Nutritional factors increasingly contribute to the rise in CVD in the modern world. High salt intake associated with increased blood pressure is one of the important nutritional factors contributing to the development and worsening of CVD(Reference Strazzullo, D’Elia and Kandala1–Reference Johnson, Raj and Trudeau6). In addition, high-salt diets and low fruit and vegetable intakes were responsible for 10 % of global disability-adjusted life years in 2010 and were the most prominent dietary risk factors globally and in twenty-one regions of the world(Reference Lim, Vos and Flaxman7).
The estimated mean salt intake is about 10 g/d worldwide, with regional variations(Reference Mozaffarian, Singh and Powles4, Reference Anderson, Appel and Okuda8, Reference Powles, Fahimi and Micha9). This is well above the <5 g/d for all adults recommended by the WHO(10).
Although many studies have raised controversy recently about the recommendations for establishing limits of salt intake for the general population(Reference Cogswell, Maalouf and Elliott11, Reference Trinquart, Johns and Galea12), estimating salt intake remains an important issue. Quantifying and recognizing the main sources of intake of salt among different populations and cultures is essential in identifying the need for intervention and targeting the relevant sources.
In Brazil, the few population-based studies on this topic used single methods to assess salt intake. For the example, a study conducted in a city in the state of Espírito Santo with 272 adults used only the 24 h urinary Na excretion method. The authors observed an average salt intake of 11·9 g/d for men and 8·8 g/d for women without, however, assessing intake sources(Reference Rodrigues, Souza Júnior and Pimentel13). Another study used data from the Brazilian Household Budget Survey (2008–2009), which is based on purchases of food for home use, and found that the individual amount of salt available in households was 11·7 g/d(Reference Sarno, Claro and Levy14), although the data were not validated with an objective measure.
Appropriate interventions aimed at promoting a healthier intake of dietary salt in the population requires knowledge about the intake quantity determined with a reliable, valid method(Reference Holbrook, Patterson and Bodner15–Reference McLean17), as well as about the main sources of dietary salt and the typical frequency of their consumption(18). Moreover, to the best of our knowledge, no Brazilian study has yet delineated the salt intake profile on a population basis, considering both self-reported and biochemical measures.
Therefore, the main objective of the present population-based study was to assess the salt intake in a Brazilian adult sample according to biochemical (24 h urinary Na excretion) and self-report methods. The secondary objective was to characterize the salt intake according to sociodemographic and disease-related variables.
Methods
The present study is a secondary analysis of a population-based cross-sectional survey aimed at evaluating salt intake and testing its relationship with cardiometabolic risk factors in an adult and elderly population of a Brazilian town. All enrolled participants provided written informed consent and the local ethics committee approved the study.
Participants
The survey included adults and elderly people aged 20–80 years living in the town of Artur Nogueira(19) (population: 51 126) in the state of São Paulo in the Southeast region of Brazil. We excluded individuals with chronic kidney or oncological diseases, communication issues and locomotor disabilities (bedridden, wheelchair-bound or lower-limb paralysis of any type) as well as pregnant women.
We estimated the sample size according to Cochran’s sample size formula for continuous data with a 95 % CI(Reference Cochran20). The calculation was based on data from Brazilian studies using 24 h urinary Na excretion to estimate salt intake(Reference Piovesana, Sampaio and Gallani21–Reference Cornélio, Godin and Rodrigues23). Thus, considering an sd of 5·8 g and a margin of error of 0·5 g within a 95 % CI, the total estimated sample size was 517 participants.
Sampling method
The sampling procedure considered data from the family register in primary health-care centres in Artur Nogueira, where the public health system covers more than 80 % of the population and the high accuracy of the register of families according to their respective territory makes it possible to track individuals, guaranteeing homogenization and sample representativeness. The number of participants from each of the ten primary health-care centres was proportionally distributed according to the number of persons registered in each centre. All household numbers from each centre were listed in a table in Microsoft® Excel version 13 and were randomized; the numbers drawn varied from thirty-three to sixty-five participants in the different centres.
Procedures
Data collection was carried out for each household selected; one of the researchers, the community health agent or the trained interviewer invited one participant from the household to participate in the research. If more than one participant was eligible, a person was selected by simple random sampling, using the lottery method. Then, the selected participant signed the consent form and the questionnaires on sociodemographic, disease-related data and salt intake were administered by the researcher or a trained interviewer. Participants were given written and verbal instructions for the 24 h urine collection at the end of the interview. They were advised to collect the urine in the provided container(s) over a full 24 h period. Urine collection was to start in the morning, discarding the first voiding and continuing collection until the following morning, including the first voiding of the second day. The container(s) were to be kept in the refrigerator until the urine was returned to the primary health-care centre within a maximum of two days after collection and then forwarded to the laboratory for analysis. The collection of the 24 h urine occurred within five days of the interview.
Measurements
Participant characteristics
Participants were characterized according to the following.
1. Sociodemographic data: age, sex, schooling, skin colour (white or not white, according to the classification proposed by the Brazilian Institute of Geography and Statistics), labour status (active or inactive), monthly family income and living condition (alone, with a partner, with children or partner, with children or others).
2. Self-reported disease-related variables: diabetes, dyslipidaemia (hypercholesterolaemia, hypertriacylglycerolaemia or mixed hyperlipidaemia according to the Brazilian Cardiology Society)(Reference Faludi, Izar and Saraiva24), stroke, coronary disease, hypertension, length of hypertension diagnosis, smoking and current alcohol use (considering all types of alcoholic beverage, regardless of the amount usually consumed in the last year).
3. Systolic and diastolic blood pressures: measured with the OMRON® HEM-742INT Digital Automatic Blood Pressure Monitor. The pressures were measured on the two upper limbs and repeated twice on the limb presenting the highest pressure value, with an interval of 1 min between measurements. The last measurement was considered(25).
4. BMI: calculated from weight and height using the same Welmy 104A anthropometric scale model (maximum capacity of 150 kg and accuracy of 100 g). All fieldworkers were trained and evaluated on these measurements.
5. Waist circumference: measured directly after the interview according to WHO recommendations(26) (using an inelastic metric tape measure 2 m in length and marked in 0·1 cm increments).
Salt intake assessment
Salt intake was assessed by biochemical and self-report methods.
Biochemical measure: 24 h urinary Na excretion
The biological measure of urinary excretion of Na over a 24 h period is considered the gold standard for quantifying salt intake. The ion-selective electrode method(Reference Pelleg and Levy27) was used for urine analysis and performed using an AU680 Chemistry Analyzer (Beckman Coulter, Brea, CA, USA). The content of urinary Na in meq was converted into grams of salt, considering the molecular weight of Na (1meq Na = 23 mg Na = 0·058 g salt)(Reference He and MacGregor28). To verify completeness of the 24 h urine sample, participants recorded the start date/time and stop date/time of urine collection and any missed collections. Urine samples were discarded if the total volume was less than 250 ml or in the case of self-reporting of missing (no more than a ‘few drops’) or spilled urine(Reference Elliott and Brown29).
Self-report method: 24 h dietary recall
Participants reported all foods and portion sizes consumed in the 24 h prior to the interview. The recall was done only at this point. After a participant reported a specific food or beverage, the portion size was estimated using a food model booklet or standard household measures, which allowed participants to accurately estimate portion sizes. Data were transferred to the Nutrition Data System for Research (NDSR) software (2015 version). The final amount of Na given by the dietary recall was the sum of the Na inherent in each of the foods consumed the previous day (1 g salt = 0·4 g Na)(Reference He and MacGregor28). The Brazilian Table of Food Composition (TACO)(30), which lists the composition of main foods in Brazil, was used for foods that were not present in the NDSR. Due to limited access to the NDSR software for a restricted period, a simple random sample scheme was performed using the software R 3.5.0 and a sub-sample of 319 out of the 517 recalls collected was analysed with the NDSR.
Self-report method: sodium FFQ
The sodium FFQ (Na-FFQ), a tool developed and validated in a previous study(Reference Ferreira-Sae, Gallani and Nadruz31) on the Brazilian population, comprises fifteen salty ultra-processed foods (ultra-processed food products are those that are ready to eat or ready to heat with little or no preparation)(Reference Monteiro, Levy and Claro32) for which participants report their usual frequency of consumption and portion size in the preceding 6 months. The frequency is evaluated on a seven-point scale: (i) ‘never’; (ii) ‘less than once a month’; (iii) ‘one to three times a month’; (iv) ‘once a week’; (v) ‘two to four times a week’; (vi) ‘once a day’; and (vii) ‘twice or more per day’. Portions are identified as small, medium or large, using the quantity relative to the average portion of each of the items as a reference. The Na content of foods was estimated in the original study(Reference Ferreira-Sae, Gallani and Nadruz31) with the TACO list; the amount of Na consumed is the sum of the product of intake frequency and portion for each food item. In our study, we used a reduced version of the Na-FFQ with thirteen items (including lean ham, mortadella, pork sausage, chicken sausage, hotdog sausage, bacon, beef burger, feijoada, canned sardines, instant noodles (Miojo), burger (as fast food), pizza and snacks). Items mentioning the use of salt were evaluated separately.
Self-report method: discretionary-salt questionnaire
The discretionary-salt questionnaire (DSQ) was validated in a previous study(Reference Cornélio, Godin and Rodrigues23, Reference Molina, Cunha and Herkenhoff33) on the Brazilian population. Participants reported the usual monthly salt consumption (number of 1 kg packages of salt consumed per month), the number of persons who ate meals at home, the age of each person and the number of meals that each person ate per week at home, to correct the salt consumption per person. Children younger than 3 years old were not considered in the calculation and the meals of children under 10 years of age were considered as half meals. The following steps were used to calculate the salt intake per person: (i) divide the amount of salt (grams) used per month at home by 30 and multiply the quotient by 7 (= grams of weekly salt intake at home); (ii) divide this amount of weekly salt intake by the total number of weekly meals (= grams of salt intake/meal); (iii) multiply the amount of salt used per meal by the number of meals eaten per week by the participant; and (iv) divide the total weekly salt intake of the participant by 7, resulting in the estimated quantity of individual daily added salt.
Self-report method: seasoned-salt questionnaire
The seasoned-salt questionnaire (SSQ) was validated in a previous study(Reference Cornélio, Godin and Rodrigues23) on the Brazilian population. Participants were asked about the brand, quantity (cubes, packets) and frequency of use of industrialized salt seasonings. The Na content of each seasoned salt was obtained from the product label. Subsequently, the procedure for calculating discretionary salt was used.
Self-report method: total reported salt intake
Total reported salt intake is the sum of salt intake obtained by the measurements of the 24 h dietary recall, Na-FFQ, DSQ and SSQ. The sum was calculated only for the sub-sample of 319 participants for whom the 24 h dietary recall was assessed.
Data analysis
Initially, the Shapiro–Wilk test was used to check the normality of the continuous variables. Since these variables presented a non-normal distribution, descriptive analyses were used to characterize the sample according to sociodemographic and disease-related variables. A correlation test (Spearman) was used to assess the relationship of salt intake measures with continuous sociodemographic and disease-related variables. In addition, the Mann–Whitney U test was used to compare the mean salt intake according to the sociodemographic and disease-related categorical variables. Lastly, multiple regression analyses using the generalized linear model approach were performed to explore the relationship of 24 h urinary Na excretion and total reported salt intake according to sex, age, monthly family income, waist circumference, hypertension and diabetes. We chose this class of models instead of the traditional linear regression because generalized linear models are less restrictive to the error term distribution(Reference Gill34). The significance level adopted was 5 %.
Results
Table 1 provides the characteristics of the whole sample and the sub-sample of 319 participants included in analysis of the 24 h dietary recall. The mean age of participants was 53·5 years; 58·4 % were women. The mean monthly family income was $US 753·20; 42·9 % lived with their children and partner. Almost half of the participants reported being hypertensive (44·5 %) and 15·1 % reported they were diabetics.
Table 1 Sociodemographic and disease-related characteristics, blood pressure, anthropometric variables and salt intake in the study population of adults and older people (n 517) aged 20–80 years, Artur Nogueira, São Paulo, Brazil, June–September 2016
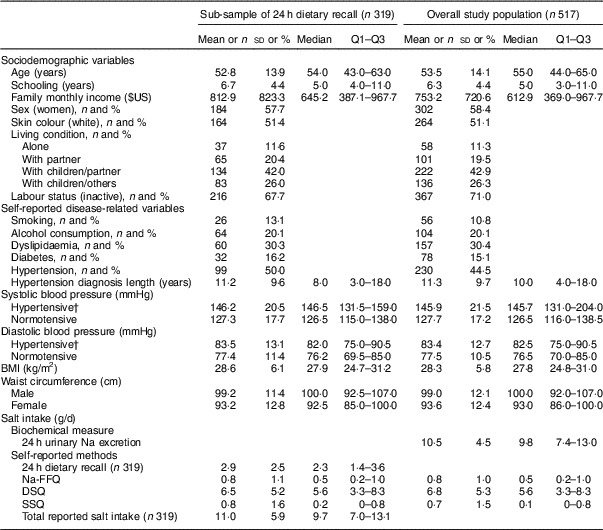
Q1–Q3, interquartile range; Na-FFQ, sodium FFQ; DSQ, discretionary-salt questionnaire; SSQ, seasoned-salt questionnaire.
Data presented are mean, sd, median and Q1–Q3 for continuous variables; or n and % for categorical variables as indicated.
† Hypertension defined as self-reported hypertension or use of antihypertensive medicine; present in 41·1 % (131/319) of the sub-sample and 44·5 % (230/517) of the overall study population.
Overall salt intake was almost the same considering 24 h urinary Na excretion and total reported salt intake: 10·5 and 11·0 g/d, respectively. As for sources of salt intake, 68·2 % came from the DSQ and SSQ; 24·0 % from the intrinsic Na assessed from 24 h dietary recall; and 7·3 % from ultra-processed foods with high salt contents assessed by the Na-FFQ (Table 1).
Table 2 gives the correlations between 24 h urinary Na excretion and the self-report methods. When the whole sample is considered, the total reported salt intake measure, the Na-FFQ and the SSQ presented significantly positive correlations with 24 h urinary Na excretion. Examining the correlations according to sex, all self-report measures, except the 24 h dietary recall, positively correlated with 24 h urinary Na excretion for women, but the correlation among men was significant only for the Na-FFQ.
Table 2 Correlations between salt intake estimated by self-reported methods and the biochemical measure, according to sex, in the study population of adults and older people (n 517) aged 20–80 years, Artur Nogueira, São Paulo, Brazil, June–September 2016
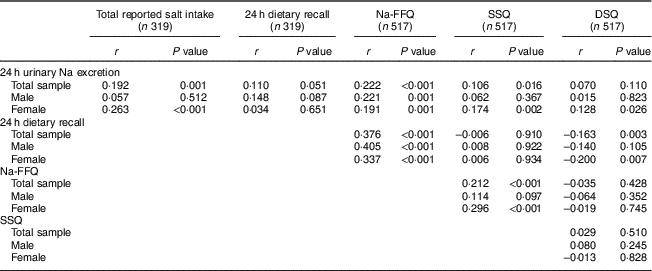
Na-FFQ, sodium FFQ; DSQ, discretionary-salt questionnaire; SSQ, seasoned-salt questionnaire, r, Spearman’s correlation coefficient.
Considering sociodemographic characteristics, we observed a higher salt intake according to 24 h urinary Na excretion among people under 65 years old, male, professionally active and with a higher family income. Specifically, regarding sex, men consumed more salt than women according to 24 h urinary Na excretion, 24 h dietary recall, Na-FFQ and total reported salt intake, but they were not different regarding salt added to meals. Participants who were professionally active and those with higher family incomes had a higher salt intake according to 24 h urinary Na excretion and the self-reported measures of 24 h recall and Na-FFQ. They reported, however, a lower use of discretionary salt in their meals (Table 3).
Table 3 Salt intake (mean and sd; g/d) according to sociodemographic variables in the study population of adults and older people (n 517) aged 20–80 years, Artur Nogueira, São Paulo, Brazil, June–September 2016
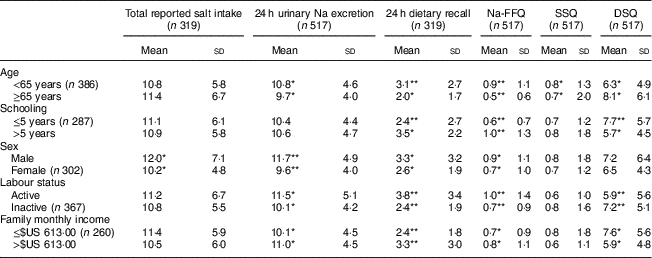
Na-FFQ, sodium FFQ; DSQ, discretionary-salt questionnaire; SSQ, seasoned-salt questionnaire, r, Spearman’s correlation coefficient.
* P<0·05
** P<0·0001 (Mann–Whitney U test).
Participants who reported they were hypertensive and/or diabetic had a lower intake of salty foods according to Na-FFQ results and/or the 24 h dietary recall, but a higher intake according to the DSQ. Thus, total salt intake – considering the sum of the self-reported methods or by 24 h urinary Na excretion – was equivalent whether the participants presented these diseases or not. Those reporting alcohol use, however, had significantly higher salt intake based on the biochemical measure, the 24 h dietary recall and the Na-FFQ (Table 4).
Table 4 Salt intake (mean and sd; g/d) according to disease-related variables, and correlation of blood pressure and anthropometric variables with salt intake measures, in the study population of adults and older people (n 517) aged 20–80 years, Artur Nogueira, São Paulo, Brazil, June–September 2016
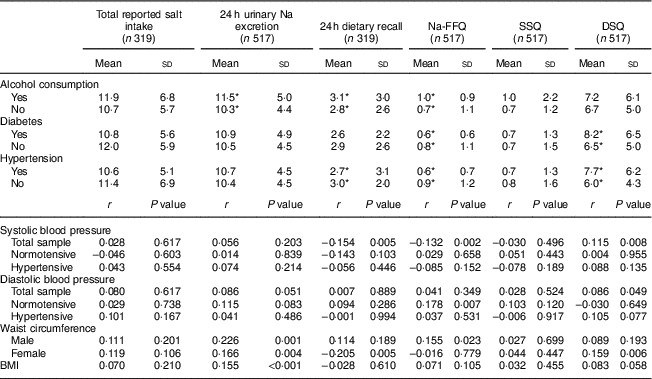
Na-FFQ, sodium FFQ; DSQ, discretionary-salt questionnaire; SSQ, seasoned-salt questionnaire, r, Spearman’s correlation coefficient.
* P<0·05 (Mann-Whitney U test).
There was no correlation between either measure of total salt intake (24 h urinary Na excretion or sum of self-reported methods) and blood pressure levels. In the total sample, however, systolic blood pressure correlated negatively with the 24 h dietary recall and Na-FFQ but correlated positively with the DSQ. In the case of diastolic blood pressure, we observed a positive correlation with the DSQ in the total sample and with the Na-FFQ among normotensive participants (Table 4).
For the anthropometric data, we observed positive correlations of 24 h urinary Na excretion with BMI and waist circumference for both men and women. For the self-report measures, waist circumference positively correlated only with the Na-FFQ among men and with the DSQ among women (Table 4).
Linear regression analyses (Table 5) indicated that being younger, male or having a higher waist circumference accounted for higher 24 h urinary Na excretion. The level of total salt intake given by the sum of the reported methods, however, was related solely to male sex.
Table 5 Multiple regression analysis of factors associated with 24 h urinary sodium excretion and total reported salt intake in the study population of adults and older people (n 517) aged 20–80 years, Artur Nogueira, São Paulo, Brazil, June–September 2016
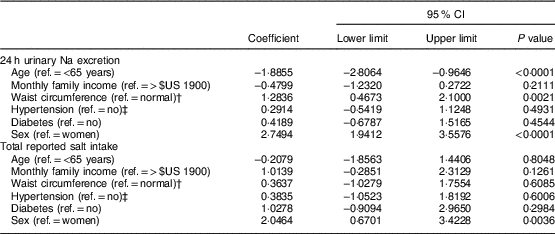
Ref., reference category.
† Normal waist circumference defined as ≤88 cm (women) and ≤102 cm (men)(25).
‡ Hypertension defined as self-reported hypertension or use of antihypertensive medicine.
Discussion
The results of the present study showed that the adult and elderly population of the town of Artur Nogueira has a daily salt intake, obtained by 24 h urinary Na excretion as well as by total reported salt intake, considerably higher than the value of 5 g salt/d recommended by the WHO(10). Elevated daily salt intake has also been found in other developing countries such as India (with variations between the North and the South: 9·4 and 10·4 g, respectively)(Reference Johnson, Mohan and Rogers35), China (11 g)(36) and South Africa (7·2 g)(Reference Swanepoel, Schutte and Cockeran37), as well as in developed countries such as Australia (9·2 g)(Reference Land, Webster and Christoforou38), Italy and Japan (10·6 g)(36).
Although many countries have high salt intake, the intake sources vary considerably, ranging from ultra-processed foods and ready-to-eat foods to the salt added to food prepared in the home or added at the table(Reference Sarno, Claro and Levy14, Reference Cornélio, Godin and Rodrigues23, Reference Perin, Cornélio and Rodrigues39–Reference Adams and White42). In our sample, salt added during cooking and at the table, as evaluated by the DSQ and SSQ, were the primary intake sources. On the other hand, ultra-processed food with high Na content accounted for only about 7 % of salt intake. Studies in Brazil using the same methodology with hypertensive and heart-failure patients observed the same pattern of salt intake(Reference Cornélio, Godin and Rodrigues23, Reference Perin, Cornélio and Rodrigues39, Reference Agondi, Gallani and Rodrigues43), being reinforced by data from the 2008–2009 Household Budget Survey within the general population that found a contribution of 74·4 % from discretionary salt and salt-based condiments to overall salt intake(Reference Sarno, Claro and Levy14). This contrasts significantly with developed countries, where the main source is usually ultra-processed foods(Reference Harnack, Cogswell and Shikany40). Although there are few studies on how the consumption of ultra-processed foods varies within a population, this pattern seems to be associated, among other factors, with the relative affordability of ultra-processed foods(Reference Moubarac, Claro and Baraldi41, Reference Adams and White42).
Our data also indicated a positive and significant correlation between the biochemical measure and the sum of the self-reported methods of salt intake. Singly, the self-reported measures were positive and significantly correlated with 24 h urinary Na excretion, particularly the Na-FFQ, in the total sample and both sexes separately. Still, women presented more significant correlations than men among the methods for estimating salt intake, which could suggest that women were probably more precise in reporting their pattern of consumption than men.
Regarding the other sociodemographic variables, we observed that participants under age 65 years, those who were professionally active and those with higher family incomes had higher salt intakes according to 24 h urinary Na excretion. Furthermore, those under 65 years of age, with more than 5 years of schooling, professionally active and with higher monthly family incomes had higher salt intakes according the 24 h dietary recall and the Na-FFQ, but lower intakes according to the DSQ. Their total intake, however, was overall equivalent to that of their counterparts. Therefore, the main source of salt consumption for these individuals was ultra-processed foods, which can obviate the need for adding salt during meal preparation. Thus, health interventions for this population must focus on optimizing the consumption of foods prepared at home, replacing processed foods.
On the other hand, those who were older, with less schooling, not professionally active or with lower incomes – who consumed homemade meals more frequently and not ultra-processed foods – seemed to compensate for this lack by adding more salt to their meals to preserve the eating pleasure related to salt intake. This is important information in designing targeted interventions. Promoting the reduction of added salt could be less effective among younger people, those with more schooling, professionally active or with higher family incomes, because that is not their main source of dietary salt.
Concerning disease-related variables, participants with known chronic diseases, such as hypertension and diabetes, reported less consumption of salty foods as assessed by the Na-FFQ, although they added more salt during meal preparation, resulting in an overall intake similar to that of participants without chronic conditions. Thus, the interventions targeting these subgroups must emphasize reducing salt added to meals. However, participants who reported alcohol use presented higher salt intake, as seen in almost all assessment measures, reproducing previous findings(Reference Mattes and DiMeglio44). The hypotheses proposed to explain this relationship are related to alcohol consumption influencing salt enjoyment and to the fact that alcoholic beverages and salty foods frequently go together(Reference Caton, Ball and Ahern45–Reference Lampuré, Schlich and Deglaire47).
Regarding the relationship between salt intake and blood pressure levels, our results differ from those found in the literature(Reference Arcand, Wong and Santos48, Reference He, Pombo-Rodrigues and Macgregor49) since, contrary to our expectations, we did not find a significant correlation between salt consumption and blood pressure levels. The lack of this relationship among the hypertensive adults in our study might be explained by use of antihypertensive medications, possibly introducing bias in the results. For the non-hypertensive participants, limited data collection might have contributed to the lack of correlation. The blood pressure measurement in the present study was based on the standardized technique advocated by the Brazilian Society of Cardiology(25) but was assessed only once. There is a growing literature on the phenomenon of masked hypertension, defined as a ‘clinical condition in which a patient’s office blood pressure level is <140/90 mmHg but ambulatory or home blood pressure readings are in the hypertensive range’(Reference Papadopoulos and Makris50). Its prevalence in the population is about one in seven or eight persons with a normal office blood pressure level. In fact, the International Consortium for Quality Research on Dietary Sodium/Salt (TRUE)(51), formed to improve the quality of research on dietary salt, highlights that much of the controversy surrounding the association between dietary salt and increased blood pressure might be due to a lack of standardization of and quality in blood pressure measurement. Thus, in future studies, the use of multiple measures of blood pressure could be important to reducing measurement bias.
Another finding of the present study refers to the association between the anthropometric measures – mainly waist circumference – and salt intake. We observed that men with greater waist circumference consumed more ultra-processed foods high in salt. In addition, women with greater waist circumference added more salt to their meals. Additionally, both sexes presented a positive correlation between waist circumference and 24 h urinary Na excretion. A recent meta-analysis showed that individuals who consumed higher amounts of salt evidenced a 4·75 cm larger waist circumference(Reference Moosavian, Haghighatdoost and Surkan52). Other studies have also found positive correlations between salt intake and different measures of obesity or visceral obesity(Reference Hoffmann and Cubeddu53–Reference Thuesen, Toft and Buhelt55). Some authors have claimed that salt intake stimulates thirst and appetite, thus promoting energy intake(Reference Oh, Han and Han54, Reference He, Marrero and MacGregor56). These results call for special attention in assessing both salt intake and overall nutritional intake, especially among those individuals presenting overweight or obesity, to better design educational activities aimed at promoting a healthier salt intake.
The multivariate analyses revealed a relationship between waist circumference, age and sex with the variation in salt intake measured by 24 h urinary Na excretion. On the other hand, sex alone accounted for the variation in consumption based on the measurement of total reported salt intake. In fact, as demonstrated in other studies(Reference Rodrigues, Souza Júnior and Pimentel13, Reference Johnson, Mohan and Rogers35, Reference Schoen, Blum and Paccaud57–Reference Nowson, Lim and Grimes61), the men presented a higher total consumption than the women according not only to 24 h urinary Na excretion, but also the self-report methods. Therefore, sex remains an important variable associated with salt intake for any measurement(Reference Rodrigues, Souza Júnior and Pimentel13, Reference Johnson, Mohan and Rogers35, Reference Schoen, Blum and Paccaud57–Reference Nowson, Lim and Grimes61). As for the age variable, other studies(Reference Assumpção, Domene and Fisberg62, Reference Hiza, Casavale and Guenther63) also observed that older individuals tend to have lower salt intake, which could be explained by wanting to have a better quality of diet.
The results of epidemiological studies shed light on the uniqueness of each population with respect to different patterns of health and health-related factors. Consequently, they inform the design of tailored and more precise interventions. Moreover, larger interventions or intervention policies on salt intake must rest on the characterization of its consumption profile in the targeted population. A survey conducted in over forty-five countries(Reference Hawkes and Webster64) aimed at identifying what approaches governments were taking to monitor salt intake. It found that the majority of the thirty countries reporting formal government salt monitoring activities were high-income countries and that less than half of them used 24 h urinary Na excretion to assess salt intake. In addition, only two of them were developing countries.
To the best of our knowledge, the present study was one of the first population studies using the gold standard measure of salt intake, i.e. 24 h urinary Na excretion, combined with self-report methods. Due to the complexity of dietary salt behaviour, the combination of self-report (Na-FFQ, 24 h dietary recall, DSQ, SSQ) and biochemical methods (24 h urinary Na excretion) is highly recommended(Reference Shim, Oh and Kim65, 66) but rarely done, especially in population-based studies(66), due to the economic and technical difficulties. Thus, our study makes a unique contribution in furthering Brazilian policies for monitoring salt intake. Besides being conducted in a small town, the present study was the first effort in the country to perform an extensive and detailed characterization of salt intake in the adult and elderly population. This study design can be reproduced in larger towns and regions to obtain a global portrait of salt consumption in Brazil’s population. With respect to the literature, the study provides important information about salt intake in developing countries.
Nevertheless, the study had some limitations. First, it was not possible to quantify salt intake from the 24 h dietary recall for 198 out of the 517 participants due to restricted availability of the nutritional software for research use. Regardless, as no differences regarding sociodemographic and disease-related characterization were observed between this sub-sample and the whole group, the results seem to reflect the overall pattern of intake according this method for the studied population. Other limitations relate to: the absence of a reliable biochemical technique to validate completeness of the 24 h urine collections due to the inadequate funds; not questioning patients diagnosed with hypertension about their restriction of salt intake; measuring blood pressure only once in the study; and the reliability of the dietary recall methods used with an older population.
Lastly, our results confirm the present study’s importance in providing a profile of the salt intake of a metropolitan population. Some interventions could be proposed to promote a healthier approach to dietary salt in the studied population. Moreover, the study can inform the design of larger studies, including in larger Brazilian towns in different regions, and thereby better support decision making on the national level regarding public policies significantly impacting the cardiovascular health of the Brazilian population. According to Webb et al.(Reference Webb, Fahimi and Singh67), a 10 % reduction in salt consumption over a 10-year period could avoid approximately 5·8 million disability-adjusted life years per year related to CVD.
Conclusion
The present study combined self-reported and biochemical measures, resulting in characterization of the salt intake and identifying its sources of consumption among adults and older people of the town of Artur Nogueira, Brazil. The findings reveal a high overall salt intake in the studied population, with the salt added during cooking as the main source. Salt intake also varied according to other factors such as sex, age and waist circumference. Thus, future research on interventions targeting the reduction of salt consumption can take account of the various intake patterns according to sociodemographic and disease-related factors.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: M.S.P., M.E.C. and M.-C.B.J.G. designed the research; M.S.P. and M.E.C. conducted the research; M.S.P., M.E.C., H.C.O. and M.-C.B.J.G. analysed the data; M.S.P., M.E.C., T.M.S.-J., C.R. and M.-C.B.J.G. wrote the paper. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Campinas (UNICAMP) Ethics Committee (protocol number 52955316.9.0000.5404). Written informed consent was obtained from all subjects.









