What role for fatty acids and what recommendations?
In brief, fatty acids are the main energy source on the basis of 1 g = 38 kJ v. 17 kJ (9 kcal v. 4 kcal) for carbohydrates (CHO) and proteins. They include SFA, MUFA, PUFA, which include n-6 and n-3 PUFA and trans fats. Recent recommendation of a healthy diet from WHO dated 24 August 2018 stated: Evidence indicates that total fat should not exceed 30% of total energy intake (TEI) to avoid unhealthy weight gain. Intake of SFA should be less than 10% TEI and trans fats less than 1% TEI, with a shift in fat consumption away from SFA and trans fats to unsaturated fats, and towards the elimination of industrial trans fats. The Dietary Reference Values for fat in UK, published by the Committee on Medical Aspects of Food Policy in 1991 and 1994(1,2) promote, as expressed as % TEI, a total fat intake ≤35 %, of which SFA ≤10 %, total PUFA 6 %, α-linolenic acid (ALA) ≥0·2 %, linolenic acid ≥1% and trans fat ≤2 %.
In 2004, the Scientific Advisory Committee on Nutrition endorsed the population recommendation (including pregnant women) of an amount of long-chain (LC) n-3 PUFA of 450 mg/d(3). However, other international recommendations have been published about optimal intake of LC n-3 PUFA(Reference Legrand, Morise and Kalonji4–Reference Rimm, Appel and Chiuve7), which may vary depending on the country and/or the physiological state and/or if secondary cardiovascular risk is taken into consideration.
Before looking at the repartition of consumption of fatty acids in Africa and their sources of production, it is useful to summarise the today burden of nutritional diseases in Africa for the following reasons : (a) fatty acids are a source of energy; (b) excess consumption of SFA and/or trans fats increases cardiovascular risk; (c) LC n-6 PUFA as a substitute for an excess amount of SFA are protective of cardiovascular risk; (d) LC n-3 PUFA are beneficial for health in many aspects: inflammation, type 2 diabetes (T2DM), metabolic syndrome, cardiovascular risk, brain and retina development, depression cognition.
Undernutrition and non-communicable diseases in Africa
Undernutrition
According to FAO UN 2017-Regional Overview of Food Insecurity Africa report(8), the prevalence of undernourishment dropped between 2000 and 2010 in all parts of Africa, especially in sub-Saharan Africa (from 28·1 to 20·6 %). From 2010 to 2015, it remained flat but rose from 2015 to 2016 (20·8–22·7 %). These trends were similar in all regions of the continent but with huge level differences. In 2016, the prevalence of undernourishment was 33·9 % in Eastern, 25·8 % in Middle, 22·7 % in sub-Saharan, 11·5 % in Western and 8 % in Southern Africa, i.e. the prevalence was four times higher in Eastern than in Southern Africa. Translated to the number of people concerned, there were, in 2016, 224·3 million people undernourished in sub-Saharan, 137·2 in Eastern, 41·6 in Western, 40·4 in Middle and 5 in Southern Africa.
Stunting in children under age 5 years involved 154·8 million in 2015 whose 34·1 % lived in sub-Saharan Africa(8). In all regions of Africa, stunting decreased steadily from 2000 to 2016; by example, it decreased from 45 to 36·7 % in Eastern Africa, where the prevalence is the highest. Wasting, on the difference of stunting, which is an indicator of chronic undernutrition is an indicator of recent and severe weight loss. In 2016, 51·7 million children (7·7 %) suffered wasting in Africa, with the highest prevalence in Western, sub-Saharan and Middle Africa. All African regions are above the 5 % target of World Health Assembly for wasting with some differences. In Southern Africa, 2/3 of countries are on track to meet the World Health Assembly target whereas the progress is lower in countries of Western and Middle Africa.
Non-communicable diseases
On the other side of nutritional status, obesity in adults is increasing in adults in every country of Africa from 2005 to 2014. The highest prevalence is observed in Southern Africa reaching 15 % in men and about 37 % in women, especially in South Africa. In all regions of Africa, the prevalence of obesity is higher in women than in men on difference with Western Europe and with Caucasian Americans. In Mauritius and Seychelles (Eastern Africa), the prevalence is as high as in South Africa(8). Obesity in children increased from 5·0 to 5·2 % from 2005 to 2014 in Africa with disparities between the regions. It increased during that period from 10·6 to 11·8 % in Southern Africa and from 8·9 to 10 % in Northern Africa, whereas it remained stable at a lower level in the three other regions(9).
Diabetes is reported in International Diabetes Federation Atlas 2017(10) to be the lowest in the world with an age-adjusted comparative prevalence of 4·4 % (prevalence in America is the highest at 11 %). However, undiagnosed diabetes is reported to be as high as 69 % as compared with 38 % in North America and in Europe. High cardiovascular risk is low in Africa as compared with other continents(11).
Fatty acids consumption in Africa
Micha et al.(Reference Micha, Khatibzadeh and Shi12) have identified by region, country, time, age and sex-specific dietary fats and oils consumption in 1990 compared with 2010 in 266 country-specific nutrition surveys. Global average consumption of SFA was 9·4 % TEI with a range across twenty-one global regions from 4·3 to 23·5 % (5·5-fold). Seventy-five countries (61·8 %; 2·73 billion people) achieved optimal intake <10 % TEI. In Africa as a whole regional average for SFA is between 8·9 and 12·5 % TEI (North: 9·1 %, Central: 12·2, Eastern: 10·7 %, Southern: 8·9 % and Western Africa: 12·5 %), so not very far from the ≤10 % TEI recommended by Dietary Reference Values and WHO. The lowest intake was in Lesotho (4·5 %) and the highest in Somalia (17·8 %).
Global average consumption of n-6 PUFA was 5·9 % TEI, with a range from 2·5 to 8·5 %. In Africa as a whole regional consumption was between 3·9 and 5·7 % (North: 5·7 %, Central: 4·7 %, Eastern: 3·9%, Southern: 5·2 % and Western: 4·2 %). The lowest intake was in Liberia (2·2 %) and the highest in Central African Republic (8·5 %).
Africa average consumption of trans fats was low 1·2 % (North: 2·4 %, Central: 0·8 %, Eastern: 0·8 %, Southern: 1 % and Western: 0·9 %). The highest level was in Egypt (6·5 %), which is very high as compared with recommendations (Dietary Reference Values <2 %; ideally 0 %). Almost all African countries had a consumption <1 % TEI.
Africa average of ALA was 836 mg/d (North: 1561 mg/d, Central: 848 mg/d, Eastern: 394 mg/d, Southern: 633 mg/d and Western: 745 mg/d). The highest consumption was in Tunisia (2215 mg/d), the lowest in Comoros (126 mg/d). It is to note that palm oil is the main type of oil available in Africa (40·8 %), which contributes to explain relatively low consumption of ALA(Reference Petrova, Dimitrov and Willett13).
Africa average of LC n-3 PUFA (60·7 mg/d) was very low (North: 66·5 mg/d, Central: 73 mg/d, Eastern: 52 mg/d, Southern: 13 mg/d and Western: 99 mg/d). The highest consumption was in Seychelles (1291 mg/d), the lowest in Zimbabwe (5 mg/d). The very low consumption of LC n-3 PUFA is explained by low fish availability (average of 127 g/week)(Reference Petrova, Dimitrov and Willett13). As a comparison, fish availability is 524 g/week in Southeast Asia and 523 g/week in Europe(Reference Petrova, Dimitrov and Willett13). In most countries in Africa both availability of fish and ALA are low (respectively, <400 g/week and <4% TEI). Several African countries have both a very low fish (<200 g/week) and ALA (<2 % TEI) availability(Reference Petrova, Dimitrov and Willett13). Per capita food fish consumption in Africa (9·9 kg/year) is with Latin America and the Caribbean (9·8 kg/year) the lowest in the world(14). As a comparison food fish consumption in Asia is 24 kg/year, in Europe 22·5 kg/year, North America 21·6 kg/year and Oceania 25 kg/year(14).
In summary, SFA consumption is globally low. Some efforts should be made in some countries to try to reach ≤10 % TEI. Trans fat is low (except in Egypt) but zero would be better everywhere else but political decisions are involved. n-3 PUFA (especially LC n-3 PUFA) consumption is too low in every African country so that the ratio n-6:n-3 is very high. This low consumption is directly explained by a low fish availability and consumption and the preferential use of palm oil.
Fatty acids intake and health
At a time where some controversy may have arisen about the deleterious and/or health beneficial effects of total fat intake (TFI) and of the different types of fatty acids intake, it is useful to try to delineate what is presently known and not only thought. Fat may have effects on total mortality, cardiovascular mortality and events and on other illnesses.
Industrially produced trans fatty acids formed during partial hydrogenation of vegetable oils to form margarine and shortening have been proved to increase cardiovascular risk(Reference Mozaffarian, Katan and Ascherio15). Wang et al.(Reference Wang, Li and Chiuve16) have assessed the specific effects of dietary fats assessed every 2–4 years, on total and cause-specific mortality in two well-known cohorts, i.e. the Nurses’ Health Study (National Health and Nutrition Examination Survey; 1980–2012; 2 464 852 person-years) and Health Professionals Follow-up Study (1986–2012; 975 102 person-years). They investigated 83 349 women from National Health and Nutrition Examination Survey and 42 884 men from Health Professionals Follow-up Study.
Comparisons were made by quintiles of fat intake. The percentage energy intakes from TFI and specific fats were calculated as cumulative average up to the start of each 2 or 4-year follow-up interval. For multivariable analyses, isoenergetic substitution models were built, which simultaneously included energy intake, the percentages of energy derived from protein and specific types of fat and other potentially confounding variables. Thus, as underlined by the authors, the coefficients from these models can be interpreted as the estimated effect of substituting a certain percentage of energy from fat for equivalent energy from CHO. The relative risk (RR) by quintiles is reported in Table 1. An RR > 1 means that the factor is a risk factor, RR = 1 the factor has no effect; if RR < 1, the factor is a protective factor. The CI indicates whether this is not by chance. If the CI (95 %) of the RR contains the value 1, it is not possible to conclude. By using these models, TFI was slightly inversely associated with total mortality (RR: 0·95; P for trend <0·001). For an amount of TFI reaching 42·2 % of TEI (highest quintile), RR of total mortality was decreased by 16 % (Table 1). When substituted for CHO, SFA were positively associated with total mortality (RR: 1·08; P for trend <0·001). PUFA (RR: 0·73; P for trend <0·001) and MUFA (RR: 0·90; P for trend <0·001) were inversely associated with total mortality. Trans fat was positively related to increased mortality (RR: 1·16; P for trend <0·001). When 5 % of energy from SFA was replaced by the same energy from PUFA or MUFA, the estimated reduction of total mortality was, respectively, decreased by 27 and 13 %. Trans fatty acids were associated with a 20 % increase in cardiovascular mortality (RR: 1·20; P for trend <0·001); SFA intake, when substituted for total CHO, was not related to cardiovascular mortality. PUFA and MUFA intakes were inversely associated with cardiovascular mortality (both P for trend <0·001). However, the replacement of 5 % of SFA by CHO from whole grains was associated with a 10 % lower risk of CHD whereas it was not if replaced with refined CHO(Reference Li, Hruby and Bernstein17, Reference Chen, Li and Sun18). When 5 % of SFA intake was replaced by MUFA or PUFA, the risk of CHD decreased respectively, by 15 and 20 %(Reference Li, Hruby and Bernstein17).
Table 1. Associations between total and specific types of fat intakes and total mortality (comparison is isoenergetic substitution for total carbohydrate; from ref 15)

RR, relative risk; MV, multivariable
In summary, as compared with an isoenergetic amount of CHO, TFI is inversely associated with mortality; as fat intake increases to the fifth quintile of intake (42·2 % of TEI), multivariate RR for total mortality decreases to 0·84. The type of fatty acids is positively associated with mortality for trans and SFA and inversely associated with MUFA and PUFA. For the prevention of CHD, an excess intake of SFA (>10 % TEI) should be replaced mainly by MUFA and PUFA and not by refined CHO.
Why Africa should increase its availability and consumption of long-chain n-3 PUFA
As reported earlier, the average consumption of LC n-3 PUFA is very low in Africa. Most international recommendations propose an average daily dose of about 500 mg/d. LC n-3 PUFA have been proved to have many beneficial health effects. In this review, we will only consider the beneficial effects towards total and cardiovascular mortality, prevention of cardiovascular risk and of T2DM and on components of metabolic syndrome.
Long-chain n-3 polyunsaturated fatty acids and insulin resistance
The effects of LC n-3 PUFA on insulin resistance have been recently reviewed(Reference Delarue and Guriec19); we will only briefly discuss this aspect. It is established that LC n-3 PUFA do not have a corrective effect on insulin resistance once installed. This is confirmed by the global meta-analysis of Abbott et al.(Reference Abbott, Veysey and Lucock20) involving twenty-six studies. However, when the analysis is done in subgroups, taking into account studies of more than 6 weeks and by sex, LC n-3 PUFA increase insulin sensitivity in women (P = 0·045) but not in men (P = 0·313). The risk of T2DM in elderly women (age 77 (sd 7) years; BMI ≥ 27) is inversely associated with quartiles of the EPA + DHA content of erythrocyte membranes (RR = 1·0 (reference), 0·82, 0·56 and 0·22; P = 0·015), which is in favour of a protective effect. Despite the same trend among men, it was NS. Experimental data and studies in human subjects have shown their ability to prevent insulin resistance(Reference Delarue and Guriec19). In a very recent meta-analysis(Reference Gao, Geng and Huang21) involving seventeen randomised control trials (RCT) using a high dose of LC n-3 PUFA (1−4 g/d EPA + DHA), their increasing effect on insulin sensitivity was concluded, but only in the subgroup of subjects with at least one metabolic alteration. Recent reviews have detailed the basic mechanisms underlying the beneficial effects of LC n-3 PUFA towards insulin resistance(Reference Lepretti, Martucciello and Burgos Aceves22–Reference Pahlavani, Ramalho and Koboziev24). The protective effect of LC n-3 PUFA towards the risk of developing T2DM is less clear, because of a great geographical heterogeneity. Sub-analysis in meta-analysis of cohorts by Chen et al.(Reference Chen, Yang and Yu25) showed that LC n-3 PUFA was inversely associated with T2DM risk in Asians (RR = 0·82, P < 0·001); whereas the risk was increased in Westerners (RR = 1·30, P < 0·001).
n-3 PUFA and cardiovascular risk
The effects of LC n-3 PUFA on cardiovascular risk have been recently reviewed(Reference Wang and Hu26–Reference O'Mahoney, Matu and Price30). These reviews as well as a recent meta-analysis including both cohort studies and RCT(Reference Alexander, Miller and Van Elswyk31) have concluded that LC n-3 PUFA have a secondary preventive effect on cardiovascular mortality, but not on primary prevention. The meta-analysis performed by Alexander et al.(Reference Alexander, Miller and Van Elswyk31) concluded that among RCT, there was a non-statistically significant reduction in CHD risk with EPA + DHA provision (RR: 0·94; 95 % CI 0·85, 1·05). Subgroup analyses of data from RCT indicated a statistically significant CHD risk reduction with EPA + DHA provision among higher-risk populations, including participants with TAG levels ≥1·50 g/l (RR: 0·84; 95 % CI 0·72, 0·98) and LDL cholesterol ≥1·30 g/l (RR: 0·86; 95 % CI 0·76, 0·98). A meta-analysis from prospective cohort studies concluded to a significant RR of 0·82 (95 % CI, 0·74, 0·92). The most recent meta-analysis and sensitivity analyses(Reference Siscovick, Barringer and Fretts32) suggested little or no effect of increasing LC n-3 on all-cause mortality (RR 0·98 in thirty-nine trials, high-quality evidence), cardiovascular mortality (RR 0·95 in twenty-five RCT), cardiovascular events (RR 0·99 in thirty-eight trials, high-quality evidence), CHD mortality (RR 0·93 in twenty-one RCT). There was a suggestion that LCn-3 reduced CHD events (RR 0·93 in twenty-eight RCT) but this was not confirmed in sensitivity analyses. Globally all evidence was of moderate GRADE quality, except as noted. The controversy that has arisen during past years over their real cardiovascular protective effect is mainly due to the confounding effect of the association of drugs given to patients with CHD, especially statins(Reference Bird, Calder and Eggersdorfer28). In addition, the difference between Alexander's and Abdelhamid's meta-analysis is the inclusion, in the former one, of prospective cohort studies. Altogether, this analysis strongly suggests that a sufficient long duration of intake of dietary LC n-3 PUFA is required to obtain a secondary preventive effect and/or that patients at high cardiovascular risk are more likely to obtain benefits for LC n-3 PUFA supplementation with a dosage about 1 g/d EPA + DHA. The Science Advisory Board from the American Heart Association proposed in 2017(Reference Siscovick, Barringer and Fretts32) this recommendation: ‘Although recent RCT evidence has raised questions about the benefits of n-3 supplementation to prevent clinical CVD events, the recommendation for patients with prevalent CHD such as a recent myocardial infarction remains essentially unchanged. Treatment with n-3 PUFA supplements is reasonable for these patients. Even a potential modest reduction in CHD mortality (10%) in this clinical population would justify treatment with a relatively safe therapy’. The same Board in 2018 also proposed in the context of 2015–2020 Dietary guidelines for American people that one to two seafood meals per week be included to reduce the risk of congestive heart failure, CHD, ischemic stroke and sudden cardiac death, especially when seafood replaces the intake of less healthy foods(Reference Rimm, Appel and Chiuve7).
Concerning ALA, Abdelhamid's meta-analysis(Reference Abdelhamid, Brown and Brainard33) concluded that increasing ALA intake probably makes little or no difference to all-cause mortality, cardiovascular mortality, CHD events and probably reduces risk of CHD mortality (1·1 to 1·0 %, RR 0·95 in three RCT), and arrhythmia (3·3 to 2·6 %, RR 0·79 one RCT). The evidence was of low-quality GRADE.
Hall, in this journal(Reference Hall34) has given a very pragmatic conclusion we reproduce here. ‘RCT investigating effects of supplementation on prevention of CHD in populations with low basal LC n-3 PUFA tissue status are lacking, and so the clinical benefits of supplementing non-fish-eating groups with vegetarian sources of n-3 PUFA remain to be determined. Refocusing dietary LC n-3 PUFA intervention studies towards those individuals with a low LC n-3 PUFA tissue status may go some way towards reconciling results from RCT with the epidemiological evidence.’
Should Africa increase its consumption of olive oil?
The protective cardiovascular effect of a Mediterranean diet was first evocated after the publication of 10-years results of cardiovascular mortality of the Seven Countries Study(Reference Mariotti, Capocaccia and Farchi35). This very well-known study including people from seven countries showed a North–South gradient of 10-years CHD mortality, the lowest been observed in Crete, the highest in East Finland. The analysis by Mariotti et al.(Reference Mariotti, Capocaccia and Farchi35) suggested that ‘major risk factors existed other than the standard widely used blood pressure, smoking, cholesterol and age, which accounted for approximately 50% of the different incidence between North and South European cohorts’. At 15-years follow-up another paper has taken into account diet as a variable, which could contribute to explain the difference of mortality between the fifteen cohorts of the Seven Countries Study(Reference Keys, Menotti and Karvonen36). The comprehensive conclusion of the authors was that differences in mean age, blood pressure, serum cholesterol, and smoking habits explained 46% of variance in death rate from all causes, 80% from CHD, 35% from cancer, and 45% from stroke. The cohorts differed in average diets. Death rates were related positively to average percentage of dietary energy from SFA, negatively to dietary energy percentage from MUFA and were unrelated to dietary energy percentage from PUFA, proteins, carbohydrates and alcohol. All death rates were negatively related to the MUFA:SFA ratio. Inclusion of that ratio with age, blood pressure, serum cholesterol and smoking habits as independent variables accounted for 85% of variance in rates of deaths from all causes, 96% CHD, 55% cancer, and 66% stroke. Oleic acid accounted for almost all differences in MUFA among cohorts. All cause and CHD death rates were low in cohorts with olive oil as the main fat.
Since the Seven Countries Study, many others have highlighted the beneficial effect of a diet rich in olive oil, especially the Mediterranean Diet. The first intervention study is the Predimed Study, which aimed to assess the primary prevention of cardiovascular disease in a population at high cardiovascular risk with a Mediterranean Diet supplemented with extra-virgin olive oil or nuts as compared with a control diet consisting in advice to reduce fat intake(Reference Estruch, Ros and Salas-Salvadó37). The primary end point was a major cardiovascular event (myocardial infarction, stroke, or death from cardiovascular causes). The follow-up was 4·8 years. The incidence of major cardiovascular event was reduced by 31 % with the Mediterranean Diet supplemented with olive oil and by 28 % with the Mediterranean Diet supplemented with nuts.
A very recent meta-analysis(Reference Eleftheriou, Benetou and Trichopoulou38) of the Mediterranean diet and its components in relation to all-cause mortality concluded, on the basis of thirty cohort studies that total mortality was decreased by 21 % in people with the highest score of adhesion to the Mediterranean diet as compared with those with the lowest score. On the basis of four studies included into this meta-analysis, olive oil was not associated with a decreased mortality.
In summary, a Mediterranean diet supplemented with olive oil or nuts decreased primary cardiovascular risk in people at high cardiovascular risk, but there is presently no real proof that olive oil by itself, i.e. independently of a Mediterranean diet has a protective cardiovascular effect or decreases total mortality. Except in North Africa, olive oil is not widely used, so that in other parts of Africa other oil sources such as oils from plants rich in ALA should be considered in addition to increased fish consumption.
n-3 PUFA and metabolic syndrome
Many studies have observed a beneficial effect of LC n-3 PUFA on the components of metabolic syndrome(Reference de la Iglesia, Loria-Kohen and Zulet39–Reference Flachs, Rossmeisl and Kopecky46).
The effects of LC n-3 PUFA on the risk of developing a metabolic syndrome are inconsistent. The more recent meta-analysis included seven case–control and twenty cross-sectional studies. A higher plasma/serum of LC n-3 PUFA was associated with a lower metabolic syndrome risk (Pooled RR = 0·63). The plasma/serum LC n-3 PUFA in controls was significantly higher than cases. There was no significant association between LC n-3 PUFA or fish dietary intake and metabolic syndrome risk(Reference Guo, Li and Shi47).
Fatty acids composition of human milk in Africa
Although this aspect is beyond the scope of this review, it is of interest to briefly summarise the available data because the fatty acid composition of mothers is of major concern for the child growth and brain development. Koletzko et al.(Reference Koletzko, Thiel and Abiodun48) have compared the fatty acid composition of milk in Europe and Africa (Nigeria, Gambia, South Africa, Tanzania, Egypt, Ivory Coast, Uganda). The content in n-3 PUFA was quasi-identical between the two continents, suggesting that metabolic processes of mammary gland are able to adjust these contents according to different dietary intakes when n-3 dietary intake is low. However, Nuyar et al.(Reference Nyuar, Min and Ghebremeskel49, Reference Nyuar, Min and Dawood50) reported in Sudanese women whose traditional diet is rich in CHO, that if the arachidonic acid content of the colostrum, transitional and mature milk were broadly comparable with published international values, in contrast, the DHA level was very low. The authors propose to advise these women the regular consumption of river fish whose content in n-3 PUFA is higher than their milk(Reference Nyuar, Min and Dawood50). Indeed, lactating African women from Nigeria and Tanzania with high fish intakes have a higher content of LC n-3 PUFA, especially of DHA in their milk(Reference Koletzko, Thiel and Abiodun51–Reference Kuipers, Luxwolda and Dijck-Brouwer53). The same is observed in Inuits with a high traditional consumption of fish and seal meat(Reference Innis and Kuhnlein54).
In summary, most of the lactating women living in Africa have a fatty acid content of their milk similar to that of European women. However, some exceptions have been reported as in Sudanese women, which justify to advise them to increase their dietary intake of LC n-3 PUFA by consuming fatty fish.
Which environmentally friendly solution can be proposed to increase fish availability and consumption in Africa?
As the main characteristics of fat intake in Africa is a very low amount in LC n-3 PUFA, it is of crucial importance to help African people to eat more fatty fish, which implies first to increase its availability and secondly to educate and advise people to eat more fish. This must be done while trying to reach the sustainable development goals.
The African Union Development Agency in its 2014–2017 Strategic Plan(55) noted the need for better fisheries management. ‘ The case of fisheries illustrates the shortfall of an inappropriate management of African resources. The resource is underpaid: financial payments (compensation) for Fisheries Access Agreements to developing countries range between 2 and 17% of the catch value, even less than other natural resources such as minerals, forestry and crude oil (usually 30%). Going down the value-chain is deterred by the fact that adding value to fish exports attracts counter-measures (custom tariffs) whereas raw fish is duty-free, quota-free. While some processing does occur in Africa, it represents only a minor share of the final price. Quality and safety standards, such as sanitary and phyto-sanitary standards, often limit the capacity of African countries to trade. Illegal, unreported and unregulated fishing amounts to approximately US $1 billion a year (around 17% of total catch) in Africa and for some countries represents a substantial share (Guinea 50%, Liberia 59%, Somalia 75%, Nigeria 40%, Cote d'Ivoire 45%). Finally, only a small part of added value is kept in Africa’(55).
The 2018 FAO State of World Fisheries and Aquaculture 2018(Reference Imamura, Micha and Khatibzadeh56) reports that world total marine catch remained stable about 80 million tonnes/year since the year 1990. Aquaculture continues to grow faster than other major food production. Its annual growth rates of the 1980 and 1990 reached 11 %, then declined to 5·8 % during the period 2000–2016, although double-digit growth still occurred in Africa from 2006 to 2010. Global aquaculture production in 2016 included 80·0 million tonnes of food fish (54·1 million tonnes of finfish). China in 2016 has produced more farmed fish than the rest of the world combined every year since 1991. The fraction of marine fish stocks fished within biologically sustainable levels has decreased from 90·0 % in 1974 to 66·9 % in 2015. In contrast, the percentage of stocks fished at biologically unsustainable levels increased from 10 % in 1974 to 33·1 % in 2015. In 2015, maximally sustainably fished stocks accounted for 59·9 % and underfished stocks for 7·0 % of the total assessed stocks(Reference Imamura, Micha and Khatibzadeh56). The only country in Africa which is in the top twenty-five of producers (marine capture) is Morocco. In contrast, five African countries (Uganda, Egypt, Tanzania, Nigeria, Democratic Republic of Congo) are in the top sixteen for inland waters capture production. Although aquaculture increases continuously in Africa, it contributes for 2 million tonnes to total fish production in the world (76 million tonnes) similar to Americas and Europe (each 3 million tonnes), but quite less than Asia without China (22 million tonnes) and China 48 million tonnes.
The very positive observation is that Africa is the continent which is projected to increase the most its fish production by 2030 (Table 2)(14).
Table 2. Projected fish production, 2030 (live weight equivalent) (FAO 2018,(Reference Imamura, Micha and Khatibzadeh56))
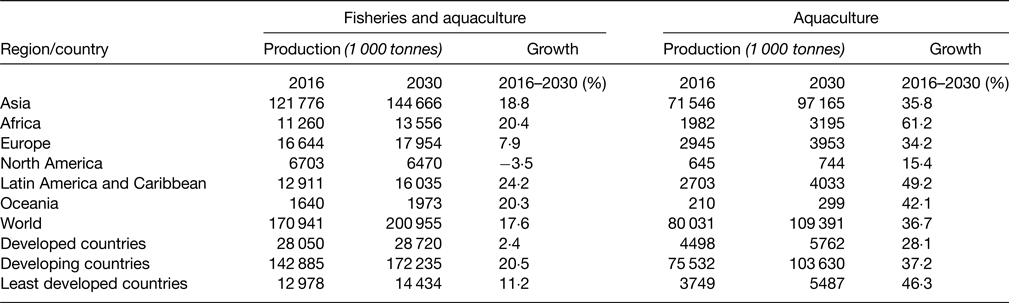
In summary, Africa has a relatively low aquaculture production especially as compared with Asia including China, but in tonnes, it is not very different from Europe and Americas.
The projected growth by 2030 is very high, which is very encouraging both for food fish availability and sustainability of marine resource.
Conclusion
Africa is now facing the triple burden of undernourishment, obesity and micronutrients deficiency. However, the cardiovascular risk and diabetes incidence are low knowing that undiagnosed diabetes seems to be very high. The traditional pattern must be respected because except for n-3 (especially LC n-3 PUFA intake too much low), the contribution of others fatty acids is not so far from Dietary Reference Values or WHO recommendation for good health maintenance. The global dietary quality pattern of Africa is one of the best among 187 countries according to Imamura et al.’s study(Reference Imamura, Micha and Khatibzadeh56). Africa's development of aquaculture provided it develops marine aquaculture as well as global politics promoting consumption of fatty fish would be very useful inasmuch as it could contribute to antagonise, through a higher LC n-3 PUFA intake, the deleterious effect of nutrition transition. Consumption of river fishes with a reasonably high content in LC n-3 PUFA several times weekly may be an alternative in African countries which do not have access to the coastal sea(Reference Nyuar, Min and Dawood50).
Financial Support
None.
Conflict of Interest
None.
Authorship
The author had sole responsibility for all aspects of preparation of this paper.






