The UK Scientific Advisory Committee on Nutrition (SACN) recently released their draft report on the impact of SFA on health. This important qualitative review included forty-six studies of randomised controlled trials (RCT) and prospective cohort studies. In brief, the working group identified adequate evidence from RCT to support both a reduction of SFA intakes and replacement of SFA with PUFA, to reduce CVD event risk and improve glycaemic control(1). This is in consensus with the WHO and the 2015–2020 Dietary Guidelines for Americans which recommended replacement of SFA with unsaturated fatty acids(2, 3). The timing of this report is pertinent, due to mixed public health messages following a number of controversial studies that contradicted the role of SFA in disease(Reference de Souza, Mente and Maroleanu4, Reference Dehghan, Mente and Zhang5).
Furthermore, the current obesity epidemic is a global issue. The prevalence of obesity has risen in recent years, and the WHO estimate that 39% of adults are overweight, of which 13% are obese(6). These figures are estimated to rise substantially by 2030(Reference Kelly, Yang and Chen7). CVD and type-2 diabetes (T2D) are two common obesity related comorbidities. CVD is the leading cause of global mortality and is responsible for 17·3 million deaths annually, a rate that is expected to increase to 23·6 million by 2030(8). Similarly, T2D incidence has increased dramatically, it is estimated that 415 million individuals are currently living with diabetes, in comparison with 30 million in 1964, and an estimated 642 million by 2040(Reference Ogurtsova, da Rocha Fernandes and Huang9). Thus, obesity is associated with a significant economic burden, with global costs estimated to exceed 2 trillion US dollars annually(Reference Dobbs, Sawers and Thompson10). Thus, effective public health strategies are required to reduce disease risk, therefore replacement of SFA with PUFA is a promising initiative to improve dietary quality without affecting habitual dietary patterns.
SFA intakes have been associated with increased risk of CVD, due to their LDL-cholesterol raising properties(Reference Griffin11). SFA probably have also been associated with adverse effects upon key biological processes including insulin sensitivity, inflammation and lipid metabolism(Reference Ralston, Lyons and Kennedy12). However, a large proportion of the population are exceeding the WHO recommendation of ≤10% of total energy (TE) from SFA(13). One such strategy to reduce SFA intakes is replacement with PUFA(1, 2). The evidence to date typically includes total PUFA, therefore further research is required to decipher if there is a difference between the PUFA subtypes. The health benefits of EPA and DHA are widely reported(Reference Calder14); however the effects of α-linolenic acid (ALA) are not as well-characterised. Therefore, the aim of this review is to discuss the public health and physiological impact of replacing SFA with PUFA, with an emphasis on ALA. We provide an overview of current dietary fat intakes, the evidence from RCT and cohort studies relating to replacement of SFA with PUFA and the associated mechanism of action.
Dietary fatty acids
Nomenclature and health impacts
Fat consists of fatty acids and glycerol or other lipids on a carbon skeleton, connected by either single or double bonds. SFA contain solely single bonds, with chain lengths ranging from one to thirty carbon atoms. These SFA can be further characterised into short-chain (<6 : 0), medium-chain (6 : 0–12 : 0) and long-chain (LC) (12 : 0–30 : 0)(Reference Calder15). Dietary guidelines typically recommend reducing SFA intakes. However, not all SFA exhibit the same biological effects due to their divergent impact on serum lipids, with lauric (12 : 0), myristic (14 : 0) and palmitic (16 : 0) acids typically associated with adverse effects. A RCT by Dreon et al. identified an association between high SFA intake (46% TE) and increased concentrations of LDL-cholesterol, which is a known risk factor for CVD. More specifically, myristic acid (14 : 0) and palmitic acid (16 : 0) intakes correlated with increased LDL particle size, with no significant association observed with stearic acid (18 : 0) and LDL(Reference Dreon, Fernstrom and Campos16). Cohort studies have reported similar observations. A meta-analysis of RCT and prospective cohort studies by Micha et al. suggested that lauric acid (12 : 0), myristic acid (14 : 0) and palmitic acid (16 : 0) were associated with adverse effects on total cholesterol (TC) and LDL-cholesterol concentrations, with no observed impact of stearic acid. In addition, the TC:HDL ratio was decreased significantly by lauric acid (12 : 0), but not by myristic (14 : 0) and palmitic acid (16 : 0)(Reference Micha and Mozaffarian17). This is in agreement with previous findings from the Nurses’ Health Study that identified increased risk of CHD with LC SFA (12 : 0–18 : 0) but not with the shorter-chain SFA (4 : 0–10 : 0)(Reference Hu, Stampfer and Manson18). It is difficult to fully elucidate the impact of individual fatty acids on disease risk, as we don't consume individual fatty acids but rather we consume foods that contain a variety of different SFA in combination. Moreover, the majority of studies have examined SFA as a collective group rather than investigating the effects of individual fatty acids. However, based on the existing evidence, instead of classifying SFA as an entire entity, reformulation strategies, in line with the recent SACN and WHO guidelines(1, 2), should consider replacement of individual fatty acids, in particular myristic (14 : 0) and palmitic acid (16 : 0), with PUFA.
PUFA are intricate fatty acids containing a minimum of two double bonds with the configuration of the fatty acid contributing to its role in metabolic processes. PUFA are further characterised as an n-3 or n-6 PUFA by the location of first double bond, which is either on the third or sixth carbon atom, respectively(Reference Calder15). As with SFA, PUFA can be further characterised based on their carbon chain length, wherein PUFA with greater than twenty carbon atoms are referred to as LC PUFA. There is conflicting evidence relating to the inflammatory status of n-6 PUFA(Reference Innes and Calder19), nevertheless evidence suggests that intakes of linoleic acid (LA) are associated with reduced adiposity(Reference Li, Brennan and Bloomfield20), CHD(Reference Farvid21) and mortality(Reference Wu, Lemaitre and King22) and improved glycaemic control(Reference Belury, Cole and Snoke23). The anti-inflammatory effects of the LC n-3 PUFA, EPA and DHA are well recognised(Reference Calder14, Reference Oliver, McGillicuddy and Phillips24, Reference Serhan and Levy25). In brief, mechanisms include attenuation of the pro-inflammatory NF-κB pathway, activation of pro-inflammatory PPARγ and production of the anti-inflammatory mediators, resolvins, protectins and eicosanoids. However, the impact of the n-3 PUFA, ALA is not as well recognised and will be detailed in the current review.
Sources of dietary fatty acids
When evaluating the impact of SFA on markers of health, it is important to consider fat quality as not all dietary SFA exert the same effects. Primary dietary sources of SFA include dairy, meat and vegetable oils(Reference De Oliveira Otto, Mozaffarian and Kromhout26). Data from eleven European countries have identified that 17–30% of dietary SFA intake comes from dairy products and 15–30% from meat products(Reference Eilander, Harika and Zock27). This is similar to reported intakes in the USA, wherein dairy contributes to 13% and meat to 15% of SFA intakes(28). Palmitic acid (16 : 0) is the most abundant dietary SFA as it is derived from animal lipids and plant seed oils. Stearic acid (18 : 0) is the next most abundant and is found in animal and vegetable lipids, while dairy fats typically comprise the odd-chain SFA, including pentadecanoic acid (15 : 0) and heptadecanoic acid (17 : 0)(Reference De Oliveira Otto, Mozaffarian and Kromhout26). Data from the US Health Professionals Follow-Up Study and the Nurses’ Health Study (n 222 234) reported that dairy fat intake was not significantly associated with risk of stroke (relative risk (RR) 0·99; 95% CI 0·93, 1·05), CHD (RR: 1·03; 95% CI 0·98, 1·09) or CVD (RR: 1·02; 95% CI 0·98, 10·5) in males and females(Reference Chen, Li and Sun29). Furthermore, evidence from the Multi-Ethnic Study of Atherosclerosis prospective cohort study reported that SFA of dairy origin are cardio-protective (hazard ratio (HR): 0·79; 95% CI 0·68, 0·92) compared with meat-derived SFA (i.e. palmitic acid (16 : 0) and stearic acid (18 : 0)) which were associated with increased CVD risk (HR: 1·26; 95% CI 1·02, 1·54; P < 0·05)(Reference De Oliveira Otto, Mozaffarian and Kromhout26). A similar effect was observed in the EPIC cohort, whereby high dairy derived SFA intakes were associated with reduced risk of IHD, however no adverse association between meat-derived SFA was observed in this Dutch cohort(Reference Praagman, Beulens and Alssema30). This was previously reported by Sjogren et al. who identified that milk-derived fatty acids were favourably associated with reduced LDL particles, and consequently CHD risk(Reference Sjogren, Rosell and Skoglund-Andersson31). The food matrix in which the lipids are contained has been shown to have a central role in their health effects. For example, dairy fat within a cheese matrix was associated with significant improvements in total and LDL-cholesterol (P < 0·05) in overweight adults, compared with alternate dairy matrices, including butter(Reference Feeney, Barron and Dible32). This is in agreement with previous evidence wherein butter increased total and LDL-cholesterol(Reference Hjerpsted, Leedo and Tholstrup33). The differences in SFA composition may partly explain this association as cheese contains lower levels of lauric acid (12 : 0) and higher levels of palmitic acid (16 : 0) and stearic acid (18 : 0) than butter(Reference de Goede, Geleijnse and Ding34). Lauric acid (12 : 0) has been associated with increased LDL-cholesterol, therefore the reduced levels in cheese may be beneficial for health(Reference Mensink, Zock and Kester35). However, further research is required as cheese also contains higher levels of protein and calcium, which may be contributing to the positive effects on lipid profiles(Reference Feeney, Barron and Dible32). The primary dietary sources of the LC n-3 PUFA, EPA and DHA are oily fish, additionally they can be synthesised endogenously from ALA(Reference Calder36). ALA, however, is an essential fatty acid, wherein it cannot be synthesised endogenously and needs to be obtained from dietary sources. Sources of ALA include green leafy vegetables, certain nuts e.g. walnuts, flaxseed, rapeseed and the respective oil counterparts(Reference Baker, Miles and Burdge37).
Typical fatty acid intakes
The SACN(1) and the WHO(2) recommend that SFA intakes are 10% less of TE intake, while the European Food Safety Authority recommends that SFA intakes should be as low as possible(38). Nevertheless, population intakes typically exceed these recommendations. Mean SFA intakes are between 8·9 and 15·5% in Europe(Reference Eilander, Harika and Zock27), 11% in the USA(Reference Hugh, Fulgoni III and Keast39), and 13·3% in Ireland(Reference Li, McNulty and Tiernery40) and 12·7% in the UK(Reference Bates, Lennox and Prentice41). It is estimated that reduction of SFA intakes to 10% TE, by replacing 3% with PUFA would infer a 10% reduction in CVD risk(Reference Minihane42). The WHO recommends that total PUFA intakes are greater than 6% TE(13). In a global review of dietary intakes, twenty out of the forty studies included met the aforementioned PUFA recommendation, with intakes ranging from 2·8 to 11·3%(Reference Harika, Eilander and Alssema43), with over half of EU countries adhering to the >6% TE recommendation(Reference Eilander, Harika and Zock27). Many studies report PUFA intakes cumulatively, however the FAO/WHO and European Food Safety Authority recommend that intakes of ALA are >0·5% TE(13, 38). Alas, population ALA intakes are not always reported, however, data from the latest Irish food survey suggest 100% adherence to the ALA recommendation, at the population level, with a mean daily intake of 0·65% TE (1·4 g)(Reference Li, McNulty and Tiernery40), which is similar to other EU counties for which an intake of 0·4–0·8% TE (0·7–2·3 g) is reported(38) and the USA (1·5 g)(Reference Papanikolaou, Brooks and Reider44). Of note, a recent review of the evidence relating to ALA and CVD risk suggests that this recommendation should be reviewed as evidence suggests that intakes of greater than 2 g/d (0·6–1·1% TE) would be more beneficial for reducing CVD risk(Reference Fleming and Kris-Etherton45), in agreement with a previous recommendation(Reference Mozaffarian46).
Health impact of dietary fatty acids
SFA have been associated with increased risk of heart disease since the seven countries study by Ancel Keys in 1958 up to the current draft SACN report on saturated fats and health, which identified improved total and LDL-cholesterol, and reduced CHD risk following reduction in SFA intakes(1). Due to the complex nature of the hypothesis and the complexity of dietary intakes it is difficult to extend the direct impact of SFA intakes on CVD risk.
The robust SACN report included a Cochrane review of fifteen RCT, which included long-term trials (minimum of 24 months). This analysis demonstrated that a reduction in SFA intake was associated with a 17% reduced risk of CVD events (RR: 0·83; 95% CI 0·72, 0·96), following sensitivity analyses(Reference Hooper, Martin and Abdelhamid47). This review also identified a 27% reduction in CVD risk (RR: 0·73; 95% CI 0·58, 0·92) following substitution of 10% SFA with PUFA(Reference Hooper, Martin and Abdelhamid47). This is in agreement with previous meta-analyses. For example, a meta-analysis including eight RCT (n 13 614) demonstrated that a 5% replacement of SFA with PUFA resulted in a 10% decrease in CHD risk (RR: 0·90; 95% CI 0·83, 0·97)(Reference Mozaffarian, Micha and Wallace48). Similarly, Mensink et al. identified a reduction in TC and HDL-cholesterol, and an improvement in the TC:HDL-cholesterol ratio with the substitution of 1% SFA with PUFA(Reference Mensink, Zock and Kester35). Thus, even slight replacement of SFA is capable of exerting health benefits. Recent evidence from a large prospective cohort study of men and women (n 126 233) from the Health Professionals Follow-up Study and the Nurses’ Health study reported an 8% increased risk (95% CI 1·03, 1·14, P < 0·001) of total mortality with the highest SFA consumption (17·9% TE) after a follow-up period of approximately 30 years(Reference Wang, Li and Chiuve49). However, the SACN report concludes that the length of follow-up in studies was not sufficient to derive a relationship between a reduction in SFA or the replacement of SFA with PUFA on overall mortality(1).
It is well established that SFA exert their adverse effects on CVD risk by increasing serum LDL-cholesterol, which directly correlates to increased CVD risk(Reference Griffin11). Despite this, a number of meta-analyses of observational studies have recently challenged this concept by reporting that dietary SFA was not associated with increased risk of CVD or total mortality(Reference de Souza, Mente and Maroleanu4, Reference Chowdhury, Warnakula and Kunutsor50). Chowdhury and colleagues concluded that their findings did not support altering the public health guidelines to reduce SFA and increase PUFA for CVD prevention. Similarly, De Souza et al. concluded that SFA are not associated with increased risk of mortality, heart disease or diabetes. However, the results of these studies need to be interpreted with caution due to study limitations. First, it is important to note that cause and effect of disease cannot be derived from observational studies. Moreover, these studies presented study selection bias, heterogeneity and residual confounding which are factors that may have influenced CVD risk, while carbon chain length and replacement SFA macronutrient also varied between studies(Reference Houston51).
Whilst recent results from the controversial PURE study, an observational study encompassing eighteen countries, reported that total fat and SFA were inversely associated with reduced total mortality risk(Reference Dehghan, Mente and Zhang5). It is important to note that there are a number of study limitations that also must be acknowledged, including the undefined carbohydrate sources, as similar to fat, different carbohydrates have been associated with divergent health effects(Reference Zong, Gao and Hu52). Furthermore, a number of countries were of low socio-economic status, hence the unusually high carbohydrate (>60%) intakes, furthermore, the main source of SFA was meat and dairy, which may have been corrected for micronutrients deficiencies in countries with poor dietary quality. Conversely, findings from the same cohort also identified an association between intakes of SFA and increased total and LDL-cholesterol(Reference Mente, Dehghan and Rangarajan53). Nonetheless, it is important to consider the macronutrient of replacement for SFA, as PUFA, and to a lesser extent MUFA, have been associated with beneficial effects, while there is inconsistent evidence relating to carbohydrate substitution(1).
Replacement of SFA with PUFA
The replacement of SFA with an alternate macronutrient to reduce risk of disease has been a controversial topic in recent years. Prospective cohort studies and RCT have presented inconsistent results, which can be partly explained by differing populations, intervention durations, doses and the nominated replacement macronutrient. One of the most important confounding factors is body weight, wherein reducing SFA may occur in conjunction with weight loss, and concomitant weight changes would also affect physiological outcomes(Reference Lefevre, Champagne and Tulley54). Nevertheless the SACN concluded that there was adequate evidence from RCT to suggest that replacement of SFA with PUFA reduces total and LDL-cholesterol, decreases CHD and CVD event risk and improves glycaemic control. They also identified a beneficial effect of MUFA replacement on blood lipids and T2D risk(1) (Fig. 1).
Perspectives from cohort studies
A limitation of cohort studies that have investigated the replacement of SFA with PUFA is that they typically do not differentiate between n-3 and n-6 PUFA. Nevertheless, the SACN committee concluded that there was adequate evidence from prospective cohort studies to support a reduced risk of CHD events and mortality with replacement of SFA with PUFA but insufficient evidence to derive an association with improved blood lipids(1).
A modelling approach using data from the US Nurses’ Health Study (n 73 147) and the Health Professionals Follow-up Study (n 426 354) suggested that isoenergetic replacement of 1% SFA with PUFA could reduce CHD risk by 8% (HR: 0·92; 95% CI 0·89, 0·96)(Reference Zong, Li and Wanders55). This complements previous results from Li et al. that identified a 25% reduction in CHD risk (HR: 0·75; 95% CI 0·67, 0·84) when 5% of dietary SFA was replaced with PUFA after a minimum of 24 years of follow-up in this US cohort(Reference Li, Hruby and Bernstein56). Similarly, Chen et al. also modelled the impact of replacing dairy fat with PUFA, and reported that 5% substitution would reduce CVD risk by 24% (RR: 0·76; 95% CI 0·71, 0·81)(Reference Chen, Li and Sun29). Furthermore, outcomes from the PREDIMED study showed that isoenergetic replacement of SFA with PUFA was associated with reduced CVD risk(Reference Guasch-Ferré, Babio and Mart57). In addition to the cardio-protective impact, complementary results from the US Nurses’ Health Study (n 83 349) and the Health Professionals Follow-up Study (n 42 884) found that replacing 5% of SFA with PUFA resulted in a 27% reduced risk of total mortality (HR: 0·73; 95% CI 0·70, 0·77)(Reference Wang, Li and Chiuve49). These studies are in agreement with previous analyses using data from prospective cohort studies that reported improvements in CVD risk following substitution of SFA with PUFA(Reference Mensink, Zock and Kester35, Reference Skeaff and Miller58–Reference Siri-Tarino, Sun and Hu60). Whilst evidence relating to the direct substitution of SFA with ALA is limited, a continuous (1-sd increase) analysis of nineteen cohort studies reported that plasma ALA was associated with a 9% reduced risk of fatal CHD (RR: 0·91; 95% CI 0·84, 0·98)(Reference Del Gobbo, Imamura and Aslibekyan61). Equivalently, Chowdhury et al. reported decrease in CHD risk following supplementation with ALA (<2 g/d) in a meta-analysis of observational studies(Reference Chowdhury, Warnakula and Kunutsor50). In agreement with a previous analysis which reported that dietary ALA intake was associated with reduced CVD risk (RR: 0·90; 95% CI 0·81, 0·99), results from the pooled dietary analysis suggesting that each 1 g increment of ALA was associated with a 10% reduced risk of CHD mortality(Reference Pan, Chen and Chowdhury62). It has been established that replacement of PUFA is a promising future health strategy, however, as with SFA, the types of PUFA should be also considered. The health benefits of EPA and DHA are well recognised, however, evidence from prospective studies suggest that ALA is also a plausible replacement for SFA. Substantial research, including RCT, is required to elucidate the optimal dose of ALA required to attain a health benefit.
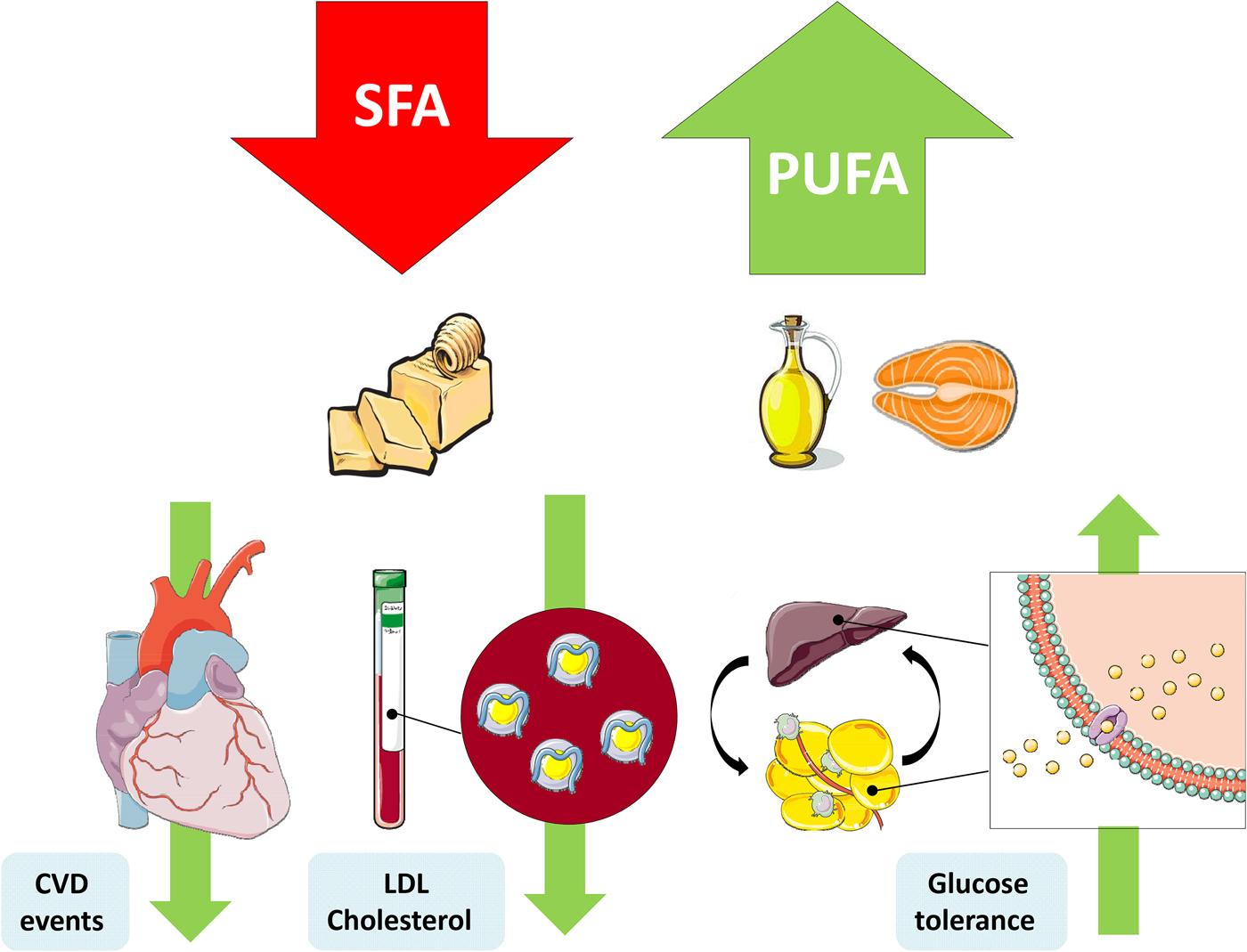
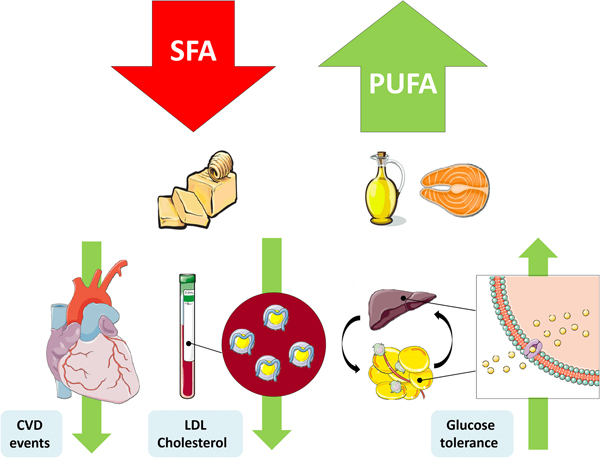
Fig. 1. (Colour online) Replacement of SFA with PUFA reduces the risk of CVD events, improves the blood lipoprotein profile to reduce LDL-cholesterol and increases glycaemic control. This figure was prepared using the SMART Servier Medical Art website (https://smart.servier.com).
Perspectives from randomised controlled trials
Prospective cohort studies provide a platform to derive associations between dietary fat intakes and disease. However, cohort studies are seriously challenged by virtue of the inherent limitations of dietary assessment methodologies, wherein fatty acid, macronutrient and energy intake is often significantly under-reported. Therefore RCT are required in order to determine the causal effect of replacing SFA with PUFA on health parameters. In terms of insulin sensitivity, a recent systematic review and meta-analysis of 102 RCT investigated the impact of PUFA replacement on glucose-insulin homoeostasis using findings. In agreement with the beneficial effects on CVD risk, 5% substitution with PUFA significantly improved fasting glucose concentrations (−0·04 mmol/l), fasting insulin (−1·6 pmol), haemoglobin A1c (−0·15%), C-peptide (+0·03 nmol/l), homoeostatic model assessment-insulin resistance (HOMA-IR; −4·1%) and insulin secretion capacity(Reference Imamura, Micha and Wu63). Although many studies fail to differentiate between the types of PUFA, a recent review of the evidence suggests that replacement of SFA with n-6 PUFA is a feasible public health initiative to reduce CVD risk(Reference Wang and Hu64). In terms of the impact of substituting SFA with n-3 PUFA, Ramsden et al. demonstrated a 21% reduced risk of CVD mortality (RR: 0·79; 95% CI 0·63, 0·99) with a combination of n-3 and n-6 PUFA, compared with a null effect following sole replacement with n-6, suggesting that n-3 PUFA is eliciting a greater cardio-protective effect(Reference Ramsden, Zamora and Leelarthaepin65). Similarly, a RCT (n 79 males) that replaced SFA with 4% fish oil for 8 weeks reported a decrease in plasma TAG and arterial blood pressure(Reference Dyerberg, Eskesen and Andersen66). Hence, current evidence suggests that replacement of SFA with n-3 PUFA may have more potent beneficial effects than replacement with n-6 PUFA.
To the best of the authors’ knowledge, no studies have investigated the impact of replacing SFA with ALA. However, a number of RCT have investigated the impact of ALA supplementation including the Alpha Omega trial in participants who had experienced previous myocardial infarction. They reported a 27% reduction in CVD events in women following daily supplementation with 2 g ALA for 40 months, with a non-significant 9% reduction in CVD incidence in the overall population(Reference Kromhout, Giltay and Geleijnse67). This is similar to previous results from the Lyon Heart Study which suggested that a diet rich in ALA, obtained from consumption of margarine containing 5% ALA, was an effective strategy for the secondary prevention of CHD, wherein there was a significantly lower rate of cardiac deaths in the intervention group (n 3) compared with the control (n 17)(Reference de Lorgeril, Renaud and Mamelle68). Evidence from a RCT suggested that diet in which two-thirds of the fat was derived from rapeseed oil (ALA) reduced TC by 12% and LDL-cholesterol by 16%, a similar magnitude to what was observed with the maize oil (LA)(Reference Lichtenstein, Ausman and Carrasco69). Taken together this suggests that replacement of SFA with ALA would infer a cardio-protective effect. Nonetheless, the earlier benefits were observed at intakes significantly greater than the current dietary guidelines (0·5% TE)(38). Therefore, further RCT are required to determine the optimal ALA dose and whether the beneficial effects of ALA are more pronounced in women. Moreover, increased ALA intakes may also improve LC n-3 PUFA status, which would consequently infer cardiovascular health benefits(Reference Calder70).
Whilst evidence suggests that replacement of SFA with PUFA has the potential to reduce CVD and T2D risk in a number of populations; the impact of inter-individual variation should also be considered. It has been established that individuals respond differently to dietary interventions depending on their baseline dietary and metabolic health status, as well as other parameters which may include genetic background and ethnicity. An example of this was observed in the LIPGENE study; a randomised dietary intervention trial intended to determine the most effective dietary approach to reduce dietary SFA in individuals with the metabolic syndrome (n 417) encompassing eight European countries. The participants were randomly assigned to one of four isoenergetic diets for 12 weeks: a high-fat, SFA-rich, high-fat MUFA enriched, low-fat with high-complex carbohydrate, or low-fat with high-complex carbohydrate with 1·2 g/LC n-3 PUFA(Reference Tierney, McMonagle and Shaw71). In this study, reducing SFA intakes in a weight-stable context in obese individuals had no effect on insulin sensitivity, cholesterol, blood pressure or inflammatory status(Reference Tierney, McMonagle and Shaw71). Of note, replacement of SFA with the low-fat, high-complex carbohydrate LC n-3 PUFA diet did significantly improve plasma TAG and NEFA concentrations in males(Reference Tierney, McMonagle and Shaw71). Further analysis of this cohort stratified participants by insulin sensitivity (HOMA-IR). Participants with the greatest HOMA-IR, higher BMI and the most adverse metabolic phenotype, responded better to dietary replacement of SFA with MUFA and PUFA. In this adverse phenotype group, fasting insulin and HOMA-IR were significantly reduced, following the dietary replacement of SFA within the dietary intervention(Reference Yubero-Serrano, Delgado-Lista and Tierney72). In contrast, individuals with the lowest HOMA-IR status, increased HOMA-IR in response to the SFA diet, wherein fasting insulin and HOMA-IR concentrations were significantly increased(Reference Yubero-Serrano, Delgado-Lista and Tierney72). Thus, incorporation of personalised nutrition into future public health strategies may improve population health by providing a tool to predict which dietary interventions are most likely to improve health. However, a recent review by Ordovas et al. on personalised nutrition and health concluded that a large body of the current evidence supporting personalised nutrition is derived from observational studies, not RCT, therefore substantial research and regulation will be required before personalised nutrition can be implemented(Reference Ordovas, Ferguson and Tai73).
Insights into the mechanism of action of altering dietary fat composition
This review seeks to present a synopsis of the biological mechanism as to how fatty acid modification affects health. A plethora of studies have investigated the in vivo effects of PUFA; however, it is important to consider the differences in doses of PUFA between studies, as some studies apply total replacement whereas others replace a proportion of the diet with PUFA, which is more physiologically relevant, furthermore a variety of different mouse models are used, all of which could lead to discrepancies between studies. Furthermore, while it is possible to achieve efficacy in animal studies, this does not always translate to human subjects.
Mechanism of action of SFA beyond cholesterol homoeostasis and CVD risk
The impact of SFA on metabolic health have been recently reviewed(Reference Ralston, Lyons and Kennedy12, Reference Hotamisligil74). In brief, SFA have been associated with negatively altered insulin sensitivity, adipose tissue and pancreatic β-cell inflammation, hepatic steatosis and mitochondrial dysfunction(Reference Ralston, Lyons and Kennedy12). A high-SFA diet negatively impacts in vivo signalling pathways, including impaired insulin signalling via downregulation of insulin receptor substrate-1 mRNA expression, which contributes to the progression of insulin resistance, and subsequently T2D. This often occurs in tandem with modulation of adipose tissue inflammation, wherein pro-inflammatory cytokine production is increased. Thus, promoting a hypertrophic adipose phenotype(Reference Finucane, Lyons and Murphy75) with increased pro-inflammatory M1 macrophage polarisation and the formation of crown-like structures, whereby macrophages surround necrotic adipocytes and secrete pro-inflammatory mediators(Reference Murphy, Thomas and Crinion76). However, the majority of this mechanistic evidence is derived from cell culture and animal studies; therefore, it is difficult to elucidate the biological impact of SFA substitution in human subjects, as 100% replacement is not a feasible dietary fat modification.
The activation of the Toll-like receptor 4 (TLR4) pathway and subsequently the NF-κB pathway has been one of the fundamental pathways thought to be involved in SFA-induced inflammation and insulin resistance(Reference Hotamisligil74, Reference McNelis and Olefsky77). Quite recently, this theory was challenged in a pertinent and refined publication which provided novel evidence that palmitate does not activate TLR4 but promotes inflammation by reprogramming macrophage metabolism(Reference Lancaster, Langley and Berglund78). The authors of this study suggest that SFA are not TLR4 agonists per se rather that TLR4-dependent priming is required and that the palmitate provides the ‘second hit’ to induce inflammation, coupled with alterations to macrophage metabolism and the lipidome(Reference Lancaster, Langley and Berglund78). This theory is in agreement with previous evidence demonstrating that SFA does not induce a rapid activation of c-Jun N-terminal and NF-κB compared with lipopolysaccharide(Reference Galic, Fullerton and Schertzer79, Reference Hernandez, Jun Lee and Young80) and neoseptin-3(Reference Wang, Su and Morin81). Moreover, it is within reason that circulating SFA which are in constant flux could not solely activate such a potent inflammatory response. It is important to note that this study only used palmitic acid, which had been previously associated with TLR4 activation, however, it is currently unknown if other types of SFA also induce inflammation in a TLR4-independent manner. Lancaster and colleagues also provided evidence to support SFA-induced metabolic endotoxaemia(Reference Cani, Amar and Iglesias82, Reference Sonnenburg and Backhed83), wherein the gut microbiota is altered and promotes lipopolysaccharide secretion, which subsequently induces TLR4 activation, adipose inflammation and pro-inflammatory adipose tissue macrophage activation(Reference Lancaster, Langley and Berglund78). This new evidence changes the classical paradigm of SFA activation via the TLR4 pathway; therefore, further research is required to fully elucidate the mechanism by which SFA contribute to inflammatory effects.
Modulation of fatty acid composition: putative health impacts of α-linolenic acid
As SFA mediate a number of adverse effects, substitution with other fatty acids has been the focus of much research. MUFA and PUFA have been associated with beneficial effects, hence supporting the proposed replacement of SFA to modulate disease risk. The role of MUFA in ameliorating adipose tissue inflammation has been recently reviewed(Reference Ralston, Lyons and Kennedy12). Briefly, oleic acid has potential to modulate the NLRP3 inflammasome to reduce IL-1β cytokine production and improve insulin sensitivity in vivo (Reference Finucane, Lyons and Murphy75). Moreover, oleic acid(Reference Finucane, Lyons and Murphy75) and palmitoleic acid(Reference Chan, Pillon and Sivaloganathan84) activate an important metabolic hub 5′-AMP-activated protein kinase. This subsequently impedes inflammatory signalling, improves glucose metabolism, promotes mitochondrial biogenesis and fatty acid oxidation(Reference Herzig and Shaw85). This review will focus on the in vivo evidence relating to ALA and metabolic health.
Evidence from in vitro studies suggests that ALA exerts beneficial effects through activation of PPARγ and subsequent inhibition of the NF-κB pathway(Reference Zhao, Etherton and Martin86), inactivation of the NLRP3 inflammasome(Reference Kumar, Gupta and Anilkumar87) and ameliorating the pro-inflammatory effects of M1 macrophages(Reference Pauls, Rodway and Winter88). Yu et al. recently illustrated attenuated high-fat diet (HFD)-induced insulin resistance in C57BL/6J mice by amelioration of metabolic activation of adipose tissue macrophages(Reference Yu, Tang and Liu89). Mice were allocated to one of five groups; low fat diet (10% energy fat), HFD (60% energy fat) or HFD with 10, 20 or 30% of fat replaced by flaxseed oil for 16 weeks. All three flaxseed groups demonstrated significant improvements in insulin sensitivity and a stepwise improvement in HOMA-IR was observed with increasing ALA replacement (P < 0·05)(Reference Yu, Tang and Liu89). Furthermore, adipose tissue inflammation was attenuated following substitution with flaxseed oil, with reduced secretion of TNFα, IL-6, IL-1β and monocyte chemoattractant protein-1 and increased adiponectin in adipose tissue(Reference Yu, Tang and Liu89). In agreement with these findings, a previous study reported improvements in insulin sensitivity and attenuation of hepatic, adipose and skeletal muscle inflammation following replacement of 10% of energies in a HFD with ALA, for 16 weeks, through induction of G protein-coupled receptor-120(Reference Oliveira, Marinho and Vitorino90). In addition, recent evidence suggests an alternate mechanism of action whereby ALA attenuates the NLRP3 inflammasome through activation of the PPARγ pathway(Reference Kumar, Gupta and Anilkumar87). Similar beneficial effects on HFD-induced hepatic steatosis, inflammation and lipid homoeostasis were observed following substituting of 10% of HFD with ALA for 12 weeks(Reference Han, Qiu and Zhao91–Reference Wang, Zhang and Feng94). Furthermore, ALA has been associated with improvements in CVD risk factors. A recent study showed that ALA supplementation increased peripheral vasodilation in Zucker rats(Reference Barbeau, Holloway and Whitfield95). These findings complement previous evidence demonstrating that a high ALA diet (7·3% w/w) reduced plaque area by 50% in apolipoprotein E−/− mice and significantly decreased plaque T-cell accumulation, vascular cell adhesion protein-1 and TNFα(Reference Winnik, Lohmann and Richter96). Therefore, there is consistent in vivo evidence to suggest that partial replacement of SFA with ALA improves insulin sensitivity, inflammation, hepatic steatosis and CVD risk factors (Fig. 2). Nevertheless, the dose of ALA administered in the aforementioned animal studies is physiologically greater than typical intakes, as a proportion of dietary fat composition. Thus, further research is required to investigate the translation of these findings to human subjects, and to whether efficacy can be achieved using a physiologically relevant dose of ALA.
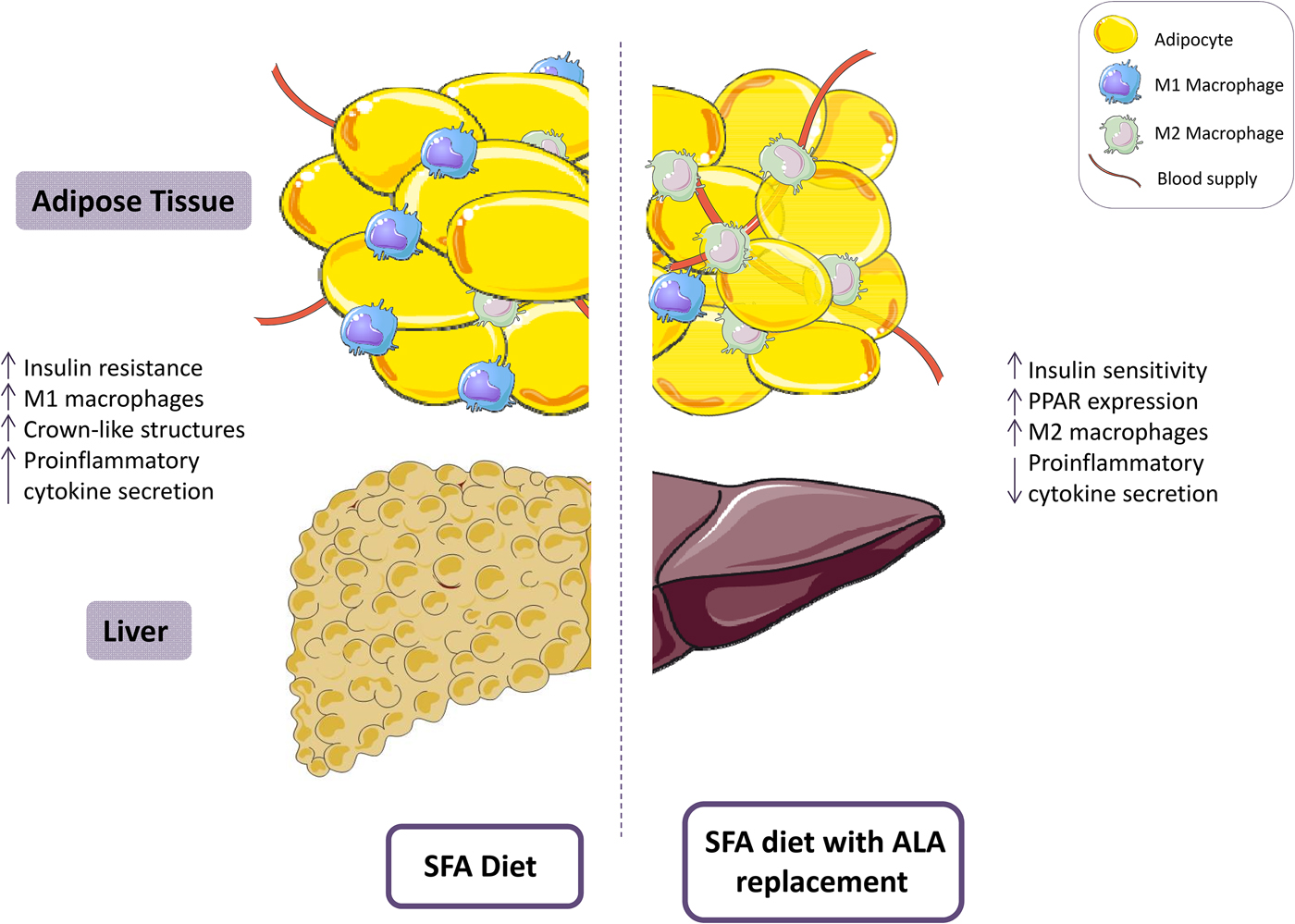
Fig. 2. (Colour online) A diet high in SFA has been associated with reduced insulin sensitivity and increased adipose tissue inflammation, including a pro-inflammatory (M1) resident macrophage population. Replacement of SFA with α-linolenic acid (ALA) ameliorates insulin sensitivity and attenuates adipose tissue inflammation. This figure was prepared using the SMART Servier Medical Art website (https://smart.servier.com).
Furthermore, ALA is a precursor of the LC n-3 PUFA, whereby it is biosynthesised to EPA, and subsequently DHA by elongases and desaturases, with delta-6 desaturase being the rate limiting enzyme(Reference Calder14). Therefore, the beneficial effects of ALA have typically been attributed to provision of a precursor for LC n-3 PUFA. However, in man this endogenous biosynthesis is poor, with 8–12% converted to EPA, a mere 1% of which is converted to DHA in males, with better rates observed in females(Reference Calder36). Interestingly, a study identified a protective effect of ALA against hepatic steatosis in the delta-6 desaturase knockout mouse model, whereby the ALA group presented lower hepatic lipid accumulation and inflammation than the comparative lard group, highlighting the ability of ALA to ameliorate steatosis independent of EPA and DHA(Reference Monteiro, Askarian and Nakamura97). However, LA, an n-6 PUFA is converted to arachidonic acid by the same elongase and desaturase enzymes as ALA, therefore there is competition for the rate-limiting delta-6 desaturase between the essential PUFA(Reference Calder14). However, LA is much more abundant than ALA in the Western diet, with an estimated ratio of 20 : 1, therefore conversion of LA to arachidonic acid is more prominent(Reference Simopoulos98). Therefore, it is evident that dietary ALA intakes need to be increased to modulate the LA:ALA ratio and increase the availability for LC n-3 fatty acid synthesis. Thus, modulation of fatty acid composition to reduce SFA and increase ALA has the potential to increase dietary ALA, and consequently ALA abundance for conversion to EPA and DHA. These LC n-3 PUFA mediate a range of potential anti-inflammatory mechanisms, we will not elaborate on this here as this evidence has been reviewed in detail(Reference Calder14, Reference Oliver, McGillicuddy and Phillips24, Reference Serhan and Levy25).
Food reformulation as a public health initiative to improve dietary quality
A substantial body of evidence supports replacement of SFA with PUFA which would reduce population SFA intakes and ultimately reduce disease risk. There are a number of strategies that could be implemented to achieve this. For example, the reformulation of dairy products to reduce fat content has proved successful in reducing population SFA intakes. A 6% reduction in contributions of whole milk and butter to SFA intakes was observed over a 10-year period in younger Irish adults, which is potentially attributable to adherence to low-fat dairy product public health messages(Reference Li, McNulty and Tiernery40). Consistent with this, data from the latest UK food consumption survey reported a 9% reduction in whole milk consumption; however the overall percentage contribution of milk and milk products to dietary fat remained unchanged(Reference Bates, Lennox and Prentice41). Future evidence from prospective cohort studies will further elucidate the benefit of low-fat dairy consumption. Interestingly, recent evidence from the UK National Diet and Nutrition Survey highlighted the efficacy of product reformulation in reducing trans-fat intakes, wherein following product reformulation, only 2·5% of adults exceeded the WHO recommendation of <1% TE, compared with 57% pre-reformulation(Reference Hutchinson, Rippin and Jewell99). Therefore, this highlights the potential of improving the profile of commonly consumed foods in reducing disease risk.
Red meat is one of the primary sources of dietary SFA, along with providing many essential vitamins and minerals. Red meat, in particular processed red meat, has been associated with increased risk of CHD(Reference Micha, Wallace and Mozaffarian100) and diabetes(Reference Pan, Sun and Bernstein101). However, the associations were derived from observational studies; therefore it is not plausible to infer causality. No association was observed between a high processed red meat dietary pattern and markers of CVD and T2D risk, including cholesterol, in the latest adult Irish food consumption survey(Reference Lenighan, Nugent and Li102). Nonetheless, due to the ingredient profile of processed red meat, recent modelling studies have demonstrated product reformulation as a potential strategy to reduce SFA and sodium intake and infer a health benefit(Reference Masset, Mathias and Vlassopoulos103, Reference Gressier, Privet and Mathias104).
Animal feeding practices to alter food composition
An additional reformulation strategy is modification of the fatty acid composition of beef and dairy products through ruminant grass-based feeding practices. This results in a reduction of SFA and an increase in PUFA concentrations, in particular ALA and conjugated linoleic acid(Reference Daley, Abbott and Doyle105). A limited number of studies have investigated the health impact of consumption of grass-fed red meat or dairy products. A RCT by McAfee et al. identified a significant increase in plasma, platelet and dietary intakes of LC n-3 PUFA after replacement of habitual red meat consumption (<500 g/week) with grass-fed beef or lamb for 4 weeks(Reference McAfee, McSorley and Cuskelly106). The impact of modifying the ruminant diet to improve milk fat was recently reviewed and concluded that it was an effective strategy to reduce population SFA intakes but that further research is required to optimise the palatability for consumers(Reference Givens107). In 2018, Benbrook and colleagues applied a dietary modelling approach to investigate the impact of grass-fed milk consumption on dietary fat intakes. Consumption of grass-fed milk was estimated to decrease LA intakes and the LA:ALA ratio, and increase intakes of ALA, which consequently improves LC n-3 PUFA precursor bioavailability(Reference Benbrook, Davis and Heins108). It is evident that further research is required to fully elucidate the impact of grass-fed meat/dairy consumption on markers of health, and also to investigate if the fatty acid profile of grass-fed beef could be further enhanced with flaxseed or alternate ALA supplementation. However, the evidence to date suggests that habitual consumption of unprocessed red meat and dairy products following reformulation with grass-based feeding practices has the potential to improve dietary fat quality, within current dietary red meat guidelines of <500 g per week(109).
Health v. food sustainability issues
Whilst grass-fed beef consumption provides a potential strategy to reduce SFA and increase PUFA intakes without altering habitual dietary consumption it is important to consider the sustainability of beef. Ruminant animals currently produce one-third of the global protein, and demand is set to increase based on the growing population. A recent review by Layman et al. recommended implementation of strategies to optimise land use and minimise environmental impact for production of high quality protein(Reference Layman110). Grass-fed beef had previously been associated with a large environmental footprint due to methane production and land use. However, a recent report suggested that both grass-fed and concentrate-fed beef elicit a similar environmental impact(Reference Garnett, Godde and Muller111). Therefore, consumption of lower quantities of high-quality beef protein, as part of a healthy diet, is a potential strategy to meet global protein requirements and reduce the environmental impact. Moreover, oily fish is the primary dietary source of LC n-3 PUFA, however due to the depletion of fish stocks and the estimated population growth, it is predicted that the fish stocks alone will not be adequate to provide sufficient LC n-3 PUFA intakes(Reference Baker, Miles and Burdge37). Hence, to sustain intakes a complementary LC n-3 PUFA source will be required. Alternate strategies include algal oil, GM oil seeds and biosynthesis from plant-based ALA(Reference Sanders112).
Conclusions
Whilst modification of foods to reduce SFA and replace it with PUFA is an attainable public health strategy to reduce SFA intakes and subsequently disease risk, there are still gaps in the knowledge base as to the adequate dose of PUFA replacement. Research needs to be completed and validated in a number of populations to establish if the replacement PUFA dose varies by age, sex and habitual dietary intakes. Furthermore, the interplay from confounding dietary and non-dietary factors needs to be considered. The food industry and nutrition researchers are faced with the challenge of implementing long-term RCT, with controlled diet and lifestyle parameters, to answer this pertinent research question in order to improve overall dietary quality and optimise health outcomes. Moreover, it is apparent that not all SFA and PUFA exert the same effects, thus replacement strategies need to consider fat quality, as well as consumer palatability to ensure adherence to the public health initiative. The global obesity epidemic is greater than fat quality alone, therefore simultaneous, effective public health strategies are required to achieve a healthy diet and lifestyle, and collectively reduce disease risk.
Acknowledgements
Y. M. L. was funded by the Irish Department of Agriculture, Food and the Marine ‘Healthy Beef’ programme (grant number 13/F/514). B. A. M. was supported by the Irish Department of Agriculture, Food and the Marine National Teen's Food Consumption Survey II (NTFS II; 17 F 231) and the National Children's Food Consumption Survey II (NCFS II; 15 F 673). H. M. R. was supported by Science Foundation Ireland (SFI) principal investigator award (11/PI/1119); Joint Programming Healthy Life for a Healthy Diet (JPI HDHL) funded EU Food Biomarkers Alliance ‘FOODBALL’ (14/JP-HDHL/B3076); the Irish Department of Agriculture, Food and the Marine, ‘Healthy Beef’ (13/F/514) and ‘ImmunoMet - dietary manipulation of microbiota diversity for controlling immune function’ (14/F/828) programmes.
Financial Support
The present work was supported by funding from the Irish Department of Agriculture, Food and the Marine under the National Development Plan (2007–2013) (grant number 13/F/514). The Irish Department of Agriculture, Food and the Marine had no role in the design, analysis or writing of the present paper.
Conflict of Interest
None.
Authorship
Y. M. L. completed the review. B. A. M. and H. M. R. advised in relation to content, and critically evaluated the manuscript. All authors have read and approved the final manuscript.