post-pasteurisation checks(Reference Lewin, Delage and Bernier70).
Exposure effects
For PDHM, donor screening is used to reduce exposure risk, although there are no global regulatory frameworks for milk banking in place(72). Therefore, screening and processing practices differ and are based on screening requirements for blood and tissue donation(Reference Hartmann, Pang and Keil9) and processing techniques used in the dairy industry as a basis(Reference Holsinger, Rajkowski and Stabel73). This results in a variable product and therefore has variable risks and benefits(Reference Hartmann74). The entry points for bacterial contamination for both PDHM and expressed milk include unhygienic expression (improperly washed hands or breast pump), unhygienic storage (incorrect temperature or storage duration), non-compliant pasteurisation, improperly sterilised feeding equipment (parenteral tube, enteral tube, bottle or cup) and through contamination during fortification of donated milk for premature infants(Reference Boo, Nordiah and Alfizah69,Reference Becker, Smith and Cooney75) .
The majority of cases of infant morbidity collected for this review were associated with unpasteurised milk (not sourced through milk banks) and expressed with both manual and electric breast pumps (online Supplemental Table 2). However, if expressed milk is handled properly, there is little evidence for any significant risk to infants, in contrast to PIF use, which is associated with a direct intrinsic and extrinsic pathogen risk as well as substantial intolerance reactions. Health authorities routinely advise against the use of expressed milk, notably if shared informally between mothers, due to the lack of regulatory control and lack of pasteurisation. However, with appropriate hygiene, there is no strong evidence that under share arrangements without monetary incentives, the quality and microbial contamination is systematically different between supervised, formally or informally shared milk(Reference Perrin, Fogleman and Davis76). It thus appears that it is specifically the education in appropriate handling and storage of expressed milk, rather than necessarily pasteurising milk (with its potential lowering of quality), that yields the greatest health benefits and that sharing as such does not increase the risk above that of bottle feeding with own milk.
Vulnerability effects
Thermal pasteurisation, as generally practiced, significantly reduces the milk’s nutritional and bioactive components(Reference Peila, Moro and Bertino77), as well as most beneficial bacteria naturally present(Reference Fernandez, Ruiz and Jara66). Despite a partial loss of function, PDHM is still associated with better clinical outcomes than reconstituted PIF in the protection against opportunistic pathogens. Studies have found that feeding preterm infants with PDHM (rather than reconstituted PIF) significantly reduces the incidence of necrotising enterocolitis(Reference Sullivan, Schanler and Kim78–Reference Quigley, Henderson and Anthony80), late onset sepsis(Reference Hair, Peluso and Hawthorne81) and bronchopulmonary dysplasia(Reference Villamor-Martínez, Pierro and Cavallaro82). However, not all studies have found a difference in necrotizing enterocolitis rates between PDHM and reconstituted PIF-fed infants(Reference Schanler, Lau and Hurst83). There are a number of reasons why PDHM might not show the expected beneficial effects. Over-treatment of PDHM may aggravate the loss of biological functions still present in expressed raw milk. Also, prolonged storage of expressed milk in a fridge (>72 h)(Reference Silvestre, Löpez and March84) or freezer (>3 months)(Reference Takci, Gulmez and Yigit85) reduces bactericidal activity. Increased vulnerability might also result from the absence, as with any form of bottle feeding, of the entero-mammary transfer of beneficial microbiota through breast-feeding(Reference Rodríguez37) and the missing adaptive responses of the maternal immune system in the wake of breast-feeding(Reference Brandtzaeg3).
Risks associated with powdered infant formula
Pathogen risk is inherent to PIF use as the manufacturing process itself introduces direct, albeit small, pathogen risks that cannot be completely eliminated(Reference Farmer86). Contamination risks are associated with both dry and wet blending methods(Reference Kent, Fitzgerald and Hill10). Although dry blending tends to have a lower contamination risk due to the dry environment(Reference Kent, Fitzgerald and Hill10), batch testing of dried milk samples does not always detect contamination due to uneven distribution(Reference Zink87). Recall of PIF due to either later-detected contamination or increased infant morbidity is a regular occurrence (online Supplemental Table 4).
In addition to the risks associated with manufacturing, PIF reconstitution also yields sources of contamination. Potential extrinsic pathogen entry points include reconstitution water, utensils, bottles and teats, the preparers’ hygiene practices or the storage duration/conditions. Contamination of reconstituted PIF with faecal bacteria and inadequate sterilisation of the bottle and teat is common(Reference Gibson, Sahanggamu and Fatmaningrum11). Some feeding bottles designed with a silicone straw inside are particularly difficult to clean according to WHO recommendations, which recommend scrubbing all internal and external surfaces with hot soapy water(12) and are thus prone to biofilm formation.
PIF are mostly based on modified bovine milk with added vegetable oils, vitamins, minerals and processing agents. Due to the heat processing of bovine milk, PIF contains a range of pro-inflammatory advanced glycation end-products(Reference Birlouez-Aragon, Pischetsrieder and Leclère88).
Use of reconstituted PIF is associated with an increased risk of gut inflammation, alteration of the gut microbiome and disruption of the epithelium barrier(Reference Walker42), all of which may increase the risk of pathogen entry into the bloodstream(Reference Claud and Walker89), thus increasing the infant’s vulnerability. Some infant formulas are supplemented with probiotics(Reference Chassard, de Wouters and Lacroix90), and some with a synthetic human oligosaccharide(Reference Coulet, Phothirath and Allais91). It should, however, be noted that there are over 200 interactive human oligosaccharides present in breast milk(Reference German, Freeman and Lebrilla92) and that the health benefits of probiotics in infant formula are reported in comparison with conventional infant formula and not benchmarked against donor milk. Thus, the reported effects are primarily a mitigation of the detrimental effects of un-supplemented infant formula.
There are a wide range of pathogens that have been linked to contamination of PIF (online Supplemental Table 3), including bacterial spores(Reference Haughton, Garvey and Rowan93) and bacteria that resist desiccation(Reference Juma, Manning and Forsythe94). Salmonella and Cronobacter are the two most critical genera based on prevalence and health impact reported in the literature. While both pathogens are regularly detected in batches of PIF (online Supplemental Table 4), morbidity is mostly associated with Salmonella enterica. However, there may also be an underappreciated link between contamination of PIF with spores and infant morbidity(Reference Barash, Hsia and Arnon95).
Hazard: Salmonella enterica
Salmonella enterica are non-spore forming gram-negative Enterobacteriaceae, with a growth range of 5·2–46·2°C, are able to survive desiccation and, in certain circumstances, are able to survive freezing and some heat treatments(96). Although there is no dose-specific model for infants <9 months, existing data suggest a very low infective dose (possibly as low as <10 CFU)(97). Salmonella contamination of milk substitutes is associated with non-typhoidal S. enterica (Reference Kent, Fitzgerald and Hill10). It affects both term and premature infants and, based on reports in the literature, is generally associated with large outbreaks (although the true incidence rates are not known). The natural reservoirs of Salmonella are foods of animal origin including raw milk, human contact with animals, the faecal-oral route or exposure to other infants with diarrhoea(97). Non-typhoidal Salmonella infection can lead to gastroenteritis, diarrhoea, fever and vomiting, or more serious and potentially fatal conditions in premature infants including bacteremia, meningitis or severe levels of dehydration(Reference Jones, Ingram and Fullerton98). This is particularly pertinent in resource-poor settings where medical attention and clean water are not readily available. While the detection of Salmonella is by far the most commonly identified reason for morbidity associated with contaminated PIF, no reports could be found for the overall morbidity or mortality rates for Salmonella associated with PIF contamination(Reference Cummings, Sorvillo and Kuo99). While reports of Salmonella outbreaks associated with PIF exist for resource-rich settings, no such reports could be found for resource-poor settings, despite high levels of Salmonella infection in infants in these regions(Reference Feasey, Archer and Heyderman100,Reference Mohan, Munusamy and Tan101) . It remains a common cause of neonatal sepsis in Africa and is thought to be associated with unhygienic conditions in the home environment(Reference Gordon102). However, the association with PIF is unknown and calls for urgent research into the prevalence and incidence of Salmonella infections in PIF-fed infants. Our meta-analysis included all reports commenting on either morbidity or mortality traceable to a Salmonella contamination of PIF. Based on the reports found, there were 407 cases of Salmonella infant infection from twelve unrelated outbreaks that could be attributed to contaminated PIF in the past 50 years. From the compiled data, one was fatal (0·2 %) (Table 4Reference Collins, Treger and Goldsby103–114). These morbidity and mortality rates for Salmonella infection from contaminated PIF may well represent a substantial under-reporting. Non-typhoidal Salmonella is a common foodborne pathogen worldwide and is the most frequently isolated enteric bacterial pathogen in children under 5 in the USA(Reference Scallan, Mahon and Hoekstra115). The source of the infection is often difficult to find and is frequently not even investigated.
Table 4 Salmonella outbreaks attributed to contamination of PIF
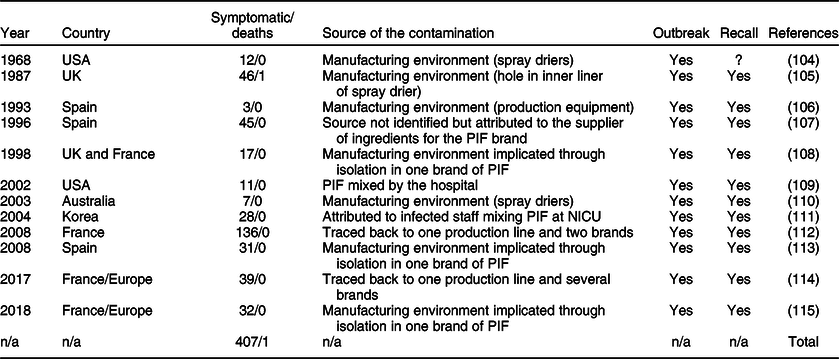
NICU, neonatal intensive care unit.
Exposure effects
All outbreaks of Salmonella infections caused by contamination of PIF were either traced back to manufacturing equipment or the raw milk product(Reference Collins, Treger and Goldsby103,Reference Louie, Paccagnella and Osei105,Reference Usera106,Reference Forsyth, Bennett and Hogben109,Reference Rowe, Rocourt and Shiferaw116,Reference Jones, de la Gandara and Herrera-Leon117) or isolated from unopened tins of PIF, implicating the manufacturing environment. The only exception was an outbreak in a neonatal intensive care unit in 2001, which was attributed to infected staff(Reference Bornemann, Zerr and Heath108). Small amounts of Salmonella may grow to an infectious dose through unhygienic reconstitution, storage and feeding conditions(Reference Rowe, Hutchinson and Gilbert104).
Vulnerability effects
As previously noted, PIF feeding can increase vulnerability to bacterial pathogens due to gut microbiome dysbiosis(Reference Lee, Lim and Kim41,Reference Bezirtzoglou, Tsiotsias and Welling44) or lack of passive maternal immune protection(Reference Brandtzaeg3).
Hazard: Cronobacter sakazakii
Cronobacter are an opportunistic gram-negative Enterobacteriaceae with a growth range from 5 to 47°C, are able to survive desiccated conditions for up to 2 years and, once ingested, can pass through the blood-brain barrier(Reference Holý and Forsythe118). Cronobacter sakazakii (or Enterobacter sakazakii prior to 2007)(Reference Holý and Forsythe118) is a ubiquitous pathogen that rarely causes disease except in vulnerable populations, including infants and the elderly(Reference Holý and Forsythe118). Cronobacter infection is almost exclusively associated with premature infants, or term infants within the first 2 months of life(Reference Jason119), and has not been associated with large outbreaks. Although the natural reservoir is not known, Cronobacter infection in infants is usually associated with PIF use(Reference Jason119). Morbidities associated with Cronobacter include meningitis, necrotising enterocolitis, bacteremia and sepsis, all of which can lead to fatality or permanent sequelae(Reference Holý and Forsythe118). Mortality rates are estimated to be between 33 and 80 %(Reference Lai120). The infectious dose for neonates is estimated to be between 1 × 103 CFU and 1 × 104 CFU in a single feed(97) which is most likely subject to the degree of vulnerability of the infant.
Estimates of invasive Cronobacter infection incidence in infants in the USA from 2003 to 2009 were 0·66 per 100 000(Reference Patrick, Mahon and Greene121). This is likely underestimated as Cronobacter is not notifiable in all states of the USA or in most countries, even in resource-rich settings(97). Data for the incidence of Cronobacter infection outside of resource-rich settings could not be found.
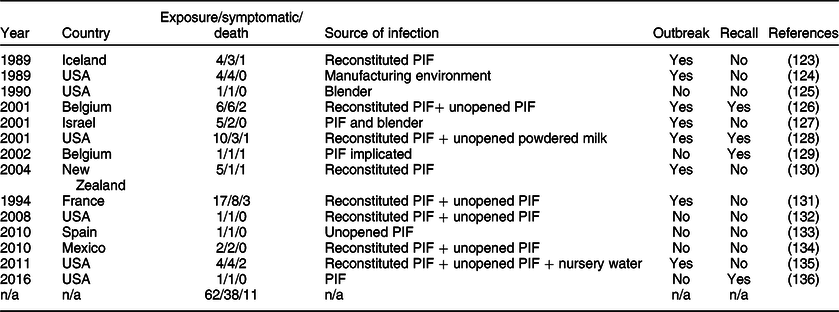
A meta-analysis of all the reports containing comments on Cronobacter-associated morbidity or mortality identified fourteen reports of Cronobacter outbreaks or cases in neonates where the source was investigated and attributed to PIF use. Of the sixty-two cases in these reports, thirty-eight cases were invasive (61·2 %) and eleven were fatal (17·7 %); eight of fourteen studies were considered to be outbreaks (Table 5Reference Biering, Karlsson and Clark122–135).
Table 5 Cases of Cronobacter in neonates attributed to contamination of PIF. Note that there are records of other Cronobacter infections in neonates; however, all cases where the source of contamination was not investigated were excluded
Exposure effects
Cronobacter contamination has been traced back to unopened tins of PIF(Reference Van Acker, De Smet and Muyldermans125,Reference Caubilla-Barron, Hurrell and Townsend130,Reference Flores, Medrano and Sánchez136) , powdered milk(Reference Himelright, Harris and Lorch127) and once back to the manufacturing environment(Reference Simmons, Gelfand and Haas123). Several other studies on the incidence of bacteria in PIF found that it is common for there to be small amounts of bacterial contamination from the manufacturing environment.(Reference Márquez, Hernández and Echevarría137) Incidence of morbidity and mortality from Cronobacter infection is often associated with unhygienic practices during reconstitution and storage, including leaving reconstituted PIF for too long before consuming(Reference Biering, Karlsson and Clark122), improper cleaning of blenders(Reference Noriega, Kotloff and Martin124,Reference Bar-Oz, Preminger and Peleg126) and reconstituting with water that is insufficiently heated(Reference Flores, Medrano and Sánchez136). A recent study demonstrated that the bacterial load on the hands of the caregiver may also pose a risk through the hand-spoon-PIF route and should also be considered in any risk assessment(Reference Cho, Hwang and Kim138). It is unclear whether the major source of the Cronobacter is the manufacturing environment or lies in the environment during reconstitution. Whatever the origin, if Cronobacter is present, unhygienic reconstitution conditions allow for growth to reach an infective dose(Reference Lu and Matthews139).
Vulnerability effects
Cronobacter infection is highly specific to preterm and <2 months old infants, suggesting that the development of the immune system, presence of maternal immune components and a healthy microbiome in the infant gut (which may outcompete ingested Cronobacter)(Reference Holý and Forsythe118) are highly relevant to the degree of vulnerability of the infant. The absence of breast milk is associated with increased gut permeability and dysbiosis and therefore increased risk of pathogen translocation through the gut lining.
Outcomes and conclusions
This review collated cases of infant morbidity or mortality where the source of infection could be traced to breast-feeding, donor breast milk or PIF use, in an effort to better compare the risks associated with infant feeding modes. However, large gaps in the literature hampered this task. It is likely that issues such as PIF contamination remain significantly under-reported, making it challenging to gauge the risks associated with major hazards such as Salmonella. While the published peer-reviewed literature may give an incomplete picture of the different risks, important observations can still be made about each feeding mode.
The primary perceived risk associated with breast-feeding is maternal virus transmission, the greatest concern being HIV. However, when the mother is provided with antiretroviral therapy and exclusive breast-feeding is practiced, the transmission rate is very low. A mixed feeding mode appears to increase the transmission rate, which may be due to increased exposure of infants to the virus as a consequence of the damaging effects of PIF on the infant digestive system(Reference Smith and Kuhn140). Moreover, mortality resulting from withholding breast milk is higher than the infant mortality rate due to HIV infection(Reference Creek, Kim and Lu25,Reference Rollins, Ndirangu and Bland26) . The design of future studies needs to pay greater attention to the definitions of exclusive (from birth) breast-feeding and the impact of early supplementation and later mixed feeding modes, which in many reports are frequently observed as confounding variables.
Proper management of breast-feeding for HTLV-1 positive mothers is complex. Although antenatal screening and strict withholding of breast-feeding for all HTLV-1 positive mothers have greatly lowered vertical transmission rates in Japan(Reference Kashiwagi, Furusyo and Nakashima141), this also carries its own risks and consequences associated with PIF use, which would be magnified in resource-poor settings. The risk of HTLV-1 transmission must be weighed against the risks associated with PIF use and the loss of the benefits associated with breast-feeding. Monitoring of the pro-viral load in the milk may be a feasible option to assess transmission risk. Additionally, clinical trials should be conducted in order to assess the efficacy of antiretroviral therapy in lowering transmission risk. Finally, further research is needed to evaluate the effect of mixed feeding on transmission risk. An alternative practicable approach might also be the systematic provision of PHDM or pasteurisation of the mothers’ own milk until the endemic presence of HTLV-1 in vulnerable communities has been removed.
Pathogen risk cannot be eliminated from the PIF manufacturing process. Salmonella is the pathogen of most concern as it has been repeatedly associated with large outbreaks traced back to PIF contamination. Recent assessments have concluded that final quality control measures may not be enough to ensure microbial safety(142). The reconstitution from powder additionally introduces extrinsic pathogen risks from contaminated water(Reference Marino143), contaminated bottles, teats or utensils and improper storage conditions which allows for bacterial growth. While all of these risks may be adequately mitigated by strict adherence to hygienic practices (using 70°C water with sterilised equipment), the formula-fed infant will still be left more vulnerable due to the lack of protective and developmental components in PIF.
A full assessment of the true risk associated with infant feeding modes also needs to encompass indirect hazard mechanisms. This includes any delay or disturbance in gut maturation through PIF-induced gut dysbiosis(Reference Walker42) or through lack of the protective and developmental factors in breast milk(Reference Brandtzaeg3), the involvement of (possibly sub-clinical) inflammation(Reference Walker42,Reference Moodley-Govender, Mulol and Stauber144) and other immune responses. Presently, there are insufficient data on the frequency of hospitalised infant morbidity linked to PIF use, and non-hospitalised infants with infections are not captured systematically. Therefore, the true incidence of PIF with primary or secondary contamination leading to illness may be greatly underestimated. Better documentation and quantification of the impact of diarrheal disease and pneumonia in infants fed with reconstituted PIF is needed, and clinical trials of PIF need to be benchmarked against health benefits of breast-feeding or donor milk.
Ultimately, a wider availability of PDHM could be a highly valuable public health measure if it decreases reliance on PIF. The available published data indicate that the risk of infant infection through PDHM or expressed milk is low. Indeed, most cases of bacterial contamination can be attributed to unhygienic handling of donor milk, including handling errors during the pasteurisation process. Proper decontamination of collection equipment and user hygiene are, therefore, key preventative strategies. While Holder pasteurisation can limit opportunistic pathogens, challenges remain around heat-stable spores(Reference Mullié, Obin and Outurquin68) and the potential impact of thermal treatment on milk quality. To resolve these issues, one solution would be to advance non-thermal pasteurisation methods. Alternatively, a greater emphasis could be placed on testing the functional integrity of the processed batches of milk and rigorously comparing the advantages associated with unpasteurised donor milk on preterm infants with pathogen-related risks(Reference Lindemann, Foshaugen and Lindemann145).
Acknowledgements
Acknowledgements: The contributions to the technical discussions by Kevin Condon and Lynne-McKensey Hall, Australian Breast Milk Bank and the editorial support by Suze Coppinger are highly appreciated by authors. Financial support: The authors acknowledge the financial support, for this study, from the University of Sydney for establishing Centre for Excellence in Advanced Food Enginomics. K.B. acknowledges support from the Joyce Beatrice Pirchan Research Scholarship. Conflict of interest: None (all authors). Authorship: R.B.B. conceptualised the review and defined the scope of the systematic review process. K.B. drafted the initial manuscript, which was extensively edited by A.S. and P.V. N.B. reworked the risk analysis section and provided a public health perspective. All authors including F.D. and N.K. provided commentary and approved the final manuscript. Ethics of human subject participation: N/A (review).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980020000555












