- Adj-BW
adjusted body weight
- BWt
total body weight
- CYP
cytochrome-P450
- FM
fat mass
- LBW
lean body weight
- PCM
protein–energy malnutrition
- Vd
volume of distribution
The clinical variability that exists among patients is well-recognized by most clinicians. This inter-individual variability includes the response to a drug which can be accounted for by a number of factors. Appropriate patient care requires an appreciation of the factors that influence the disposition and effect of medication. As used here, the term disposition refers to the absorption, distribution and elimination of a drug; while the term effect reflects the physiologic action of the drug at the target tissue or cell. The sources of variability that influence medication are numerous and include age, life stage, sex, genotype, disease state and nutrition status(Reference Rowland and Tozer1). The latter is the focus of this review and encompasses primary protein–energy malnutrition (PCM) and obesity.
The influence that nutrition status can have on drug disposition and effect is included as one of the five broad categories in the classification of drug–nutrient interactions(Reference Santos and Boullata2, Reference Boullata, Boullata and Armenti3). As a precipitating factor to the interaction, nutrition status may result in drug treatment failure or drug toxicity related to the degree of malnutrition(Reference Mehta4, Reference Walter-Sack and Klotz5). Related to the other areas of drug–nutrient interactions, the data available to clinicians are limited for the influence of obesity on drug disposition and even less for PCM(Reference Compher, Boullata, Boullata and Armenti6, Reference Boullata, Boullata and Armenti7). Drug evaluations in special patient populations (e.g. renal or hepatic impairment) take place routinely, but far less frequent is the influence of PCM or obesity evaluated. For example, despite longstanding descriptions of the potential influences on drug metabolism, the nutrition status of subjects enrolled in clinical drug trials is rarely described(Reference Walter-Sack and Klotz5, Reference Conney and Burns8, Reference Cheymol9). In the meantime, a rational and conceptual approach that uses available information can be helpful in making clinical decisions.
Definitions
The introduction of a few additional terms would be useful to the remaining sections of this review. Pharmacokinetics can be simply referred to as the actions of the body on a medication; for systemic effect, a drug first needs to be absorbed by the body, it is then distributed within and excreted from the body with many drugs being metabolized in the process. These four pharmacokinetic processes: absorption, distribution, metabolism and excretion, are described by a number of parameters with mathematical derivations and relationships(Reference Rowland and Tozer1). Apart from direct intravenous injection, after a drug is administered to a patient, it requires absorption by the body to reach the systemic circulation. From the vascular space, the drug undergoes distribution to the presumed site of drug action as well as to other tissues that include those responsible for the metabolism and excretion of the drug. These latter two terms (metabolism and excretion) are often collectively referred to as drug elimination. Most of the known influences of nutrition status are seen in drug distribution and drug elimination. The pharmacokinetic parameters that reflect these processes are volume of distribution (Vd) and clearance, respectively.
The distribution or movement of an active drug from the bloodstream to the site(s) of effect are determined by several factors related to the drug (e.g. lipophilicity, plasma protein binding, degree of ionization and tissue affinity) and to the body (e.g. body composition, blood flow, tissue size and permeability)(Reference Rowland and Tozer1, Reference Summers, Samra and Humphreys10, Reference Wada, Bjorkman and Ebling11). As a result, the distribution of a given drug is not the same to all tissues of the body. The Vd is an expression that uniquely reflects the distribution of each drug and is described in litres per kilogram of body weight (litres/kg). Higher values indicate extensive distribution and tissue binding. This is not an anatomical volume, but a theoretical body volume that the drug would have to distribute into if the drug concentration everywhere in the body were the same as is found in the blood following the administration of a known dose. In other words, the Vd relates the serum drug concentration to the amount of drug in the body. Drug elimination can be viewed in two parts; metabolism and excretion(Reference Rowland and Tozer1). Although some drugs are excreted from the body unchanged, most undergo some form of metabolism first which transforms the drug's chemical structure through an enzymatic reaction. These reactions may generally be described as either phase I or phase II depending on whether they involve, respectively, addition/subtraction of a functional group or conjugation with an endogenous substance. These metabolic reactions take place not only in the liver, but also in other tissue sites (e.g. intestinal mucosa) to some degree. The removal of the drug and any metabolite(s) from the body occurs predominantly through renal and biliary routes of excretion. The expression clearance is used to define the elimination of a drug from the body and is described in litres per hour (litres/h). Physiologically, this reflects the volume of blood that is cleared of a drug per unit of time as observed from sequential serum drug concentrations following an administered dose. The term clearance refers to the sum of multiple routes of elimination when more than one route exists for a drug. The size of the initial or loading dose of a drug depends on its Vd, while maintenance doses are based on drug clearance. Many drugs rely on weight-based (mg/kg) dosing.
Malnutrition and body weight
PCM and obesity occur as a result of nutrient and metabolic needs being inadequately met over time; reflecting chronic deficits or imbalances in macronutrients, but both including micronutrient deficits as well(Reference Torún, Shils, Shike and Ross12, Reference Rolfes, Pinna and Whitney13). PCM results in growth failure in children and unintended weight loss in adults and is associated with increased morbidity(Reference Torún, Shils, Shike and Ross12, Reference Gross and Webb14). Overweight and obesity are also implicated in higher morbidity and mortality(Reference Mokdad, Ford and Bowman15–18). Consequently, PCM and obesity are disproportionately found in healthcare settings(Reference Stratton and Elia19, Reference Santry, Gillen and Lauderdale20). As a part of usual clinical practice, the height and weight are obtained to calculate the BMI. These patient parameters should actually be measured, as visual estimates are often inaccurate(Reference Goutelle, Bourguignon and Bertrand-Passeron21). The BMI is frequently used in adults to define PCM (e.g. <18·5 kg/m2) and varying degrees of obesity (e.g. ≥30 kg/m2). The BMI is not perfect, but remains the most valid indicator of body habitus and morbidity/mortality risk(Reference Keys, Fidanza and Karvonen22, 23). There are still some using percentage of ‘ideal’ body weight as an expression of PCM (e.g. ⩽70–80%) and obesity (e.g. ≥130%). In clinical drug trials, obesity has also often been defined arbitrarily as ≥120% or 130% of ‘ideal’ body weight. The use of ‘ideal’ body weight in practice or in drug studies is problematic(Reference Boullata, Boullata and Armenti7, 23, Reference Knapp24). The equations often used to establish ‘ideal’ body weight are empirically derived and only take height and sex into account rather than being based on actual body composition data(Reference Robinson, Lupkiewicz and Palenik25–Reference Devine27). As a result, the ‘ideal’ body weight is not a true reference weight and cannot be used as a surrogate for a patient's lean body weight (LBW). A body metric that goes beyond just height and weight to include body composition would be more useful clinically. BMI is not easily applied in determining drug dosing, in particular, because at a given value it does not distinguish between excess adipose tissue and muscle mass. Weight-based drug dosing presumes a healthy BMI that is assumed to represent ‘normal’ body composition. In clinical practice, drug doses are often based on the total body weight (BWt) with the value arbitrarily scaled up or down in PCM and obesity.
BWt represents many compartments as can be appreciated in images from CT scans and MRI. Reflecting all body compartments, BWt cannot by itself represent body composition for weight-based dosing in PCM or obesity. An appreciation of the simple two-compartment view of body composition allows further discussion. Body composition can be conceptually divided into fat mass (FM) and fat-free mass, which resemble the anatomic adipose tissue and lean body mass, respectively, allowing recognized distinctions(Reference Shen, Wang and Punyanita28). The clinical term LBW is used to reflect fat-free mass. As expected, and documented by MRI, BMI is strongly correlated with FM, while LBW includes body water and is correlated with body cell mass(Reference Sarkar, Kuhlmann and Kotanko29). So if ‘ideal’ body weight is not appropriate, and BWt fails to differentiate between FM and LBW, then what dosing weight should be used for the patient with obesity or PCM?
The answer to this question depends on the drug being dosed and how it is handled differently, if at all, in the patient with obesity or PCM. In other words, the ‘dosing weight’ should be based on the characteristic behaviour of the drug rather than in relation to a standard weight-for-height metric. The body size metric should account for age and ethnicity, as well as height, weight, sex and remain robust at the extremes of BMI(Reference Green and Duffull30). For practical purposes, the BWt is used as the dosing weight most often except for the volume overloaded patient and for the patient with obesity. LBW may be used as the dosing weight for some drugs in PCM and obesity. In other cases, an adjusted body weight (Adj-BW) that lies between BWt and LBW is often used clinically to empirically adjust the altered body composition expected in obesity. The Adj-BW is calculated as
where the correction factor (cf) represents the fraction of the FM that normalizes the drug's Vd in obese patients to that of the non-obese. As each drug has a characteristic distribution between FM and LBW, the correction factor is expected to differ by drug. Unfortunately, this data is not readily available for many drugs. Some of the variability in distribution to FM reflects the various adipose tissue compartments (i.e. subcutaneous (superficial and deep), internal (visceral, including intraperitoneal; non-visceral)) each with their own blood supply and metabolic activity(Reference Summers, Samra and Humphreys10, Reference Shen, Wang and Punyanita28). In the obese patient, the focus on drug dosing should go beyond excess FM, to appreciate that the totality of change in body composition and function influence the tissue distribution of a drug, its clearance and ultimately its clinical effects. Arbitrary dose reductions or dose capping in obesity may be counterproductive by adversely influencing the clinical outcome(Reference Barras, Duffull and Atherton31, Reference Thompson, Rosner and Matthay32).
The best approach would be to use a body size metric that closely reflects body composition. When body composition has been taken into account as part of a clinical pharmacokinetics trial, significant influence on drug distribution and clearance can be identified(Reference Thompson, Rosner and Matthay32). Given the impracticality of obtaining body composition data on each patient, the incorporation of accurate height and weight data into validated equations for LBW derived from individuals across the BMI spectrum is recommended. Such equations do exist:

The first pair of equations was based on a report that included data from three studies(Reference Cheymol9, Reference James33). There were a total of 133 subjects in which less than 10% had BMI >30 kg/m2(Reference Hume and Weyers34–Reference Womersley, Boddy and King36). Although this pair of equations works well at typical BMI values, they may not be appropriate at more severe BMI levels. The latter pair of equations has been suggested as the best currently available equation for determining LBW(Reference Green and Duffull37). These were developed from a semi-mechanistic model in which body composition was taken into account, and uses the same easily obtained patient parameters (height, weight and sex). The model was developed from a group of 303 subjects, 18–82 years of age, with BMI between 17 and 70 kg/m2, 70% of whom were overweight and obese(Reference Janmahasatian, Duffull and Ash38). This pair of equations have good precision and no bias in predicting LBW, performing as well as the first pair of equations at modest BMI, but remaining predictive at higher BMI(Reference Janmahasatian, Duffull and Ash38). The application of these equations performed much better clinically in critical drug dosing in obesity than previously used equations(Reference La Colla, Albertin and La Colla39).
The distribution of a drug in the body can involve both compartments, while drug clearance is correlated with LBW. This correlation between systemic drug clearance and LBW is recognized(Reference Morgan and Bray40). Although BWt may correlate with liver volume, LBW was the only variable to correlate with hepatic drug clearance following stepwise multi-regression analysis(Reference Nawaratne, Brien and Seeman41). The renal function is also closely related to LBW(Reference Janmahasatian, Duffull and Chagnac42).
Both PCM and obesity induce changes in body composition and function. These physiologic changes seen in PCM and obesity in turn can influence drug disposition. The degree of difference in body composition and function ultimately influences drug distribution and clearance. Assuming a similar structure and function in PCM and obesity does not account for the influence of body composition on drug disposition and effect following administration. Having a sense of the patient's LBW (e.g. as calculated by an appropriate equation) is valuable(Reference Janmahasatian, Duffull and Ash38, Reference La Colla, Albertin and La Colla39).
Physiologic changes influence pharmacokinetics in protein–energy malnutrition
Individuals with PCM have reductions in both adipose tissue and lean tissue (skeletal muscle and organ mass). The degree of loss depends on the severity of PCM but may include a FM of only 5% of body weight compared with normal of 20–25%(Reference Detsky, McLaughlin and Baker43, Reference Hoffer, Shils, Olson and Shike44). Additionally, extracellular fluid volume may expand relatively from 20 to 40% of body mass(Reference Detsky, McLaughlin and Baker43, Reference Hoffer, Shils, Olson and Shike44). PCM is also characterized by reductions in cardiac output with reduced hepatic blood flow and glomerular filtration rate(Reference Hoffer, Shils, Olson and Shike44, Reference Owen, Felig and Morgan45). Hepatic protein synthetic rates are also reduced in PCM(Reference Hoffer, Shils, Olson and Shike44). The altered body composition along with reduced transport proteins and regulatory hormones can influence drug distribution, while the reduced cardiac output, glomerular filtration rate, organ mass and function influence drug elimination.
In PCM, the human pharmacokinetic data are limited. A drug's Vd may be increased or decreased in PCM(Reference Bravo, Arancibia and Jarpa46–Reference Lares-Asseff, Zaltzman and Guillé49). Renal drug clearance may be reduced or unchanged in severe PCM(Reference Buchanan, Davis and Eyberg50–Reference Berlinger, Park and Spector52). Severe PCM can decrease oxidative metabolism and possibly conjugation reactions(Reference Mehta, Nain and Sharma51, Reference Ashton, Boime and Alemayehu53). Mild PCM may increase or have no effect on oxidative drug clearance by the cytochrome-P450 (CYP) isoenzymes. When examined, it appears that CYP3A is not influenced by PCM, but CYP1A2, CYP2E1, and others are reduced in PCM(Reference Lee, Suh and Lee54). Data from an animal model of PCM suggest supplementation with the S-containing amino acid cysteine can improve drug metabolizing enzyme activity(Reference Cho, Kim and Lee55).
Physiologic changes influence pharmacokinetics in obesity
Obese individuals have absolute and relative increases in adipose tissue, with absolute increases in both lean tissue and body water based on MRI and 2H data(Reference Sarkar, Kuhlmann and Kotanko29, Reference Sartorio, Malavolti and Agosti56). Approximately 20–40% of the excess body weight in obesity is made up of lean mass across BMI of 29–47 kg/m2(Reference Forbes and Welle57). All tissue compartments (e.g. visceral organs, muscle mass, adipose tissue and body water), with the possible exception of bone, have a higher mass at higher BMI values(Reference Sarkar, Kuhlmann and Kotanko29, Reference Sartorio, Malavolti and Agosti56). Functionally, these individuals have a larger cardiac output with tissue blood flow and the glomerular filtration rate. There is a low proportion of blood flow to adipose tissue(Reference Rosell and Belfrage58). Altered body composition and carrier proteins (e.g. increased lipoprotein and α1-acid glycoprotein) could influence the distribution, while an increased cardiac output, filtration rate and inflammation can influence drug clearance. Again the degree of altered physiology and function varies in patients, realizing that the body composition of every person with a BMI of 45 kg/m2 is not identical(Reference Gallagher, Visser and Sepulveda59, Reference Fernández, Heo and Heymsfield60). This makes predictions difficult. The degree of difference in body composition and function ultimately influences drug distribution and clearance. Although drug clearance is known to increase with BWt, the relationship is not linear as it is with LBW(Reference McLeay, Morrish and Kirkpatrick61).
A few dozen drugs have been investigated for their influence by obesity(Reference Han, Duffull and Kirkpatrick62). Generally, these studies use equations (e.g. ‘ideal’ body weight) without the benefit of actual body composition data. A better understanding of the quantitative relationship between body composition and function with drug distribution and elimination is required. Lipophilic drugs may have a larger Vd in obesity, but not necessarily(Reference Greenblatt, Abernathy and Locniskar63, Reference Flechner, Kilbeinsson and Tam64). Differences in Vd of hydrophilic drugs are just as unpredictable in obesity. This should not be too surprising as the oil–water partition coefficient (i.e. degree of lipophilicity) is only one of the characteristics of a drug that determines its distribution, and does not necessarily overcome the other factors (e.g. blood flow and tissue binding). Hepatic drug clearance may be increased, decreased, or unchanged in obesity(Reference Milsap, Plaisance and Jusko65, Reference Abernathy and Greenblatt66). It appears that there is no influence of obesity on CYP1A2, but reductions may exist in CYP3A, while increases in CYP2E1 and uridine diphosphate-glucuronyl-transferase activity have been suggested(Reference Caraco, Zylber-Katz and Berry67–Reference Kotlyar and Carson70). Renal drug clearance is likely to be increased in obesity(Reference Reiss, Hass and Karki71). Individualizing drug dosing in obese individuals can reduce adverse effects without sacrificing therapeutic efficacy(Reference Barras, Duffull and Atherton31).
It should be clear, based on the available data so far, that generalizations cannot be made about the influence of PCM or obesity on drug disposition. Drug-specific data needs to be determined or closely evaluated when available.
Antimicrobial examples
Both PCM and obesity may predispose patients to infection(Reference Falagas, Athanasoulia and Peppas72). Of the 11 studies representing 3159 hospitalized patients which were included in a systematic review, 64% describe worse outcome from infection in obese and PCM patients compared with other patient groups(Reference Falagas, Athanasoulia and Peppas72). The role of inappropriate antimicrobial dosing is a factor to be seriously considered. Given the existing morbidity/mortality risk from infection, any altered drug disposition in PCM or obesity should be taken into account to assure optimal drug dosing. A recent study of 839 hospital admissions identified that obese patients are at a significantly greater risk for errors with weight-based drug dosing(Reference Miller, Johnson and Harrison73). Of the antimicrobial orders, under dosing was reported in 11% and overdosing in 5%, significantly greater than in the control group, although clinical outcomes were not described. In PCM, the Vd of aminoglycosides (e.g. tobramycin) is increased, while the clearance is reduced(Reference Bravo, Arancibia and Jarpa46, Reference Ronchera-Oms, Tormo and Ordovas48, Reference Buchanan, Davis and Eyberg50). This lends itself to a higher (mg/kg) dose with longer intervals between doses. Recall that maintenance doses are based on drug clearance, while the initial or loading doses are based on Vd. It has been suggested that loading doses of aminoglycosides be increased by a factor of 1·1 in underweight patients(Reference Traynor, Nafziger and Bertino74). Drug clearance is also reduced in PCM for chloramphenicol, penicillin, quinine and isoniazid(Reference Mehta, Nain and Sharma51, Reference Ashton, Boime and Alemayehu53, Reference Bolme, Eriksson and Paazlow75–Reference Eriksson, Bolme and Habte78). The additive influence of acute infection on drug disposition may also play a distinct role(Reference Renton79, Reference Boucher, Wood and Swanson80).
In obesity. the weight-corrected Vd of the aminoglycosides is unchanged or reduced, while the clearance is increased. The mg/kg dosing of these drugs should then be based on an Adj-BW using a correction factor of 0·4 to represent the proportion of the excess body weight into which the aminoglycosides distribute(Reference Traynor, Nafziger and Bertino74, Reference Leader, Tsubaki and Chandler81). There is very little data on the antifungal agents, but dosing based on BWt has been suggested for amphotericin as both Vd and clearance may be increased in obesity(Reference Gillum, Johnson and Lavoie82–Reference Cohen, DiBiasio and Lisco84). Although the clearance of the β-lactam antimicrobials (e.g. penicillins and cephalosporins) is increased in obesity requiring more frequent dosing to maintain adequate drug concentrations, the available data on Vd appear to differ by drug(Reference Kampmann, Klein and Lumholtz85–Reference Newman, Scheetz and Adeyemi87). For example, the Vd for ampicillin and piperacillin is increased, while Vd for ertapenem is reduced in obesity(Reference Kampmann, Klein and Lumholtz85, Reference Newman, Scheetz and Adeyemi87). The Vd for vancomycin is not appreciably different in obesity, but the clearance of the drug is increased(Reference Blouin, Bauer and Miller88–Reference Penzak, Gubbins and Rodvold90). Dosing of this antibiotic should be based on BWt with a dosing interval frequent enough so that the serum trough concentration remains at a therapeutic level.
Much of the data are derived from single case reports or case series rather than well-designed comparative trials. Therefore, it may be instructive to review a couple of examples.
A 43-year-old man hospitalized with bacterial cellulitis received a course of linezolid at the usual dose of 600 mg twice daily after 7 d of cefazolin(Reference Mersfelder and Smith91). This patient weighed 286 kg (BMI 86 kg/m2) and had a measured creatinine clearance of 75 ml/min. The measured linezolid Vd in this patient was 135·7 litres (0·47 litre/kg) as compared with the normal Vd of approximately 40–50 litres (about 0·64 litre/kg). Given the information from this case, that the weight-corrected Vd is lower in obesity compared with normal (0·47 litre/kg divided by 0·64 litre/kg=0·73), an Adj-BW should be used for dosing this drug. This further indicates that a correction factor of 0·27 (1−0·73) can be used in estimating the Adj-BW to account for the difference in Vd. This would need to be prospectively studied to document that such a dosing regimen would adequately and safely maintain drug concentrations.
A 60-year-old man admitted with a presumptive diagnosis of herpes encephalitis was treated with acyclovir 1000 mg intravenously every 8 h(Reference Hernandez, Norstrom and Wysock92). This patient weighed 108·9 kg (BMI 38 kg/m2) with blood urea nitrogen and creatinine serum values of 9 mg/dl (3·2 mmol/l) and 0·7 mg/dl (61·9 μmol/l). By the third hospital day, he developed acyclovir-induced nephrotoxicity with peak blood urea nitrogen and creatinine serum values of 57 mg/dl (20·1 mmol/l) and 5·5 mg/dl (486 μmol/l). The dose of acyclovir appeared to be within normal limits at 9·2 mg/kg BWt. However, because this drug has a known reduction in Vd (litres/kg) in obesity, it should have been dosed based on the patient's estimated LBW. Given that this patient had a LBW of approximately 60 kg, the dosing of acyclovir was actually 17 mg/kg of dosing weight and would be much more likely to cause the documented toxicity.
Recommendations
Antimicrobial dosing should be individualized to patient BMI/body composition based on the available data for each agent in order to maximize effectiveness and safety(Reference Falagas, Athanasoulia and Peppas72). Dose individualization in PCM and obese individuals will be advanced by further exploration of the influence that body composition has on drug disposition(Reference Han, Duffull and Kirkpatrick62). Weight-based drug dosing needs to take into account the relative distribution into various tissue compartments, as well as drug competition for transport proteins, lean tissue and adipose tissue binding characteristics. From an academic perspective, pharmacokinetic and pharmacodynamic evaluations in obesity should include a measurement of body composition; at least a two-compartment assessment. This requires a close collaboration between those with expertise in the evaluation of drug disposition and those with expertise in body composition. Body composition analysis methods associated with the least biologic variability or technical error (e.g. 2H dilution and dual-energy X-ray absorptiometry) should be directly applied to the study of drug disposition in PCM and obesity. If these studies are able to evaluate data across a wide spectrum of BMI and allow stratification by body composition, this would very helpful. Moving forward, it would be beneficial if all new drugs were required to perform these evaluations to ensure safety in posology. Dosing adjustments for PCM and obesity should be incorporated into the process of new drug development and regulatory review(Reference Boullata, Boullata and Armenti7, Reference Falagas and Karageorgopoulos93). Covariates to explain variability allow better pharmacokinetic and pharmacodynamic modelling, whether descriptive or predictive. Mechanistic covariates should be physiologically appropriate (e.g. eye colour is less useful than renal function).
Given the limited data on the impact of PCM on most drugs, the use of caution in managing these patients is recommended especially when using narrow therapeutic index agents. When available, drug-specific data should be integrated with patient-specific data to develop an appropriate dosing and monitoring regimen in PCM. In obesity, the use of drug-specific data and close patient monitoring is also important for designing optimal dosing strategies. The general approach in obesity would include evaluating the influence on Vd when the loading dose is being determined, and the influence on drug clearance when maintenance doses are being evaluated (Table 1)(Reference Boullata, Boullata and Armenti7). An empirical clinical approach integrates available data (in the absence of prospective trial results) to determine which dosing weight should be used for weight-based drug dosing. If a case suggests that the Vd (litres/kg) in obesity is equal to or greater than the expected Vd in the non-obese individual, then BWt can be used for the loading dose. If that comparison reveals a Vd (litres/kg) in obesity that is less than the expected value in the non-obese individual, then the LBW or possibly an Adj-BW should be used. The LBW estimates should be based on an appropriate equation(Reference Janmahasatian, Duffull and Ash38). For maintenance dosing, the total clearance (litres/h) in obesity is compared with the expected clearance in the non-obese individual. If clearance is equal to or greater in obesity then BWt can be used, but if clearance is less than the expected value in the non-obese the LBW should be used. This is most important to evaluate weight-based dosing of narrow therapeutic index drugs or those drugs that possess concentration- or time-dependent action (e.g. antimicrobials). Of course these empirical recommendations necessitate close patient monitoring.
Table 1. Empirical guide to weight-based dosing in obesity

Vd, volume of distribution in litres per kilogram of total body weight; LBW, lean body weight; Adj-BW, adjusted body weight; BWt, total body weight; Cl, clearance in litres per hour.
For clinicians, as part of routine patient assessments, any unexpected clinical presentation may be an indication to rule out the influence of PCM or obesity on drug disposition and drug effect. In this way, the clinician may be able to better explain (or better yet to predict) altered drug effects in the patient with PCM or obesity. Clinicians are encouraged to publish drug-specific findings from their patients with obesity (or PCM) providing pharmacokinetic parameters as well as body composition data when available(Reference Penzak, Gubbins and Rodvold90, Reference Mersfelder and Smith91). The dosing weight suggestions listed in Table 2 are based on limited data mostly from case reports. Prospective evaluations with body composition data will hopefully allow stronger recommendations to be made in the future.
Table 2. Suggested dosing weight for antimicrobial examples in obesity
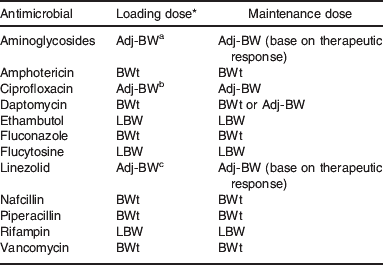
LBW, lean body weight; Adj-BW, adjusted body weight; BWt, total body weight.
* Suggested correction factor: a0·4; b0·5; c0·3.
Acknowledgements
The author declares no conflicts of interest. The work presented received no specific grant from any funding agency in the public, commercial or non-profit sectors.






