- BMC
bone mineral content
- BMD
bone mineral density
- DHT
dihydrotestosterone
- ER
oestrogen receptor
Equol (7-hydroxy-3-(4′-hydroxyphenyl)-chroman) is a non-steroidal oestrogen that is formed exclusively via the bacterial metabolism of the soya isoflavone daidzein in the intestine. Equol was first identified in the urine of pregnant mares in 1932(Reference Marrian and Haslewood1), but was not discovered in human urine until the 1980s(Reference Axelson, Kirk and Farrant2). The oestrogenic effects of equol first became apparent in the 1940s when sheep grazing on pastures containing clover in South Western Australia became infertile(Reference Bennetts, Underwood and Shier3). This ‘clover disease’ was later attributed to the rich quantities of formononetin present in the clover, which is converted to daidzein in the rumen(Reference Shutt4). The isoflavone intake associated with clover disease (20–100 g/d) was, however, far in excess to that of human subjects who consume a soya-rich diet (isoflavone intake among older adults in Japan is about 40 mg/d)(Reference Messina, Nagata and Wu5).
Daidzein, one of the major soya isoflavones, is present in most soya foods as the glycoside daidzin. Upon consumption, daidzin is hydrolysed by intestinal β-glycosidases to form the bioavailable aglycone, daidzein. Daidzein is further metabolised by colonic bacteria to form equol or O-desmethylangolensin via a pathway that involves the formation of the intermediate dihydrodaidzein(Reference Setchell, Brown and Lydeking-Olsen6, Reference Yuan, Wang and Liu7) (Fig. 1). The importance of the gut microflora in the biotransformation of daidzein to equol is evident from studies that have demonstrated that germ-free animals(Reference Bowey, Adlercreutz and Rowland9) and infants below the age of 4 months(Reference Setchell, Zimmer-Nechemias and Cai10–Reference Hoey, Rowland and Lloyd12) who have a poorly developed gut microflora, do not produce equol when fed a soya diet.
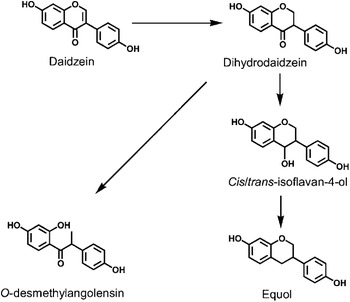
Fig 1. Metabolism of the soya isoflavone daidzein to O-desmethylangolensin and equol. (From Lampe(Reference Lampe8); reproduced with permission.)
In comparison with other isoflavones, equol is unique in structure, having a chiral carbon atom at position C-3 of the furan ring. Equol can therefore exist in two distinct enantiomeric forms, S-equol and R-equol (Fig. 2). It has recently been shown that human intestinal bacteria exclusively synthesise the S-equol enantiomer from daidzein(Reference Setchell, Clerici and Lephart14). Not every individual can, however, produce equol; approximately 30% of adults among western populations(Reference Lampe, Karr and Hutchins15–Reference Setchell and Cole17) and 60% of Asian adults(Reference Tanaka, Fujimoto and Chihara18, Reference Liu, Liu and Uchiyama19) have the ability to produce equol from daidzein following a soya challenge. Inter-individual variation in the ability to produce equol has been attributed to a number of factors, primarily inter-individual variation in gut microflora composition. Bacterial species that have been identified as being capable of converting daidzein to equol in vitro include Streptococcus intermedius, Ruminococcus products and Bacteriodes ovatus (Reference Ueno and Uchiyama20). It is evident that different bacteria are involved in different stages of the biotransformation of daidzein to equol and that these bacteria may differ between individuals(Reference Yuan, Wang and Liu7). Lactococcus garvieae can efficiently convert daidzein to S-equol and has recently been used to produce the natural S-equol-containing supplement SE5-OH(Reference Yee, Burdock and Kurata21). Diet has also been associated with the ‘equol producer’ phenotype, with equol producers reported to consume higher amounts of carbohydrate(Reference Lampe, Karr and Hutchins15, Reference Rowland, Wiseman and Sanders16) and lower amounts of fat(Reference Rowland, Wiseman and Sanders16) as a percentage of energy than non-producers. Fibre(Reference Lampe, Karr and Hutchins15), meat(Reference Hedlund, Maroni and Ferucci22), PUFA and alcohol intake(Reference Bolca, Possemiers and Herregat23) have also been positively associated with the ability to produce equol among western populations. The frequency of equol producers among vegetarian adults has been reported to be much higher than non-vegetarians, with levels comparable to those of Asian adults(Reference Setchell and Cole17). Dietary modification may therefore potentially influence equol producer status. Consequently, prebiotics and probiotics have been studied as an aid to induce equol production in adults; however, studies to date have been unsuccessful(Reference McMullen, Hamilton-Reeves and Bonorden24–Reference Larkin, Astheimer and Price26). Familial correlation and segregation analysis has suggested that the ability to produce equol is under a certain degree of genetic control with other non-genetic factors also involved(Reference Frankenfeld, Atkinson and Thomas27). The involvement of host genetics is credible given that the ability to produce equol appears to remain stable over time(Reference Frankenfeld, Atkinson and Thomas28).

Fig 2. Comparison of the conformational structures of the diastereoisomers of equol, showing the site position of the chiral carbon centre. (From Brown et al.(Reference Brown, Belles and Lindley13); reproduced with permission.)
Individuals with a plasma equol concentration of <40 nmol/l (10 μg/l) can be classified as ‘equol non-producers’ and those with concentrations >83 nmol/l (20 μg/l) classified as ‘equol producers’(Reference Setchell, Brown and Lydeking-Olsen6). Cut-offs can also be applied to urinary concentrations with an equol producer being defined as excreting >1000 nmol/l(Reference Lampe, Karr and Hutchins15, Reference Rowland, Wiseman and Sanders16). Defining thresholds for equol producer status is complicated by variations in the methodologies used to detect S-equol. Indeed, with sensitive methods, small quantities of equol can be detected in the urine of most individuals, as small amounts of equol can be obtained via animal foods in the diet e.g. cow's milk(Reference Adlercreutz, Bowey and Heinonen29). Many studies have defined equol producers as those in whom equol is detectable according to the assay system used. Professor Ken Setchell has developed a more accurate approach to define an ‘equol producer’ that is independent of isoflavone intake and the analytical technique used. Thus, a threshold value of log10-transformed urinary S-equol:daidzein ratio of greater than −1·75 can be used to define an equol producer(Reference Setchell and Cole17).
Biological properties of equol
Approximately 50% of equol circulates in the ‘free’ form, unbound to serum protein(Reference Nagel, vom Saal and Welshons30) in comparison with only 18·7% daidzein and 4·6% oestradiol(Reference Setchell, Brown and Lydeking-Olsen6). Equol can be classified as a selective oestrogen receptor (ER) modulator, having a selective affinity for ERβ. S-equol binds to ERβ with approximately 20% as much affinity as 17β-oestradiol and has a much stronger affinity for ERβ compared to R-equol(Reference Setchell, Clerici and Lephart14). Equol also has stronger in vitro antioxidant activity compared to other isoflavones(Reference Hodgson, Croft and Puddey31–Reference Arora, Nair and Strasburg33). S-equol is somewhat unique in that in addition to its oestrogenic properties, it potently antagonizes dihydrotestosterone (DHT) in vivo (Reference Lund, Munson and Haldy34). In addition, equol inhibits binding of oestradiol, testosterone and sex hormone binding globulin in a dose-dependent manner(Reference Martin, Haourigui and Pelissero35). Due to the biological properties of equol, it has been postulated that the ability to produce equol may be beneficial to health, in particular, in relation to hormone-dependent diseases.
A wealth of clinical studies investigating the effects of soya and isoflavones on health has been conducted to date, yielding somewhat inconsistent findings. In 2002, Professor Ken Setchell postulated that the beneficial effects of soya/isoflavones may be dependent on equol producer status; a theory now known as the ‘equol hypothesis’(Reference Setchell, Brown and Lydeking-Olsen6). This paper will outline research to date that has examined the effects of equol on the risk and progression of a number of chronic diseases including breast and prostate cancer and CVD. Effects on bone health and menopause will also be discussed.
Equol and breast cancer
Breast cancer is the most common cancer in women worldwide, accounting for about 23% of all female cancers(36). Incidence in western countries, including the UK and Ireland, is between two- and four-fold higher compared to Asian countries(Reference Ferlay, Shin and Bray37). In the 1980s, Setchell and Adlercreutz were the first to postulate that soya isoflavones may confer protection against breast cancer(Reference Setchell, Adlercreutz and Rowland38) and Adlercreutz et al. have demonstrated that individuals living in countries with high rates of breast cancer excrete low amounts of isoflavones(Reference Adlercreutz, Fotsis and Heikkinen39–Reference Adlercreutz, Fotsis and Bannwart41) in comparison with those living in areas at low risk(Reference Adlercreutz, Hockerstedt and Bannwart42–Reference Adlercreutz, Goldin and Gorbach46).
Few epidemiological studies have investigated the association between equol and breast cancer risk, albeit two studies have reported positive findings. In a case–control study conducted in women (144 pairs, aged 30–84 year) resident in Perth, western Australia, Ingram et al.(Reference Ingram, Sanders and Kolybaba47) reported that equol excretion was associated with a substantial reduction in risk of breast cancer (OR 0·27, 95% CI 0·1, 0·69 highest v. lowest quartile equol excretion). Furthermore, a nested case–control study conducted within the Multiethnic Cohort Study in Hawaii and Los Angeles reported similar findings for white post-menopausal women, with the highest quartile of equol excretion associated with a reduced risk of breast cancer compared to the lowest quartile (OR 0·27 (95% CI 0·08, 0·95))(Reference Goodman, Shvetsov and Wilkens48). This finding, however, was not statistically significant (P=0·07) possibly due to the small sample size (51 white cases and 96 controls). In contrast to the above findings, a prospective study conducted within the UK arm of the European Prospective Investigation of Cancer and Nutrition study, analysed 114 spot urines and 97 serum samples from women (aged 41–76 years) who developed breast cancer 4–8 years after sampling(Reference Grace, Taylor and Low49). Samples were matched to 219 urine and 187 serum controls. Exposure to daidzein and equol was significantly associated with increased breast cancer risk (log2 OR=1·34 (95% CI 1·06, 1·7)), P=0·013 for urinary equol). It was noted, however, that dietary intake was low, with only 3% of the study population consuming soya foods and mean urinary equol excretion was 0·5 (sd 2·1) μg/mmol creatine. A follow-up to this study that included 237 cases and 952 controls (aged 45–75 years), however, reported no association between serum or urinary equol and breast cancer risk, although urinary equol excretion was associated with an increased risk of cancer in those women with ER-positive tumours(Reference Ward, Chapelais and Kuhnle50). It is plausible that inconsistent findings from epidemiological studies conducted in western populations investigating the relationship between equol and breast cancer risk are due to the low isoflavone intakes among these groups. Isoflavone intake in Europe and USA is about 3 mg/d(Reference Messina51) and the European Prospective Investigation of Cancer and Nutrition study outlined earlier(Reference Grace, Taylor and Low49) reported mean dietary intakes of only 0·2 mg/d daidzein and 0·2 mg/d genistein.
Breast cancer biomarkers have also been studied, to a limited extent, in relation to equol excretion in human subjects. Duncan et al. (Reference Duncan, Merz-Demlow and Xu52) supplemented the diet of fourteen premenopausal women with 0·15, 1·0 and 2·0 mg isoflavones/kg bodyweight per day and reported that for all doses tested, equol producers had a more favourable hormonal profile compared to the equol non-producers. Equol producers generally had lower concentrations of oestrone, oestrone-sulphate, testosterone, androstenedione, dehydroepiandrosterone, dehydroepiandrosterone-sulphate and cortisol and higher levels of sex hormone binding globulin, a profile associated with lower risk of breast cancer. In addition, Nettleton et al. (Reference Nettleton, Greany and Thomas53) have shown that in post-menopausal women, equol producer status is associated with increased concentrations of urinary 2-hydroxyestrogens and an increased 2-hydroxyestrogen:16α-hydroxyestrone ratio. 2-hydroxyestrogen:16α-hydroxyestrone has previously been reported to be inversely associated with breast cancer risk(Reference Kabat, Chang and Sparano54–Reference Fishman, Schneider and Hershcope56) although not all studies support this finding(Reference Ursin, London and Stanczyk57, Reference Adlercreutz, Fotsis and Hockerstedt58). Equol producer status may also confer protection on mammographic density, an established marker positively associated with breast cancer risk. Frankenfeld et al. (Reference Frankenfeld, McTiernan and Aiello59) studied fifty-five overweight post-menopausal women given a soya challenge (soya protein containing >10 mg daidzein/d for 3 d) to determine equol status and reported that mammographic density was 39% lower in equol producers compared to non-producers (P=0·04). Although Fuhrman et al.(Reference Fuhrman, Teter and Barba60) found no independent association of equol status or soya intake with percent mammographic density, among post-menopausal equol producers (n 75), those who consumed soya on a weekly basis had a significantly lower breast density. In contrast, for the equol non-producers (n 157), weekly soya intake was associated with increased mammographic density, suggesting that soya may exert differential effects on breast tissue depending on equol producer status. In a recent study among premenopausal women in USA (n 200), no differences were found in mammographic breast density between equol producers and non-producers(Reference Atkinson, Newton and Aiello Bowles61).
Although the mechanisms of action of soya isoflavones in breast and prostate cancer have been well studied(Reference Magee and Rowland62), little research has focused on equol specifically. In vitro studies have demonstrated that equol, at concentrations ⩽10 μm, stimulates the proliferation of ER positive breast cancer cells(Reference Zava, Blen and Duwe63–Reference Magee, Raschke and Steiner65), whereas at higher concentrations (≥10 μm), proliferation of the oestrogen-insensitive breast cancer cell line MDA-MB-231 is inhibited(Reference Schmitt, Dekant and Stopper64, Reference Magee, Raschke and Steiner65). We have also demonstrated that racemic equol and S-equol inhibit the invasion of MDA-MB-231 breast cancer cells through matrigel and that racemic, but not S-equol, protects MCF-10A breast cells against DNA damage induced by 2-hydroxy-4-nonenal or menadione(Reference Magee, Raschke and Steiner65). This latter finding is of particular interest as it implicates the R-equol rather than the S-equol enantiomer as being responsible for this antigenotoxic effect and warrants further investigation. In support of our findings, another recent study(Reference Brown, Belles and Lindley13) has highlighted the potential beneficial effects of the unnatural R-equol enantiomer in breast cancer. In this study, the effects of a diet supplemented with 250 mg/kg of S- or R-equol on dimethylbenz(a)anthracene-induced mammary tumours in rats were investigated. While S-equol was found to have no chemopreventive effects, R-equol significantly reduced the number of tumours detected, increased tumour latency and reduced the invasive capacity of the tumours. Both R- and S-equol enantiomers therefore deserve further investigation as potential anti-carcinogenic agents in the breast.
There have been concerns over the safety of isoflavones due to their oestrogen-like properties, particularly in relation to breast cancer. The suggestion that isoflavones may have adverse effects in women with breast cancer or at increased risk of the disease has arisen mainly from animal studies and is generally not supported by clinical and epidemiological studies. However, little is known regarding the effects of isoflavones on breast tissue and so these women should perhaps be cautious with regard to their isoflavone intake(Reference Messina and Wu66). The European Food Safety Authority is currently conducting a safety evaluation and will assess the hazards to human health and also the potential benefits of isoflavones, foods rich in isoflavones and isoflavone supplements.
Equol and prostate cancer
Prostate cancer is the second most common cancer in men worldwide(36). Similar to breast cancer, prostate cancer incidence is much higher (3–30-fold) in the western world in comparison with Asian countries(Reference Ferlay, Shin and Bray37). A case–control study(Reference Akaza, Miyanaga and Takashima67) that examined the equol producer status among residents with prostate cancer and controls in Japan, Korea and USA reported that the number of equol producers was significantly lower in the prostate cancer group compared to controls for both the Japanese (29% cases, 46% controls) and Korean (30% cases, 59% controls) cohorts, suggesting that the ability to produce equol is protective against prostate cancer. As expected, the number of equol producers in USA cohort (17% cases, 14% controls) was lower compared to the Asian groups. In another case–control study nested in a community-based cohort in Japan(Reference Ozasa, Nakao and Watanabe68), equol status was examined in relation to prostate cancer risk in 52 cases and 151 controls aged ≥40 years from 45 areas of Japan. Serum equol was inversely associated with prostate cancer risk, with the highest tertile of serum equol associated with a 60% reduction in disease risk.
As first reported in 2004, equol is a potent antagonist of DHT(Reference Lund, Munson and Haldy34), one of the key sex hormones implicated in prostate cancer development. Unlike other 5α-reductase inhibitors, equol does not affect the synthesis of DHT. Instead equol appears to bind directly to DHT, thus preventing DHT binding to the androgen receptor. In a recent Japanese intervention trial(Reference Tanaka, Fujimoto and Chihara18), twenty-eight healthy volunteers were given 60 mg soya isoflavones (containing 19·1 mg daidzein) daily for 3 months and the effects on sex hormones implicated in prostate cancer development was investigated. Following supplementation, serum sex hormone binding globulin increased and serum-free testosterone and DHT decreased significantly compared to baseline concentrations. When subjects were stratified according to equol status, these beneficial effects were only significant in those men who were equol producers (64%), further implicating equol as a chemopreventive agent in prostate cancer. Of interest, in this study, two of the volunteers who had no detectable serum equol (<0·5 μg/l) at baseline, had detectable concentrations of equol in their serum (1·1 μg/l and 24·1 μg/l) following three months of soya supplementation, suggesting that equol producer status can be manipulated with prolonged and consistent soya isoflavone consumption. This theory is further supported by Hedlund et al.(Reference Hedlund, Maroni and Ferucci22) who reported that Caucasian men who consumed ≥30 mg soya isoflavones/d for at least 2 years were 5·3 times more likely to produce equol than men who consumed ⩽5 mg/d.
In vitro studies have demonstrated that equol inhibits the proliferation of benign human prostatic epithelial cells, causing an accumulation of cells in G0/G1 of the cell cycle(Reference Hedlund, Johannes and Miller69), with the effects of equol being 10-fold more potent than that of daidzein. Equol has also been shown to inhibit the proliferation of the prostate cancer cell lines 22Rv1(Reference Hedlund, Johannes and Miller69), DU145(Reference Hedlund, Johannes and Miller69), LAPC-4(Reference Magee, Raschke and Steiner65, Reference Hedlund, Johannes and Miller69), LNCaP(Reference Magee, Raschke and Steiner65, Reference Mitchell, Duthie and Collins70) and PC-3(Reference Mitchell, Duthie and Collins70). Inhibition of growth occurred at physiologically relevant equol concentrations (⩽10 μm) given that equol concentrations in the prostatic fluid of men consuming a soya-rich diet can be as high as 13·5 μm(Reference Hedlund, Johannes and Miller69, Reference Morton, Chan and Cheng71). Equol has also been shown to inhibit the invasion of PC-3 prostate cancer cells through matrigel(Reference Magee, Raschke and Steiner65) , an effect that was only significant at an equol concentration of 50 μm. Although tissue concentrations of equol are unknown, daidzein concentrations in prostate tissue have been reported to be ten-fold higher in comparison with serum levels(Reference Gardner, Oelrich and Liu72) and therefore the possibility of a concentration of 50 μm equol being physiologically relevant in the tissue cannot be excluded.
Equol and CVD
CVD is one of the leading causes of premature death in the UK(73). High soya intake has been associated with a lower incidence of CVD(Reference Lichtenstein74, Reference Nagata75) and in 1999 the Food and Drug Administration approved a health claim for CHD based on the cholesterol-lowering effects of soya protein(76), a claim supported by the Joint Health Claims Initiative in the UK in 2002. Recent meta-analyses, however, demonstrate that the cholesterol-lowering effects of soya protein, though still relevant, are much smaller (about 3–5%)(Reference Sacks, Lichtenstein and Van Horn77–Reference Taku, Umegaki and Sato80) than initially reported (12·9%)(Reference Anderson, Johnstone and Cook-Newell81). European Food Safety Authority recently concluded that a cause and effect relationship has not been established between the consumption of soya protein and the reduction of LDL-cholesterol concentrations(82). In addition, the Food and Drug Administration is currently re-evaluating its soya protein health claim.
Although a wealth of studies has investigated the effects of soya on heart health, few have stratified subjects according to equol producer status. In a randomized, placebo-controlled crossover trial in which twenty-six mildly hypercholesterolaemic subjects consumed soya foods containing 80 mg isoflavones/d or placebo for 5 weeks, no significant differences in plasma lipids, blood pressure or arterial compliance were observed(Reference Meyer, Larkin and Owen83). Retrospective analysis, however, showed that in the equol producers (35% of cohort), significant reductions in total cholesterol (8·5%), LDL cholesterol (10%), LDL:HDL (13·5%), plasma TAG (21%) and lipoprotein(a) (11%) were observed with the soya diet, suggesting that it may only be those capable of producing equol that benefit from the lipid-lowering effects of soya. In contrast, in another crossover study, Hall et al.(Reference Hall, Vafeiadou and Hallund84) found no effect of an isoflavone-enriched cereal bar (providing 50 mg isoflavones/d) on plasma lipids or blood pressure in post-menopausal women, with no differences observed between equol producers (28%) and non-producers. This study, however, involved healthy post-menopausal women (aged 45–70 years) who likely had a low risk of CVD at baseline which may explain why no significant effects were detected. Furthermore, in addition to the fact that a lower isoflavone dose was used in this study, in contrast to the previous study(Reference Meyer, Larkin and Owen83), isolated isoflavones added to a cereal bar were used rather than a whole soya food, which highlights the potential importance of the food matrix used in these studies.
In a 12-month double-blind, randomized trial, Kreijkamp-Kaspers et al.(Reference Kreijkamp-Kaspers, Kok and Bots85) compared the effects of soya protein (providing 99 mg isoflavones/d) to milk protein on blood pressure and endothelial function in 202 post-menopausal women aged 60–75 years. For the equol producers within the soya group (28%), both systolic and diastolic blood pressure decreased and endothelial function improved following intervention, whereas blood pressure increased and endothelial function deteriorated in the non-producers (Fig. 3). These beneficial outcomes in the equol producers were not significantly different from the non-producers possibly due to the small number of equol producers within the study.

Fig 3. The effects of a soya supplement (99 mg isoflavones/d for 12 months) on blood pressure (BP) and flow-mediated dilation (FMD) in healthy postmenopausal women(Reference Kreijkamp-Kaspers, Kok and Bots85). (P values represent repeated-measures ANOVA for time×equol-status interaction.)
In one of the first studies to examine the relationship between equol production and CVD risk markers within a Chinese population(Reference Guo, Zhang and Chen86), it was observed that equol producers, who comprised 50% of the cohort of 202 adults aged 20–69 years, had lower serum uric acid, TAG and waist:hip ratio and tended to have higher LDL cholesterol compared to non-producers, suggesting that equol phenotype may influence CVD risk.
Equol and bone health
Due to the oestrogenic properties of isoflavones, their effects on bone have been widely investigated(Reference Ma, Qin and Wang87); however, the European Food Safety Authority has recently concluded that there is insufficient evidence to establish a cause and effect relationship between the consumption of soya isoflavones and the maintenance of bone mineral density (BMD) in post-menopausal women(88). Few studies have stratified subjects according to equol producer status. In a two-year randomized, placebo-controlled trial, Lydeking-Olsen et al.(Reference Lydeking-Olsen, Beck-Jensen and Setchell89) investigated the effects of soyamilk (providing 76 mg isoflavones/d), natural transdermal progesterone or the combination, on BMD and bone mineral content (BMC) in the lumbar hip and spine in post-menopausal women who either had established osteoporosis or had three or more risk-factors for the disease. The percentage change in BMD and BMC in the lumbar spine was not significantly different from baseline in the soya isoflavone or transdermal progesterone group, whereas BMD and BMC significantly decreased in the groups receiving the combined treatment (−2·8% BMD loss, −2·4% BMC loss) or the placebo (soyamilk without isoflavones) (−4·2% BMD loss, −4·3% BMC loss). Equol producers (n 10) showed a 2·4% and 2·8% increase in BMD and BMC, respectively, in comparison with equol non-producers (n 12) who had corresponding increases of 0·6% and 0·3%. Although these findings were not statistically significant, doubtless due to the small numbers involved, they highlight the potentially important role of equol in bone health.
In contrast to the above study, in exploratory analyses, Frankenfeld et al.(Reference Frankenfeld, McTiernan and Thomas90) reported that among post-menopausal women who regularly consumed soya (>1 serving of tofu or soyamilk per week, n 10), spinal BMD was 20% lower among equol producers than non-producers. Numbers investigated in this study were, however, low (five equol producers and five non-producers) and clearly further studies that are appropriately designed and adequately powered are needed to investigate this association sufficiently.
Equol and menopausal symptoms
In 1992, Adlercreutz postulated that the oestrogen-like effects of soya isoflavones may account for the low prevalence of hot flushes and other menopausal symptoms in Japanese women(Reference Adlercreutz, Honjo and Higashi43). Subsequently, studies have shown that soya isoflavones reduce menopausal symptoms, in particular hot flushes, by up to 50%(Reference Kurzer91). In a study of 180 Japanese women(Reference Uchyama, Ueno and Shirota92)in which the severity of menopausal symptoms was assessed by questionnaire, it was found that only 5% of these women suffered from hot flushes. Of interest was the finding that the equol producers (53·5% of the group) reported the least severe menopausal symptoms. More recently, Ishiwata et al.(Reference Ishiwata, Melby and Mizuno93) investigated the effect of a natural S-equol-containing supplement on menopausal symptoms in a randomized placebo-controlled trial in Japanese women. The supplement used in this study, as mentioned previously, was developed by a pharmaceutical company in Japan, and was prepared by the fermentation of soya germ with the bacteria L. garvieae (Reference Yee, Burdock and Kurata21). In Ishiwata's study, 134 Japanese women were randomly assigned to receive either placebo, 10 mg equol/d or 30 mg equol/d (10 mg three times daily) for 12 weeks, with habitual isoflavone intake limited to 20 mg/d. Menopausal symptom and Profile of Mood States questionnaires were completed at baseline and post intervention. Mood-related symptom scores were significantly improved following supplementation with 30 mg equol/d in perimenopausal/post-menopausal equol non-producers, the group most likely to benefit from equol supplementation. Follow-up studies have demonstrated that 10 mg S-equol significantly improved shoulder stiffness and reduced the frequency and severity of hot flushes in Japanese post-menopausal equol non-producing women(Reference Aso94). Thus, S-equol supplementation may be a potential alternative treatment for the management of menopausal symptoms.
Conclusions
Despite the abundance of studies that have investigated the effects of soya and isoflavones on health, a few studies have been adequately designed and appropriately powered to examine the influence of equol producer status. Although research to date suggests that the ability to produce equol may confer protection against breast and prostate cancer, CVD, bone disease and menopausal symptoms, clinical intervention trials with S-equol are needed to confirm or refute the ‘equol hypothesis’. Such trials are now possible with the development of the equol-rich soya product SE5-OH, a new functional food ingredient that relies on the bacterial conversion of daidzein to S-equol(Reference Yee, Burdock and Kurata21). The effects of this supplement on menopausal symptoms have been investigated as outlined above. SE5-OH is not genotoxic and the no-observed-adverse-effect-level was 2000 mg/kg per d (the highest dose tested) in a subchronic feeding study in rats(Reference Yee, Burdock and Kurata21). In addition, reproductive and developmental toxicity studies in rats have defined no-observed-adverse-effect-levels of 1000 mg/kg per d (6·5 mg equol/kg per d) and 2000 mg/kg per d (13 mg equol/kg per d), respectively(Reference Matulka, Matsuura and Uesugi95). The pharmacokinetics of SE5-OH has also been investigated in healthy post-menopausal women(Reference Setchell, Zhao and Shoaf96). Following oral ingestion, S-equol was reported to be rapidly absorbed from the supplement, was highly bioavailable and had a terminal elimination half-life of 8 h.
Limited in vitro research has examined the mechanisms of action of equol specifically and it is important that more research is conducted in this area so that, together with clinical intervention studies, the precise role of equol in human health can be elucidated.
Acknowledgements
The author declares no conflict of interest. Some of this work was supported by the Commission of the European Communities specific RTD programme ‘Quality of Life and Management of Living Resources’ (project no. QLK-2000-00266 and Marie-Curie Programme Training Site grant no. HPMT-CT-2001-00287). It does not necessarily reflect its views and in no way anticipates the Commission's future policy in this area.







