Vitamin B12 plays an important role in haematopoiesis and nervous system development, and as a cofactor it participates in the conversion of methylmalonyl CoA to succinyl CoA and of homocysteine to methionine( Reference Stabler 1 , Reference Honzik, Adamovicova and Smolka 2 ).
Children are at increased risk of vitamin B12 deficiency, particularly in the first 6 months of life when the lowest serum vitamin B12 concentrations are seen. Levels increase again from 6 months reaching a peak at 3–7 years, and thereafter the concentrations decrease gradually to those observed in adults( Reference Bjørke-Monsen and Ueland 3 ). The main cause of vitamin B12 deficiency in infants is low vitamin B12 content in the breast milk of vitamin B12-deficient mothers( Reference Honzik, Adamovicova and Smolka 2 ). The most common manifestations of severe deficiency in infants are failure to thrive, developmental delay( Reference Honzik, Adamovicova and Smolka 2 , Reference Strand, Taneja and Ueland 4 ), convulsions and weakness( Reference Demir, Kok and Ustyol 5 ). In older children, other factors may be associated with vitamin B12 deficiency, such as the absence of animal-derived foods or fortified foods( Reference McLean, Allen and Neumann 6 ), a vegetarian diet( Reference Pawlak, Parrott and Raj 7 ), low socio-economic level( Reference Villamor, Mora-Plazas and Forero 8 ) and infection by gastrointestinal parasites( Reference Allen 9 ). Clinical presentations of vitamin B12 deficiency include erythrocyte deformability( Reference Tancer-Elci, Isik-Balcu and Bor-Kucukatay 10 ) and neurological changes, which can occur in the absence of haematological abnormality( Reference Rasmussen, Fernhoff and Scanlon 11 ). The early diagnosis and treatment of vitamin B12 deficiency in infants and children is important as long-term deficiency can cause developmental delay, failure to thrive, and clinical and neurological symptoms that can be irreversible( Reference Bjørke-Monsen and Ueland 3 , Reference Demir, Kok and Ustyol 5 ).
Estimates of the global prevalence of vitamin B12 deficiency in childhood are scarce and vary widely according to geographic location and threshold used, ranging from 8 % to 30% among infants or children <6 years of age( Reference Strand, Taneja and Ueland 4 , Reference Cuevas-Nasu, Mundo-Rosas and Shamah-Levy 12 , Reference Jones, Ramirez-Zea and Zuleta 13 ) and from 1·6 % to 32·5 % in older children( Reference McLean, Allen and Neumann 6 , Reference Villamor, Mora-Plazas and Forero 8 ).
In addition to extrinsic factors, analysis of the contribution of polymorphisms in genes involved in B-vitamin metabolism may be helpful in providing further information about the predictors of vitamin B12 status early in life( Reference Haggarty 14 ). Recent genome-wide association studies have shown that genetic polymorphisms can influence serum vitamin B12 concentrations( Reference Tanaka, Scheet and Giusti 15 – Reference Hazra, Kraft and Lazarus 17 ). Hazra et al.( Reference Hazra, Kraft and Selhub 16 ) demonstrated that women homozygous for the rs492602 G allele of fucosyltransferase 2 (FUT2) had higher vitamin B12 concentrations. Other common FUT2 variants such as rs602662 and rs601338 were also shown to be associated with levels of vitamin B12 ( Reference Tanaka, Scheet and Giusti 15 , Reference Hazra, Kraft and Lazarus 17 , Reference Tanwar, Chand and Kumar 18 ). The most commonly studied polymorphisms of enzymes involved in folate and homocysteine metabolism that are also dependent on vitamin B12 metabolism are mutations in the gene encoding the 5,10-methylenetetrahydrofolate reductase (MTHFR) enzyme (mutations identified are 677 C→T and 1298 A→C)( Reference Bailey and Gregory 19 , Reference Frosst, Blom and Milos 20 ). This enzyme catalyses the biologically irreversible conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. 5-Methyltetrahydrofolate is converted by the cobalamin-dependent methionine synthase reductase (MTRR; mutation identified: 66 A→G) to tetrahydrofolate( Reference Palladino, Chiusolo and Reddiconto 21 ). Methionine synthase and 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR; mutation identified: 2756 A→G) are required for the remethylation of homocysteine to methionine( Reference Bailey and Gregory 19 ). Studies have shown that the TT genotype of the MTHFR C677T variant and the CC genotype of the MTHFR A1298C variant are associated with low serum vitamin B12, and also with low serum folate and high homocysteine concentrations( Reference Thuesen, Husemoen and Ovesen 22 , Reference Ozarda, Sucu and Hizli 23 ).
The present study describes the prevalence of vitamin B12 deficiency and factors associated with vitamin B12 status in Amazonian children. To our knowledge, the study is the first to report vitamin B12 status, including genetic factors, in Brazilian children.
Materials and methods
Study area and population
The population-based, cross-sectional study described here was performed in 2007 in Acrelândia, a frontier town located 112 km from Rio Branco, the capital of the state of Acre, in the western Brazilian Amazon region. By 2007 Acrelândia had 11520 inhabitants of whom 44 % resided in the urban area. Sampling strategies and field procedures were as previously reported( Reference Cardoso, Scopel and Muniz 24 ). Briefly, all households from the urban area with children up to 10 years of age (n 749) were identified. This resulted in 1225 children living in 734 households being enrolled in the study.
A structured questionnaire, pilot-tested previously, was administered through face-to-face interview to the mothers or guardians of 1151 children (94·0 % of those eligible). It included demographic characteristics (child’s sex, age and race/ethnicity, classified as white, black, ‘pardo’ (brown), yellow or indigenous, according to skin colour, as used in the Brazilian census( Reference Travassos and Williams 25 )), socio-economic status and environmental conditions, reproductive health variables, history of infant feeding practices, frequency of habitual food intake and morbidities.
The study protocol was approved by the institutional review board of the School of Public Health, University of São Paulo, Brazil (No. 1681/07) and it was conducted according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was obtained from all parents or guardians of participating children prior to enrolment.
Anthropometric assessment
Anthropometric measurements were performed by trained research assistants following standardized procedures using calibrated equipment( Reference Lohman, Roche and Martorell 26 ). Among children aged <24 months, recumbent length was measured using a locally made infant measuring board; weight was measured with an electronic paediatric scale (model 1583; Tanita, Tokyo, Japan). Among children aged ≥24 months, height was measured using a stadiometer (model 208; SECA, Hamburg, Germany) and weight was measured using an electronic scale (model HS-302; Tanita, Tokyo, Japan). Each measurement was repeated and the mean value was calculated. Z-scores for length/height-for-age (HAZ) and BMI-for-age (BAZ) were calculated according to WHO guidelines( 27 ). The cut-off defined for stunting was HAZ <−2 and that for overweight was BAZ >1( Reference de Onis and Lobstein 28 ).
Dietary assessment
For children <24 months, a diet history( Reference Garcia, Granado and Cardoso 29 ) was collected by trained nutritionists. The interviewers were provided with household measures to help mothers or guardians estimate the habitual amounts of foods or beverages. The World Food Dietary Assessment System (version 2·0; University of California, USA) was used to estimate food intake. For children ≥24 months, an FFQ, based on a validation study in this area( Reference Scagliusi, Garcia and Indiani 30 ), was used to estimate the frequency of food consumption (fruit, green vegetables, root vegetables, dairy, beans, meat, eggs and fish) within the last month.
Biochemical measures
Approximately 5 ml of fasting venous blood was collected from 1131 children (98·3 % of those eligible) by trained phlebotomists. Serum folate and vitamin B12 concentrations were measured using commercial fluoroimmunoassays (Perkin Elmer, Wallac Oy, Turku, Finland). The cut-offs for vitamin B12 and folate deficiency were <150 pmol/l and <10 nmol/l( 31 ), respectively. Plasma homocysteine and serum vitamin A concentrations were determined by HPLC (Shimadzu, Kyoto, Japan) with fluorimetric detection and isocratic elution( Reference Gomes, Alves and Sevanian 32 ). Vitamin A concentrations <0·70 µmol/l were used to define vitamin A deficiency( 33 ). Anaemia, Fe deficiency and Fe-deficiency anaemia were defined according to Hb, serum ferritin and soluble transferrin receptor concentrations, respectively( Reference Cardoso, Scopel and Muniz 24 , 34 ). The normal range of soluble transferrin receptor concentration, as determined by the immunoassay manufacturer, was 2·9–8·3 mg/l. Fe deficiency was defined when serum ferritin concentrations were low (<12 µg/l for children aged <5 years or <15 µg/l for those ≥5 years) or when soluble transferrin receptor concentrations were high (>8·3 mg/l). Fe-deficiency anaemia was defined when Fe deficiency occurred in anaemic children; the cut-off for Hb concentration considered was 110·0 g/l for children aged 6 months to 5 years, and 115 0 g/l for children ≥5 years. Plasma C-reactive protein concentration was measured using the Immulite high-sensitivity chemiluminescent assay (DPC, Los Angeles, CA, USA). The cut-off for high C-reactive protein as an indicator of inflammation was >5 mg/l( Reference Thurnham, McCabe and Haldar 35 ).
Stool samples were collected from 1016 children (97·0 % of those eligible) and analysed for eggs, cysts and larvae of parasites, according to the qualitative technique of sedimentation( Reference Hoffman, Pons and Janer 36 ), as described elsewhere( Reference Cardoso, Scopel and Muniz 24 ). Geohelminths found in this population included Ascaris lumbricoides, Trichuris trichiura and Strongyloides stercoralis. Children with anaemia, nutritional deficiencies or intestinal parasitic infections received free treatment prescribed by the research clinicians.
Genotyping
SNP genotyping was performed using allele-specific PCR with the molecular beacons assay( Reference Myakishev, Khripin and Hu 37 ), under contract by Prevention Genetics (Marshfield, WI, USA). SNP included those in folate-metabolizing enzyme-encoding genes: MTHFR C677T (rs1801133), MTHFR A1298C (rs1801131), MTR A2756G (rs1805087), MTRR A66G (rs1801394) and reduced folate carrier gene (RFC1) G80A (rs1051266), as well as FUT2 AG (rs492602).
The homogeneous assay used two-tailed allele-specific primers, a common reverse primer and two different fluorescently labelled universal primers in a single-well reaction. Submicrolitre PCR reactions were carried out with Array Tape instrumentation and allele calls were generated based on clustering of fluorescent signals( Reference Rusch, Dickinson and Che 38 ). The internal quality of genotype data was assessed by typing 10 % of blinded samples in duplicate; the resulting concordance was >99 %. Allelic and genotypes frequencies for each SNP were calculated from the Hardy–Weinberg equilibrium (P>0·05) using an available online tool.
Statistical analysis
Children were stratified into age categories (<24, ≥24–60 and ≥60 months) for the descriptive analyses in which covariates are reported as absolute frequencies and percentages or as medians and interquartile ranges.
The outcome of interest was serum vitamin B12 concentration (natural log-transformed). Explanatory variables comprised the above described polymorphisms, socio-economic status, maternal and child characteristics, diet, morbidities and biochemical indicators. The definitions of variables are as follows.
A genetic risk score (GRS) was developed based on polymorphisms in genes encoding the folate-metabolizing enzymes and in the FUT2 gene. One-way ANOVA was tested for multiple comparisons of means between serum vitamin B12 concentrations for each genetic polymorphism. Mean differences with P value ≤0·10 for low vitamin B12 concentration were observed for MTHFR C677T, MTHFR A1298C and FUT2 AG, which were selected to comprise the GRS. A code value was then assigned to each gene, ranging from 0 for the lowest-risk allele to +1 for heterozygote and +2 for the increased-risk allele, according to the present study. The GRS for each individual was created by summing these values for each SNP in the GRS. GRS was examined as a continuous variable.
Principal component analysis was used to derive a wealth index representing a proxy of household income( Reference Filmer and Pritchett 39 ), based on the presence of twelve household assets, as described elsewhere( Reference Cardoso, Scopel and Muniz 24 ). The wealth index was used as a continuous variable.
Maternal schooling was categorized as <5 years v. ≥5 years. Maternal age at the child’s birth was categorized as ≥20 years v. <20 years and the child’s birth weight as <2500 g v. ≥2500 g.
Regarding dietary information, for children <24 months of age, we quantified animal-derived protein in g/d from breast milk, cow’s milk and dairy products, eggs, meat, fish and chicken. This variable was then dichotomized, according to tertiles, as low intake (first tertile, <13·5 g/d) v. high intake (second tertile, 13·5–30·0 g/d; and third tertile, ≥30·0 g/d). For older children (≥24 months), we created a score for animal-derived food (ADF) intake based on the FFQ, as follows. The frequencies of dairy products, meat and egg consumption were grouped and coded into three categories: 0=low consumption (rarely/never; 1–3 times/month; 1–3 times/week; 4–6 times/week); 1=intermediate consumption (1 time/d); and 2=high consumption (≥2 times/d). The ADF score was created by summing the codes for each child, ranging from 0 to 6. In order to quantify whether the low consumption of ADF contributes to vitamin B12 variability, the ADF score was then dichotomized into below the median (<4) v. above the median (≥4).
Indicators of morbidities were presence of geohelminth infection and reported diarrhoea in the past 15 d; plasma C-reactive protein >5 mg/l was used as an indicator of inflammation.
Crude and multiple linear regression models were conducted separately for children aged <24 months and for those ≥24 months of age, due to the different methods of collecting dietary data, as stated in ‘Dietary assessment’. Crude linear regression analyses were first conducted between the outcome, serum vitamin B12 concentration, and the covariates. The covariates were first selected for the multiple models using P<0·20, adjusted by sex and age, following a hierarchical conceptual approach( Reference Cardoso, Scopel and Muniz 24 ), and were retained in the final model if they were associated with the outcome at P<0·10. Missing observations were included by creating missing-value categories. We compared results from the model with missing-value categories with those from a complete case analysis. Because the magnitudes and directions of all associations were similar, we decided to preserve all children in the multiple models. Interaction terms between GRS and biochemical measures and the outcome were tested in the models. P values reported are two-sided. All analyses were performed using the statistical software package Stata version 11·0.
Results
Of the 1151 participants, serum vitamin B12 was measured for 988 (85·8 %). Of these, the mean age was 5·2 (sd 2·8) years (range: 2·8 months to 10·4 years). Only 13·5 % of children were exclusively breast-fed until 6 months of age. Table 1 shows the characteristics of these children. The prevalence of stunting (12·0 %) and overweight (30·5 %) was higher in children aged <24 months, and this age group also saw a highest prevalence of anaemia and Fe deficiency. In addition, they presented the highest prevalence of vitamin B12 deficiency: 13·6 (95 % CI 8·8, 19·7) %. The overall prevalence of vitamin B12 deficiency was 4·2 (95 % CI 3·0, 5·6 %) % and the prevalence of vitamin B12 insufficiency (<221 pmol//l) was 31·7 (95 % CI 28·8, 34·6) %. Only 2·6 % of children had a low plasma folate concentration, while vitamin A deficiency was found in 14·1 % of children. Mean animal-derived protein intake was 24·4 (sd 17·7) g/d in younger children. Overall, 50·3 %, 55·1 % and 35·7 % of children aged ≥24 months were observed to be in the high consumption category (≥2 times/d) for milk, meat and eggs, respectively (data not shown).
Table 1 Characteristics of urban children aged <10 years included in the study according to age group, Acrelândia, western Brazilian Amazon, 2007

IQR, interquartile range.
* Total may be less because of missing values.
† Cut-off for anaemia: Hb<110·0 and <111·5 g/l for children 6–59 months and ≥60 months, respectively.
‡ Serum ferritin concentration <12 µg/l for children <59 months or <15 µg/l for those aged ≥60 months, or serum transferrin receptor concentration >8·3 mg/l.
§ Fe-deficiency anaemia was defined when Fe deficiency occurred in anaemic children.
Gene allele distributions are described in Table 2. Based on the estimated risk for low serum vitamin B12 concentrations, the mutant allele for MTHFR C677T, the wild-type allele for MTHFR A1298C and the wild-type allele for FUT2 were established as increased-risk alleles.
Table 2 Gene allele distribution in urban children aged <10 years (n 988), Acrelândia, western Brazilian Amazon, 2007

* Total may be less because of missing values.
† Increased-risk allele for low serum vitamin B12 concentration according to the present study. Mean differences in serum vitamin B12 concentrations for MTR A2756G, MTRR A66G and RFC1 G80A were not observed according to allele.
Table 3 shows the factors associated with serum vitamin B12 adjusted for sex and age among younger children. The final model explained 44 % of the variability in natural log-transformed B12 concentrations. Wealth index and animal-derived protein intake were positively associated with vitamin B12 status, whereas serum homocysteine level and GRS were negatively associated. Among older children (Table 4), vitamin B12 status was positively associated with wealth index, HAZ and serum vitamin A; age, low maternal schooling, serum homocysteine, serum folate, geohelminth infection, low ADF consumption and GRS were negatively associated with vitamin B12 status. A significant interaction was found in this age group between GRS and homocysteine (P=0·020).
Table 3 Factors associated with vitamin B12 status in urban children aged <24 months, Acrelândia, western Brazilian Amazon, 2007
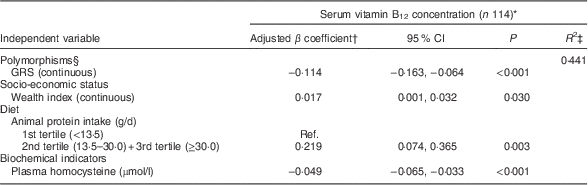
GRS, genetic risk score; Ref., referent category.
* Dependent variable, serum vitamin B12, was natural log-transformed before analysis; total may be less because of missing values.
† The model was adjusted for sex and age (continuous).
‡ Final adjusted R-squared.
§ GRS was calculated on the basis of three polymorphisms (MTHFR C677T, MTHFR A1298C and FUT2 AG) representing increased-risk alleles. Interaction term, GRS×homocysteine: P=0·053.
Table 4 Factors associated with vitamin B12 status in urban children aged ≥24 months, Acrelândia, western Brazilian Amazon, 2007
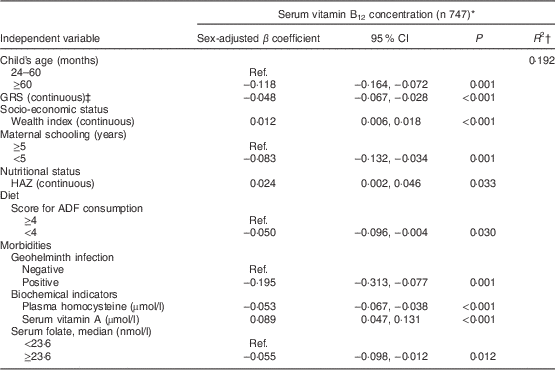
GRS, genetic risk score; HAZ, height-for-age Z-score; ADF, animal-derived food; Ref., referent category.
* Dependent variable, serum vitamin B12, was natural log-transformed before analysis.
† Final adjusted R-squared.
‡ GRS was calculated on the basis of three polymorphisms (MTHFR C677T, MTHFR A1298C and FUT2 AG) representing increased-risk alleles. Interaction term, GRS×homocysteine: P=0·020.
Discussion
Overall, the prevalence of vitamin B12 deficiency found in the present study was 4·2 %, with the highest proportion in children aged <24 months (13·6 %). This latter prevalence is higher than that observed in a national study among Mexican children aged 3 years (3·3 %)( Reference Cuevas-Nasu, Mundo-Rosas and Shamah-Levy 12 ) and in Venezuelan children (9·7 %)( Reference García-Casal, Osorio and Landaeta 40 ). Highest prevalence was found in other developing countries, such as 30 % in Guatemala( Reference Jones, Ramirez-Zea and Zuleta 13 ) and 27 % in India( Reference Strand, Taneja and Ueland 4 ), where children have low dietary intake of animal products or fruits and vegetables due to poverty or strictly vegetarian mothers, resulting in poor vitamin B12 concentrations in breast milk. In older children (≥24 months), the prevalence reported in our study (2·2 %) was much lower than that observed in Indian children (17·4 %)( Reference Osei, Houser and Bulusu 41 ) or in Kenyan children (32·5 %)( Reference McLean, Allen and Neumann 6 ), but similar to that observed in Colombian children (1·6 %)( Reference Villamor, Mora-Plazas and Forero 8 ).
In our study, only 2·6 % of children had folate deficiency, which suggests that mandatory folate fortification of wheat flour implemented in Brazil since 2003 is proving effective. However, the prevalence of vitamin B12 deficiency was higher among children <24 months of age. This might occur by the fact that infants and young children are at increased risk for vitamin B12 deficiency and the most common factors that contribute to this are poor maternal nutritional status during pregnancy; this causes a lower micronutrient concentration of breast milk and influences the infant’s stores of the vitamin( Reference Demir, Kok and Ustyol 5 , Reference Allen 42 , Reference Allen 43 ). Furthermore, exclusive breast-feeding for long periods (over 6 months of age) followed by the introduction of inadequate complementary foods lacking sufficient vitamin B12 can worsen the deficiency( Reference Allen 9 ).
In our analysis, the GRS was negatively associated with serum vitamin B12 status. We observed that children with polymorphisms for the mutant MTHFR C677T allele and wild-type alleles in MTHFR A1298C and FUT2 had the lowest mean serum vitamin B12. Based on these findings, the GRS was created. The mutations in MTHFR (677 C→T and 1298 A→C) result in a thermolabile enzyme that impairs the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. The latter is the circulating and active form of folate( Reference Bailey and Gregory 19 ). Under normal conditions, 5-methyltetrahydrofolate is essential for the conversion of homocysteine to methionine, which involves the vitamin B12-dependent enzyme methionine synthase reductase( Reference Bailey and Gregory 19 ).
As previously reported, the 677T variant is associated with low plasma folate levels and hyperhomocysteinaemia( Reference Ozarda, Sucu and Hizli 23 ). In contrast, some studies have shown that low serum vitamin B12 was significantly associated with the TT genotype of the MTHFR C677T polymorphism( Reference Thuesen, Husemoen and Ovesen 22 ) and with the CC genotype of the MTHFR A1298C polymorphism( Reference Ozarda, Sucu and Hizli 23 ). However, Huemer et al.( Reference Huemer, Vonblon and Födinger 44 ) found no significant difference in vitamin B12 concentrations between either genotype.
Data on the prevalence and significance of the recently described FUT2 polymorphism and its relationship to vitamin B12 are scarce( Reference Tanaka, Scheet and Giusti 15 – Reference Hazra, Kraft and Lazarus 17 ). In our sample, children homozygous for the G allele of rs492602 had higher vitamin B12 concentrations, as seen in a study conducted on women of self-reported European ancestry( Reference Hazra, Kraft and Selhub 16 ). The secretor enzyme α-1,2-fucosyltransferase, encoded by FUT2, catalyses the addition of fucose to form H type 1 and H type 2 antigens( Reference Kelly, Rouquier and Giorgi 45 ). A possible mechanism that has been suggested for the association between FUT2 and low vitamin B12 concentration is that individuals with FUT2 polymorphisms are more susceptible to Helicobacter pylori infection than those with the non-secretor status( Reference Hazra, Kraft and Selhub 16 , Reference Tanwar, Chand and Kumar 18 ). This could lead to reduced secretion of intrinsic factors and consequently to vitamin B12 malabsorption( Reference Carmel, Aurangzeb and Qian 46 ). In contrast, Oussalah et al.( Reference Oussalah, Besseau and Chery 47 ), who evaluated the FUT2 461 G→A (rs601338) polymorphism in two different populations, found associations with plasma vitamin B12 concentration but no association with positive H. pylori serologic status. More recently, Chery et al.( Reference Chery, Hehn and Mrabet 48 ) demonstrated that individuals carrying the FUT2 secretor variant who were also heterozygous for a GIF mutation had low vitamin B12 concentration independent of H. pylori-related gastritis. Unfortunately, in our study we could not assess the H. pylori infection status to better explore this relationship. More studies are necessary to elucidate the influence of FUT2 on cobalamin concentrations.
In the present study, wealth index and maternal schooling were associated with serum vitamin B12 concentrations. As in other developing countries, socio-economic status is an important determinant of both deficient and marginal serum vitamin B12 concentrations( Reference Villamor, Mora-Plazas and Forero 8 , Reference García-Casal, Osorio and Landaeta 40 ), where the consumption of ADF is limited because of high costs and/or cultural and religious beliefs( Reference Allen 9 ).
In our analyses, the lowest tertile of animal protein intake in children <24 months, as well as the lowest score of ADF in older children, were associated with vitamin B12 status after adjusting for other variables. The quality of diet among young Amazonian children has previously been assessed( Reference de Onis and Lobstein 28 ). These authors found that, from an early age, this group has low intakes of fruit, vegetables and ADF, and substantial consumption of unhealthy foods (almost a third of them had already experienced cookies, sweet bread and instant noodles among other processed foods), which may partially explain the higher prevalence of vitamin B12 deficiency in younger children in our study.
Another factor that contributes to cobalamin deficiency because of poor absorption is intestinal parasite infection( Reference Allen 9 ). In our study, no sanitation system was available in the town( Reference Cardoso, Scopel and Muniz 24 ) and cases of geohelminth infection were noted despite routine distribution of anti-helminthic medication under the Family Health Program of the municipality( Reference Cardoso, Scopel and Muniz 24 ); such infection was negatively associated with serum vitamin B12 concentration in children older than 24 months as this age group is at higher risk for intestinal parasite infection.
As expected, plasma homocysteine was negatively associated with serum vitamin B12 in the present study, which is consistent with other studies in children( Reference Hanumante, Wadia and Deshpande 49 ). Impaired folate or cobalamin function in tissues leads to high plasma homocysteine levels( Reference Ueland and Monsen 50 ); however, because vitamin B12 deficiency is becoming more prevalent than folate deficiency( Reference Villamor, Mora-Plazas and Forero 8 ), it can be said that vitamin B12 constitutes an important modifiable risk factor for hyperhomocysteinaemia( Reference Carmel, Green and Rosenblatt 51 ).
Vitamin A deficiency prevalence was 14·1 %, which is considered a moderate public health problem by the WHO( 33 ). Moreover, serum vitamin A was strongly associated with serum vitamin B12 status in older children. Our sample consisted of low-income children who, in the presence of an inadequate diet since early childhood( Reference de Onis and Lobstein 28 ), frequent exposure to infections and insufficient basic sanitation and water treatment, have a compromised nutritional status. It is noteworthy that animal-source foods contain large amounts of retinol (preformed vitamin A)( 52 ) as well as vitamin B12, so deficiency becomes prevalent when the intake of these foods is low( Reference Stabler 1 , 52 ). Although plasma retinol is not considered a good biomarker for dietary intake because it is tightly regulated by the mobilization of hepatic reserves( Reference Tanumihardjo 53 ), plasma retinol nevertheless increases rapidly when vitamin A-deficient children are fed dietary vitamin A( Reference Carmel, Aurangzeb and Qian 46 ) or foods fortified with vitamin A( Reference Lopez-Teros, Quihui-Cota and Méndez-Estrada 54 ). Thus, a good vitamin A nutritional status can also reflect the nutritional status of vitamin B12. This may explain the positive association between vitamin A and vitamin B12 concentrations in our analysis.
Our study has limitations that should be considered. Because of its cross-sectional design, caution should be taken in interpreting the findings. In addition, we did not investigate methylmalonic acid levels, a sensitive marker for clinical cobalamin deficiency, or mutations in genes related to the transport of vitamin B12. Despite these limitations, the study has yielded estimates of factors associated with serum vitamin B12, including the joint effects of genetic polymorphisms, in a population-based study with children living in poor conditions.
Conclusion
We found a non-negligible prevalence of vitamin B12 deficiency in young Amazonian children. The factors associated with vitamin B12 status were genetic factors, poverty, low consumption of ADF, geohelminth infection, and vitamin A and folate status. Early diagnosis of vitamin B12 deficiency is important to prevent long-term adverse consequences. More effective public health policies to promote accessibility to and consumption of healthy foods are necessary to improve vitamin B12 status of young children.
Acknowledgements
Acknowledgements: The authors are profoundly grateful to all children and their families who participated in the study and to the fieldwork research team for valuable assistance. Financial support: The study was funded by the National Council for Scientific and Technological Development of Brazil (CNPq; grant numbers 551359/2001-3, 502937/2003-3, 307728/2006-4 and 47573/2007-4); the São Paulo Research Foundation (FAPESP; grant number 2007/53042-1); and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Ministry of Education of Brazil). F.C. and R.A.A. received postdoctoral scholarships from CNPq (grant number 560988/2010-9) and CAPES (grant number 0091/08-1), respectively. CNPq, FAPESP and CAPES had no role in the design, analysis or writing of this article. Conflict of interest: None. Authors’ contributions: F.C. and M.A.C. analysed and interpreted data, and wrote the initial draft of the manuscript; L.Y.T. and R.A.A. contributed to the analysis; V.D.A. gave significant advice concerning genetic matters. All authors reviewed the manuscript and approved the final version submitted for publication. Ethics of human subject participation: The study was conducted according to the guidelines laid down in the Declaration of Helsinki. The institutional review board of the School of Public Health, University of São Paulo, Brazil (No. 1681/07) approved the study protocol. Written informed consent was obtained from all parents or guardians of participating children prior to enrolment.
Appendix The ACTION (ACre nutriTION) Study Team
Pascoal Torres Muniz, Orivaldo Florencio Souza, Cristieli Sergio de Menezes Oliveira and Thiago Santos de Araujo (Department of Health Sciences, Federal University of Acre, Rio Branco, Brazil); Suely de Godoy Agostinho Gimeno and Luciana Yuki Tomita (Department of Preventive Medicine, Federal University of São Paulo, São Paulo, Brazil); Marcelo Urbano Ferreira (Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil); Kézia K.G. Scopel (Department of Parasitology, Immunology and Microbiology, Federal University of Juiz de Fora, Juiz de Fora, Brazil); Barbara Hatzlhoffer Lourenç̧o, Pablo Secato Fontoura, Fernanda Serra Granado, Fernanda Cobayashi, Rosangela Aparecida Augusto and Marly Augusto Cardoso (Department of Nutrition, School of Public Health, University of São Paulo, São Paulo, Brazil).









