Introduction
Carbon dioxide (CO2) is an important sensory cue for animals across diverse phyla, including Nematoda (Lahiri and Forster, Reference Lahiri and Forster2003; Shusterman and Avila, Reference Shusterman and Avila2003; Bensafi et al., Reference Bensafi, Frasnelli, Reden and Hummel2007; Smallegange et al., Reference Smallegange, Verhulst and Takken2011; Carrillo and Hallem, Reference Carrillo and Hallem2015). While the CO2 concentration in ambient air is approximately 0.038% (Scott, Reference Scott2011), many nematodes encounter much higher levels of CO2 in their microenvironment during the course of their life cycles. For instance, parasitic nematodes may encounter high CO2 concentrations released from potential hosts as a byproduct of respiration or from the host feces within which they develop (Byrnes et al., Reference Byrnes, Dinarevic, Shinebourne, Barnes and Bush1997; Buszewski et al., Reference Buszewski, Kesy, Ligor and Amann2007; Carrillo and Hallem, Reference Carrillo and Hallem2015). CO2 concentrations are also high in specific tissues such as the venous bloodstream, lungs and intestine (Jensen and Jorgensen, Reference Jensen and Jorgensen1994; Rotbart et al., Reference Rotbart, Yao, Ha, Chrispa, Muir, Gibson, Kalantar-Zadeh and Ou2017), suggesting CO2 may be an important intra-host cue for parasitic nematodes (Hawdon and Schad, Reference Hawdon and Schad1990; Bekelaar et al., Reference Bekelaar, Waghorn, Tavendale, McKenzie and Leathwick2018, Reference Bekelaar, Waghorn, Tavendale, McKenzie and Leathwick2019). Moreover, many free-living nematodes are found in rotting vegetation, where CO2 levels are often high (Burg and Burg, Reference Burg and Burg1965; Felix and Duveau, Reference Felix and Duveau2012). Therefore, nematodes must detect and respond appropriately to elevated CO2 concentrations to survive, navigate through their microenvironment and propagate.
CO2 may serve as a beneficial or detrimental cue for nematodes depending on specific circumstances (Carrillo and Hallem, Reference Carrillo and Hallem2015). For instance, in the case of parasitic nematodes, CO2 may be necessary to promote parasite–host interactions and thus support their parasitic life cycle. For free-living nematodes, high CO2 levels present in their natural habitats may act as signals for food, predators, pathogens or conspecifics (Carrillo and Hallem, Reference Carrillo and Hallem2015). Because CO2 is a complex cue that can have either a positive or negative valence, it is not surprising that different species of nematodes have developed distinct behavioural and physiological responses to CO2. Moreover, many species, both free-living and parasitic, exhibit CO2 responses that vary with context, previous experience and/or life stage. Recent studies of the free-living model nematode Caenorhabditis elegans have provided insight into the cellular and molecular mechanisms that drive and modulate CO2-evoked responses. In contrast, the mechanisms that promote the diverse responses of parasitic nematodes to CO2 have not yet been elucidated due to the historic lack of tools required for molecular genetic studies of these worms. However, as a result of recent developments in molecular genetic techniques, we are now in a position to interrogate the neural circuits and molecular signals that promote CO2 responses in parasitic nematodes. The findings from these studies will enhance our understanding of the role of CO2 in sculpting parasite–host interactions and may enable the development of novel strategies to combat harmful nematode infections. Here, we review our existing knowledge of how various nematode species respond behaviourally and physiologically to CO2. We also discuss how CO2 responsiveness can be modulated based on context, previous experience and life stage.
Responses of mammalian-parasitic nematodes to CO2
Introduction to parasitic nematodes of mammals
Mammalian-parasitic nematodes infect over a billion people worldwide and are a major cause of morbidity in low-resource areas (Boatin et al., Reference Boatin, Basanez, Prichard, Awadzi, Barakat, Garcia, Gazzinelli, Grant, McCarthy, N'Goran, Osei-Atweneboana, Sripa, Yang and Lustigman2012). Infections with soil-transmitted nematodes can cause chronic gastrointestinal distress, stunted growth and cognitive impairment in children, anaemia and even fatality in infants and immunocompromised individuals (Lustigman et al., Reference Lustigman, Prichard, Gazzinelli, Grant, Boatin, McCarthy and Basanez2012). Infections with vector-transmitted nematodes can cause severe symptoms such as permanent disfigurement and blindness (Lustigman et al., Reference Lustigman, Prichard, Gazzinelli, Grant, Boatin, McCarthy and Basanez2012). Additionally, parasitic nematodes that infect livestock are an enormous source of economic burden (Jasmer et al., Reference Jasmer, Goverse and Smant2003). Current treatments for infections depend on anthelminthic drugs that reduce the worm burden in heavier infections but do not prevent reinfections, with the result that reinfection is common in endemic areas (Prichard et al., Reference Prichard, Basanez, Boatin, McCarthy, Garcia, Yang, Sripa and Lustigman2012). In addition, drug resistance resulting from mass drug administration is a major challenge for the treatment of nematode-infected livestock (Kumar et al., Reference Kumar, Rao, Varghese and Rathor2013; Roeber et al., Reference Roeber, Jex and Gasser2013; Emery et al., Reference Emery, Hunt and Le Jambre2016; Learmount et al., Reference Learmount, Stephens, Boughtflower, Barrecheguren and Rickell2016) and is expected to be a concern for the treatment of nematode-infected humans in the near future (Keiser and Utzinger, Reference Keiser and Utzinger2008; Diawara et al., Reference Diawara, Schwenkenbecher, Kaplan and Prichard2013; Repetto et al., Reference Repetto, Ruybal, Batalla, Lopez, Fridman, Sierra, Radisic, Bravo, Risso, Gonzalez Cappa and Alba Soto2018). The drugs currently available are also not sufficient to eliminate human infections in all cases, at least by following the administration schedules under practice (Repetto et al., Reference Repetto, Ruybal, Batalla, Lopez, Fridman, Sierra, Radisic, Bravo, Risso, Gonzalez Cappa and Alba Soto2018).
Many of these nematodes are gastrointestinal parasites with a developmentally arrested infective larval stage that inhabits the environment and infects hosts following either skin penetration or passive ingestion, depending on the species (Gang and Hallem, Reference Gang and Hallem2016; Bryant and Hallem, Reference Bryant and Hallem2018). The infective larval stages of these species respond robustly to a diverse array of host and environmental sensory cues, including CO2 (Gang and Hallem, Reference Gang and Hallem2016; Bryant and Hallem, Reference Bryant and Hallem2018). In addition, many parasitic nematodes may rely on sensory cues inside the host body, including CO2, to re-initiate development upon host entry, direct somatic migration and establish a successful infection (Hawdon and Schad, Reference Hawdon and Schad1990, Reference Hawdon and Schad1992; Hawdon et al., Reference Hawdon, Volk, Pritchard and Schad1992; Bekelaar et al., Reference Bekelaar, Waghorn, Tavendale, McKenzie and Leathwick2018, Reference Bekelaar, Waghorn, Tavendale, McKenzie and Leathwick2019).
Responses of skin-penetrating nematodes to CO2
Skin-penetrating nematodes such as the human-parasitic hookworms Ancylostoma duodenale and Necator americanus and the human-parasitic threadworm Strongyloides stercoralis are gastrointestinal parasites that infect hosts as developmentally arrested third-stage larvae (iL3s) (Roberts et al., Reference Roberts, Janovy and Schmidt2005; Nutman, Reference Nutman2017; Velikkakam et al., Reference Velikkakam, Fiuza, Gaze and Singh2017). The iL3s are soil-dwelling and actively engage in host seeking using a variety of host-associated sensory cues (Gang and Hallem, Reference Gang and Hallem2016; Bryant and Hallem, Reference Bryant and Hallem2018). These parasites generally have narrow host ranges, infecting only a limited number of host species (Haley, Reference Haley1961; Bezubik, Reference Bezubik1965; Nolan et al., Reference Nolan, Zhu, Ketschek, Cole, Grant, Lok and Schad2007; Viney and Lok, Reference Viney and Lok2007; Viney and Kikuchi, Reference Viney and Kikuchi2017). After invading a host by skin penetration, the iL3s resume development inside the host, a process called activation (Stoltzfus et al., Reference Stoltzfus, Massey, Nolan, Griffith and Lok2012, Reference Stoltzfus, Bart and Lok2014). The nematodes then migrate through the host body to their final destination, the small intestine, where they reside as parasitic adults (Roberts et al., Reference Roberts, Janovy and Schmidt2005; Nutman, Reference Nutman2017; Velikkakam et al., Reference Velikkakam, Fiuza, Gaze and Singh2017). The adults reproduce in the small intestine, and then the eggs or young larvae, depending on the species, exit the host body in feces. The nematodes inhabit the feces until they develop into iL3s (Roberts et al., Reference Roberts, Janovy and Schmidt2005; Nutman, Reference Nutman2017; Velikkakam et al., Reference Velikkakam, Fiuza, Gaze and Singh2017). In most species, all of the progeny of the parasitic adults develop directly into iL3s. However, Strongyloides species can cycle through one or a limited number of free-living generations on the feces before developmentally arresting as iL3s (Roberts et al., Reference Roberts, Janovy and Schmidt2005). Strongyloides stercoralis can also pass through multiple generations inside the same host through autoinfective cycles (Roberts et al., Reference Roberts, Janovy and Schmidt2005).
Many skin-penetrating nematodes show behavioural responses to CO2. For example, iL3s of the dog hookworm Ancylostoma caninum display increased nictation in the presence of CO2 (Granzer and Haas, Reference Granzer and Haas1991). Nictation is a specialized behaviour displayed by many parasitic nematodes in which the worm stands on its tail and waves its head in the air to facilitate attachment to mobile hosts (Granzer and Haas, Reference Granzer and Haas1991; Bryant and Hallem, Reference Bryant and Hallem2018). In addition, both Ancylostoma caninum and Strongyloides stercoralis iL3s exhibit increased movement when exposed to human breath, and this behaviour is not observed when CO2 is removed from the breath (Sciacca et al., Reference Sciacca, Forbes, Ashton, Lombardini, Gamble and Schad2002). Similarly, the human-parasitic hookworms Ancylostoma duodenale and Necator americanus display increased activity in response to CO2 in combination with heat and/or moisture (Haas et al., Reference Haas, Haberl, Idris, Kallert, Kersten and Stiegeler2005). The similar responses of Strongyloides stercoralis and hookworms to CO2 is particularly notable given their phylogenetic divergence, with Strongyloides stercoralis in clade IV and hookworms in clade V (Blaxter and Koutsovoulos, Reference Blaxter and Koutsovoulos2015; Blaxter et al., Reference Blaxter, Koutsovoulos, Jones, Kumar, Elsworth, Olson, Hughes and Cotton2016). However, these studies did not look at migration in CO2 gradients, and whether CO2 was an attractant or repellent was not clear.
More recent studies demonstrated that skin-penetrating iL3s of the human parasites Strongyloides stercoralis and Ancylostoma ceylanicum and the rat parasites Strongyloides ratti and Nippostrongylus brasiliensis are repelled by CO2 in CO2-chemotaxis assays (Fig. 1A, B) (Castelletto et al., Reference Castelletto, Gang, Okubo, Tselikova, Nolan, Platzer, Lok and Hallem2014; Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017). A lack of attraction towards CO2 is consistent with the route of infection of skin-penetrating nematodes, since mammalian skin surfaces emit low concentrations of CO2 (Alkalay et al., Reference Alkalay, Suetsugu, Constantine and Stein1971). On the other hand, fecal deposits contain high levels of CO2 resulting from aerobic respiration of fecal bacteria (Jensen and Jorgensen, Reference Jensen and Jorgensen1994; de Lacy Costello et al., Reference de Lacy Costello, Amann, Al-Kateb, Flynn, Filipiak, Khalid, Osborne and Ratcliffe2014; Rotbart et al., Reference Rotbart, Yao, Ha, Chrispa, Muir, Gibson, Kalantar-Zadeh and Ou2017), and CO2 repulsion may drive these iL3s off of host feces and into the environment in search of new hosts.
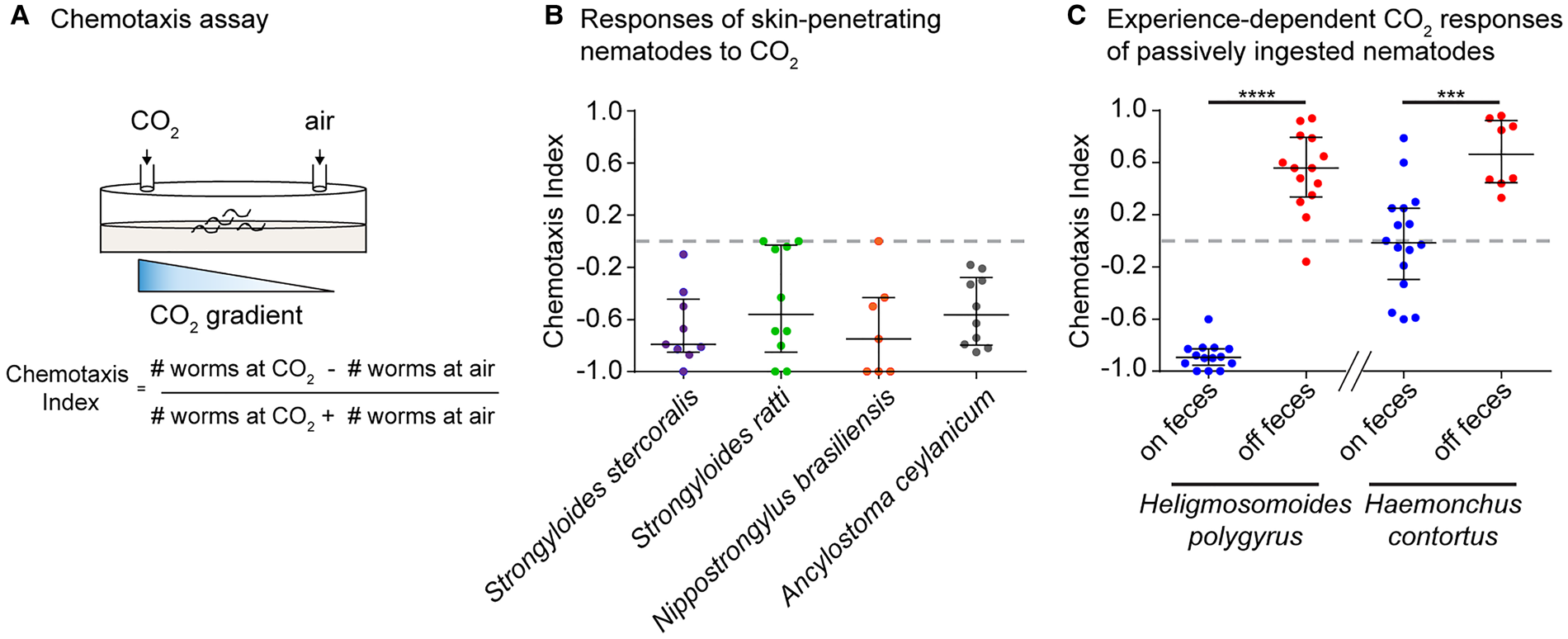
Fig. 1. Behavioural responses of mammalian-parasitic nematodes to CO2. (A) A CO2-chemotaxis assay. CO2 and air are pumped into opposite sides of a 10 cm plate. Infective larvae (iL3s) are placed at the centre and allowed to migrate for 1 h. A chemotaxis index is then calculated according to the formula indicated; a positive index indicates attraction and a negative index indicates repulsion. (B) Behavioural responses of skin-penetrating iL3s to 10% CO2. All species tested are repelled by CO2. Data are from Castelletto et al. (Reference Castelletto, Gang, Okubo, Tselikova, Nolan, Platzer, Lok and Hallem2014) and Ruiz et al. (Reference Ruiz, Castelletto, Gang and Hallem2017). (C) Experience-dependent changes in CO2 responsiveness in the passively ingested nematodes Heligmosomoides polygyrus and Haemonchus contortus. CO2 responses switch from repulsion (in Heligmosomoides polygyrus) or neutral (in Haemonchus contortus) to attraction following removal from host feces for days to weeks. Heligmosomoides polygyrus was tested with 10% CO2; Haemonchus contortus was tested with 15% CO2. Figure adapted from Ruiz et al. (Reference Ruiz, Castelletto, Gang and Hallem2017). Graphs show medians and interquartile ranges. ****P < 0.0001, ***P < 0.001, Mann–Whitney test for each species.
Responses of passively ingested nematodes to CO2
Many passively ingested gastrointestinal nematodes have a motile environmental iL3 stage that invades hosts after being swallowed. For example, iL3s of the ruminant parasite Haemonchus contortus inhabit the soil and infect after being swallowed by grazing animals (O'Connor et al., Reference O'Connor, Walkden-Brown and Kahn2006). After entering a host, the nematodes exsheath in the rumen and travel to the abomasum, where they develop into parasitic adults (Laing et al., Reference Laing, Kikuchi, Martinelli, Tsai, Beech, Redman, Holroyd, Bartley, Beasley, Britton, Curran, Devaney, Gilabert, Hunt, Jackson, Johnston, Kryukov, Li, Morrison, Reid, Sargison, Saunders, Wasmuth, Wolstenholme, Berriman, Gilleard and Cotton2013). Similarly, the murine gastrointestinal parasite Heligmosomoides polygyrus has an iL3 stage that can infect mice either from feces during coprophagy or from the fur during grooming (Hernandez and Sukhdeo, Reference Hernandez and Sukhdeo1995). Despite their passive route of infection, both Haemonchus contortus and Heligmosomoides polygyrus actively migrate towards host-associated sensory cues. This suggests that these species use host-associated cues to position themselves in the vicinity of potential hosts, where they are more likely to be ingested (Hernandez and Sukhdeo, Reference Hernandez and Sukhdeo1995; Castelletto et al., Reference Castelletto, Gang, Okubo, Tselikova, Nolan, Platzer, Lok and Hallem2014; Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017; Bryant et al., Reference Bryant, Ruiz, Gang, Castelletto, Lopez and Hallem2018).
Examination of the CO2-evoked behaviours of Haemonchus contortus and Heligmosomoides polygyrus revealed that both species show experience-dependent responses to CO2 (Fig. 1C) (Castelletto et al., Reference Castelletto, Gang, Okubo, Tselikova, Nolan, Platzer, Lok and Hallem2014; Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017). In the case of Heligmosomoides polygyrus, iL3s extracted directly from feces are repelled by CO2, while iL3s that have been removed from feces for multiple days – a condition designed to mimic the soil environment of iL3s – are attracted to CO2 (Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017). This shift in CO2 preference appears to occur as a result of the drop in ambient CO2 levels experienced by the iL3s after they migrate off feces, since cultivating iL3s off feces under high CO2 conditions (2.5% CO2) prevents the behavioural switch. The initial repulsion from CO2 experienced by Heligmosomoides polygyrus iL3s on feces may enable them to disperse off of feces and into the environment to host seek. Following a prolonged period without feces, CO2 attraction may drive them towards new hosts or fresh host feces to increase their chances of host entry through ingestion (Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017).
In the case of Haemonchus contortus, iL3s directly removed from feces are neutral to CO2, whereas iL3s that have been removed from feces for a week or more are attracted to CO2 (Castelletto et al., Reference Castelletto, Gang, Okubo, Tselikova, Nolan, Platzer, Lok and Hallem2014; Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017). This experience-dependent shift in CO2-evoked behaviour may enable the iL3s to migrate towards the mouths of grazing ruminants, whose breath emits high concentrations of CO2 (Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017). In contrast to Heligmosomoides polygyrus and Haemonchus contortus, the skin-penetrating nematodes Ancylostoma ceylanicum, Strongyloides stercoralis and Strongyloides ratti do not display this flexibility in their behavioural responses to CO2. Thus, experience-dependent plasticity towards CO2 may be unique to passively ingested nematodes (Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017). However, skin-penetrating nematodes do show other forms of sensory plasticity, including experience-dependent thermal plasticity and temperature-dependent olfactory plasticity (Lee et al., Reference Lee, Dillman and Hallem2016; Bryant et al., Reference Bryant, Ruiz, Gang, Castelletto, Lopez and Hallem2018). This suggests that experience-dependent responses to CO2 may not be beneficial for skin-penetrating nematodes, likely because the skin surface of mammals emits only very low levels of CO2 (Alkalay et al., Reference Alkalay, Suetsugu, Constantine and Stein1971).
The role of CO2 in mammalian-parasitic nematode development and physiology
In addition to being a robust behavioural cue for parasitic nematodes, CO2 is also an important regulator of their development and physiology. For example, CO2 stimulates exsheathment and activation (exit from the developmentally arrested iL3 stage) in passively ingested ruminant parasites such as Haemonchus contortus (Rogers and Sommerville, Reference Rogers and Sommerville1960; Taylor and Whitlock, Reference Taylor and Whitlock1960; Sommerville, Reference Sommerville1964; Bekelaar et al., Reference Bekelaar, Waghorn, Tavendale, McKenzie and Leathwick2018, Reference Bekelaar, Waghorn, Tavendale, McKenzie and Leathwick2019). However, the requirement for CO2 during exsheathment varies across species. CO2 is an absolute requirement for the exsheathment of Haemonchus contortus iL3s, whereas CO2 enhances but is not required for exsheathment in other passively ingested abomasal nematodes (Bekelaar et al., Reference Bekelaar, Waghorn, Tavendale, McKenzie and Leathwick2018). In the dog hookworm Ancylostoma caninum, CO2 is not required for activation but results in a slight increase in the rate of activation (Hawdon and Schad, Reference Hawdon and Schad1990).
The role of CO2 is not limited to exsheathment and activation. CO2, in combination with O2, also regulates the development of Strongyloides ratti into either free-living adults or iL3s (Taylor and Weinstein, Reference Taylor and Weinstein1990). In addition, CO2 stimulates egg hatching in the giant roundworm Ascaris lumbricoides, a human-parasitic species that infects when eggs containing developmentally arrested infective larvae are swallowed by hosts as a result of fecal–oral contamination (Fairbairn, Reference Fairbairn1961; Dold and Holland, Reference Dold and Holland2011). Finally, CO2 is required for the in vitro development of parasitic larvae in the pig roundworm Ascaris suum (Douvres and Urban, Reference Douvres and Urban1983). Thus, CO2 influences both behaviour and development in many if not all mammalian-parasitic nematode species.
Responses of entomopathogenic nematodes to CO2
Introduction to entomopathogenic nematodes
Entomopathogenic nematodes (EPNs) are parasites that infect and kill insects (Dillman and Sternberg, Reference Dillman and Sternberg2012). They are considered beneficial for humans due to their role as biological agents for pest control, and are likely also important for maintaining balanced ecosystems in nature. EPNs of the genera Heterorhabditis and Steinernema have been successfully employed commercially against insect agricultural pests (Liu et al., Reference Liu, Poinar and Berry2000; Grewal et al., Reference Grewal, Ehlers and Shapiro-Ilan2005; Dillman and Sternberg, Reference Dillman and Sternberg2012; Labaude and Griffin, Reference Labaude and Griffin2018). The geographical distribution of EPNs spans all continents except Antarctica (Hominick, Reference Hominick and Gaugler2002). Some EPNs, such as Steinernema carpocapsae and Heterorhabditis bacteriophora, are generalists that can infect many different insects; in contrast, other EPNs have very narrow host ranges (Peters, Reference Peters1996). For example, the specialist Steinernema scapterisci specifically infects mole crickets, and the specialist Steinernema diaprepesi specifically infects the larval stages of the citrus pest Diaprepes abbreviatus (Nguyen and Smart, Reference Nguyen and Smart1991; Nguyen and Hunt, Reference Nguyen and Hunt2007; Ali et al., Reference Ali, Alborn and Stelinski2010). EPNs infect only as third-stage larvae called infective juveniles (IJs); the IJ stage of EPNs is equivalent to the iL3 stage of mammalian-parasitic nematodes (Dillman et al., Reference Dillman, Chaston, Adams, Ciche, Goodrich-Blair, Stock and Sternberg2012a). IJs enter their insect hosts through a body orifice such as the mouth, spiracles or anus; IJs of some species can also penetrate directly through the cuticle (Bedding and Molyneux, Reference Bedding and Molyneux1982; Kaya and Gaugler, Reference Kaya and Gaugler1993). The IJs then enter the insect haemocoel and release a bacterial symbiont from their intestine (Bedding and Molyneux, Reference Bedding and Molyneux1982; Kaya and Gaugler, Reference Kaya and Gaugler1993). Toxins secreted by the nematode and the bacteria kill the insect, typically within 48 h (Kaya and Gaugler, Reference Kaya and Gaugler1993; Lu et al., Reference Lu, Macchietto, Chang, Barros, Baldwin, Mortazavi and Dillman2017; Chang et al., Reference Chang, Serra, Lu, Mortazavi and Dillman2019). The nematodes then feed on the insect cadaver and complete their parasitic life cycle. The nematodes can cycle through multiple generations in the host cadaver until resources are depleted, at which point new IJs form and disperse into the environment to seek out new hosts (Kaya and Gaugler, Reference Kaya and Gaugler1993).
The role of CO2 in the host-seeking behaviours of EPNs
The host-seeking strategies of EPNs vary across species. Some species are considered ‘cruisers’ that actively migrate towards stationary hosts, other species are considered ‘ambushers’ that remain relatively stationary and nictate to facilitate attachment to mobile hosts, and still other species use an intermediate strategy (Campbell and Gauger, Reference Campbell and Gauger1993; Lewis, Reference Lewis and Gauger2002; Lewis et al., Reference Lewis, Campbell, Griffin, Kaya and Peters2006). However, both ambushers and cruisers are capable of migrating towards host-emitted chemosensory cues, suggesting that all EPNs engage in chemosensory-driven navigation towards hosts (Schmidt and All, Reference Schmidt and All1979; Pye and Burman, Reference Pye and Burman1981; O'Halloran and Burnell, Reference O'Halloran and Burnell2003; Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a; Dillman et al., Reference Dillman, Guillermin, Lee, Kim, Sternberg and Hallem2012b; Castelletto et al., Reference Castelletto, Gang, Okubo, Tselikova, Nolan, Platzer, Lok and Hallem2014; Lee et al., Reference Lee, Dillman and Hallem2016). Some EPNs in the genus Steinernema also engage in a unique jumping behaviour where the IJ stands on its tail and then propels itself into the air, presumably to facilitate host attachment as well as transport to new niches (Campbell and Kaya, Reference Campbell and Kaya1999). Jumping can be stimulated by exposure to host-emitted chemosensory cues (Campbell and Kaya, Reference Campbell and Kaya1999, Reference Campbell and Kaya2000; Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a; Dillman et al., Reference Dillman, Guillermin, Lee, Kim, Sternberg and Hallem2012b).
Many EPNs, including Heterorhabitis bacteriophora, Steinernema carpocapsae, Steinernema riobrave, Steinernema scapterisci and Steinernema glaseri, are attracted to CO2 (Fig. 2A) (Gaugler et al., Reference Gaugler, LeBeck, Nakagaki and Boush1980, Reference Gaugler, Campbell and Gupta1991; Lewis et al., Reference Lewis, Gauger and Harrison1993; Robinson, Reference Robinson1995; Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a; Dillman et al., Reference Dillman, Guillermin, Lee, Kim, Sternberg and Hallem2012b; Lee et al., Reference Lee, Dillman and Hallem2016). This group includes both specialists and generalists, and both ambushers and cruisers. In addition, CO2 stimulates jumping in Steinernema carpocapsae, Steinernema riobrave and Steinernema scapterisci IJs at concentrations as low as 0.08% (approximately two times higher than atmospheric levels), suggesting that jumping is highly sensitive to environmental CO2 (Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a; Dillman et al., Reference Dillman, Guillermin, Lee, Kim, Sternberg and Hallem2012b). Both the attractive responses of EPNs towards the odour of live insect hosts and jumping responses to host odour are decreased when CO2 is chemically removed using a soda lime filter, illustrating the importance of CO2 for host seeking (Gaugler et al., Reference Gaugler, Campbell and Gupta1991; Dillman et al., Reference Dillman, Guillermin, Lee, Kim, Sternberg and Hallem2012b). However, the extent to which host attraction is reduced in the absence of CO2 varies across different EPN–host combinations (Dillman et al., Reference Dillman, Guillermin, Lee, Kim, Sternberg and Hallem2012b). Thus, EPNs use CO2 in combination with host-specific olfactory cues to migrate towards insects. CO2 also acts synergistically with plant root volatiles to attract some EPNs to plants infested with insects (Turlings et al., Reference Turlings, Hiltpold and Rasmann2012).

Fig. 2. Behavioural responses of entomopathogenic nematodes (EPNs) to CO2. (A) Behavioural responses of the infective juveniles (IJs) of various EPN species to CO2 in a chemotaxis assay (Fig. 1A). All EPNs tested showed attraction to 2.5% CO2. Data are from Dillman et al. (Reference Dillman, Guillermin, Lee, Kim, Sternberg and Hallem2012b) and Hallem et al. (Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a). (B) Age-dependent changes in the CO2 preferences of Steinernema scapterisci IJs. IJs were grown at room temperature (approximately 22 °C) and then incubated at 15 °C until the indicated time points (days post-collection). CO2 responsiveness changes from repulsion to attraction with age. IJs were tested with 1% CO2. Data are from Lee et al. (Reference Lee, Dillman and Hallem2016). The graph shows medians and interquartile ranges.
Like some mammalian-parasitic nematodes, some EPNs exhibit plasticity in their olfactory responses to CO2. For example, the IJs of Steinernema scapterisci are repelled by CO2 immediately after emergence from the insect host, but their response shifts to robust attraction over the course of weeks (Fig. 2B) (Lee et al., Reference Lee, Dillman and Hallem2016). In addition, the rate at which this shift occurs varies depending on the cultivation temperature of the IJs, with the shift occurring more rapidly in IJs cultured at 15 °C relative to IJs cultured at 25 °C (Lee et al., Reference Lee, Dillman and Hallem2016). The change in CO2 preference correlates with a corresponding change in responses to host odours, including the odour of its natural host, the mole cricket (Lee et al., Reference Lee, Dillman and Hallem2016). The strong repulsion of newly emerged Steinernema scapterisci IJs to CO2 and host odours may serve as a dispersal mechanism to drive them towards new niches. The mechanisms that drive the change in CO2 preference following host emergence in Steinernema scapterisci have not yet been elucidated. An intriguing possibility is that the CO2 repulsion exhibited by IJs immediately following host emergence could result from the elevated levels of CO2 experienced inside the decaying insect cadaver, similar to the way in which CO2 repulsion in Heligmosomoides polygyrus iL3s results from the elevated levels of CO2 experienced on mammalian feces (Ruiz et al., Reference Ruiz, Castelletto, Gang and Hallem2017). However, additional experiments will be necessary to determine whether the change in CO2 preference over time (or age) in Steinernema scapterisci is in fact regulated by ambient CO2 levels.
Responses of plant-parasitic nematodes to CO2
Introduction to plant-parasitic nematodes
Plant-parasitic nematodes (PPNs) are a major cause of agricultural crop damage throughout the world. It has been estimated that PPNs are responsible for approximately 100 billion dollars of crop loss per year worldwide (Jasmer et al., Reference Jasmer, Goverse and Smant2003; Wrather et al., Reference Wrather, Koenning and Anderson2003). Of over 4100 species of PPNs that have been identified (Decraemer and Hunt, Reference Decraemer, Hunt, Perry and Moens2006), the ones that cause the most severe economic loss are the nematodes that infect the roots of major agricultural crops (Bernard et al., Reference Bernard, Egnin, Bonsi, Shah and Mahamood2017). These PPNs prevent water and nutrient uptake by plant roots, which results in greatly reduced crop quality and yield (Bernard et al., Reference Bernard, Egnin, Bonsi, Shah and Mahamood2017).
Responses of PPNs to CO2
CO2 is ubiquitously produced by the roots of plants. Several studies have demonstrated an important role for CO2 in mediating attraction of PPNs to their host plants. For example, the stem nematode Ditylenchus dipsaci, which infects onion and garlic, migrates towards CO2 (Klingler, Reference Klingler1972; Viglierchio, Reference Viglierchio1990). Many other PPNs, including species from the genera Ditylenchus, Meloidogyne, Heterodera and Pratylenchus, are also attracted to CO2 (Johnson and Viglierchio, Reference Johnson and Viglierchio1961; Prot, Reference Prot1980; McCallum and Dusenbery, Reference McCallum and Dusenbery1992; Robinson, Reference Robinson1995). In the case of Meloidogyne incognita, attraction to tomato root volatiles appears to be due to the presence of O2 and CO2 in the volatile mix (McCallum and Dusenbery, Reference McCallum and Dusenbery1992). However, a more recent study found that for Meloidogyne hapla, the attractant is not CO2 itself but rather the low pH environment created by dissolved CO2 (Wang et al., Reference Wang, Bruening and Williamson2009). In the case of the pine wilt nematode Bursaphelenchus xylophilus, the fourth-stage juveniles (JIVs) are repelled by CO2. CO2 repulsion by JIVs plays an important role in dispersal from its insect vector, the pine sawyer beetle, into the pine tree (Wu et al., Reference Wu, Wickham, Zhao and Sun2019). Bursaphelenchus xylophilus JIVs enter the beetle tracheal system, where they are transported by the beetle to new pine trees. As the beetle matures and feeds on the pine tree, CO2 levels in the beetle tracheal system increase. Once CO2 levels reach a certain concentration, CO2 repulsion drives the JIVs out of the beetle spiracles and into the pine tree (Wu et al., Reference Wu, Wickham, Zhao and Sun2019). Thus, the responses of PPNs to CO2 vary greatly across species. A better understanding of how other PPNs respond to CO2 may enable the development of new biocontrol strategies.
Responses of free-living nematodes to CO2
Introduction to free-living nematodes
Free-living nematodes are found in a wide range of ecological habitats. These include various types of soil, sediment and organic matter, as well as marine and freshwater environments. Free-living nematodes use a wide variety of sensory cues to navigate their environment in search of food and mates, and to escape from predators and pathogens. CO2 is universally present in terrestrial and aquatic habitats, and may serve as an important cue for survival and propagation of these nematodes. The most well-studied free-living nematode is the model worm Caenorhabditis elegans. C. elegans is commonly found in microbe-rich environments such as those of fallen rotting fruits (Felix and Duveau, Reference Felix and Duveau2012), where CO2 is produced as one of many microbial byproducts. Consequently, C. elegans displays several behavioural and physiological responses to CO2.
Behavioural responses of C. elegans to carbon dioxide
The first studies of CO2 responsiveness in C. elegans demonstrated that these worms undergo rapid changes in locomotion in response to changes in CO2 concentrations (Dusenbery, Reference Dusenbery1985). These responses are characterized by an overall decrease in movement and an increase in turning frequency (Dusenbery, Reference Dusenbery1985). A more recent study examining the effects of acute CO2 exposure found that freely moving well-fed adults reverse rapidly when their head is exposed to high levels of CO2, indicating that CO2 is an aversive cue for well-fed C. elegans adults (Hallem and Sternberg, Reference Hallem and Sternberg2008). In addition, well-fed C. elegans adults avoid high CO2 areas when allowed to migrate along a CO2 gradient in a CO2-chemotaxis assay (Fig. 3A) (Bretscher et al., Reference Bretscher, Busch and de Bono2008). For well-fed adults, CO2 may indicate the presence of potential predators or pathogens, and repulsion from CO2 may function as an escape mechanism.

Fig. 3. Behavioural responses of C. elegans to CO2. (A) Responses of wild-type C. elegans adults and dauers to CO2 in a chemotaxis assay (Fig. 1A). Dauers are developmentally arrested third-stage larvae that are similar to parasitic iL3s and IJs (Hotez et al., Reference Hotez, Hawdon and Schad1993; Viney et al., Reference Viney, Thompson and Crook2005; Crook, Reference Crook2014). Animals were either well-fed adults cultivated at ambient CO2, well-fed adults cultivated at high CO2, starved adults cultivated at ambient CO2 or dauer larvae cultivated at ambient CO2. Adults were tested in a 20 min assay; dauer larvae were tested in a 1 h assay. Responses shown are to 2.5% CO2 (for adults cultivated at high CO2) or 10% CO2 (for all other conditions). For the high CO2 condition, adults were cultivated at 2.5% CO2 for one generation prior to the assay. For the starvation condition, adults were starved for 3 h prior to the assay. Data are from Guillermin et al. (Reference Guillermin, Carrillo and Hallem2017), Rengarajan et al. (Reference Rengarajan, Yankura, Guillermin, Fung and Hallem2019) and Hallem et al. (Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a). ****P < 0.0001, one-way ANOVA with Dunnett's post-test. The graph shows medians and interquartile ranges. (B) Previously experienced hypoxic conditions modulate CO2 responsiveness in C. elegans adults. Animals cultivated at low (1%) oxygen for 1 h prior to assays showed decreased CO2 avoidance. This change is mediated by hypoxia inducible factor-1 (HIF-1), since hif-1 mutants are not affected by prior O2 exposure. ns, not significant relative to N2 (wild-type); ***P < 0.001 relative to N2; +++P < 0.001 relative to N2 exposed to 1% O2 for 1 h. The N2 (air) condition represents a control condition in which animals were not exposed to a CO2 gradient. The graph shows means and SEMs. Figure is from Bretscher et al. (Reference Bretscher, Busch and de Bono2008), copyright 2008 National Academy of Sciences. (C) The BAG sensory neurons and GCY-9 are required for CO2 repulsion in C. elegans. BAG-ablated animals and gcy-9 loss-of-function (lf) mutants are neutral to CO2. ****P < 0.0001, one-way ANOVA with Dunnett's post-test. The graph shows medians and interquartile ranges. Data are from Carrillo et al. (Reference Carrillo, Guillermin, Rengarajan, Okubo and Hallem2013). (D) Calcium activity in BAG neurons in response to 10% CO2, as measured using the ratiometric calcium indicator yellow cameleon 3.60 (YC3.60). Green traces show responses of individual neurons; black line shows median response. Data are from Rengarajan et al. (Reference Rengarajan, Yankura, Guillermin, Fung and Hallem2019). (E) A model for experience-dependent modulation of CO2 responsiveness in C. elegans adults. Under ambient conditions, CO2 repulsion is mediated by activation of the RIA and RIG interneurons and inhibition of the AIY interneurons, and by both dopamine and neuropeptide signalling involving the neuropeptide gene nlp-1. CO2 attraction in animals cultivated at high CO2 is mediated by activation of AIY, inhibition of RIA and silencing of RIG, as well as neuropeptide signalling involving the neuropeptide gene flp-16. Finally, CO2 attraction in starved adults is mediated by silencing of RIG and by a change in AIY responses such that activation and inhibition are observed with approximately equal frequency. Octopamine signalling and neuropeptide signalling via the neuropeptide genes nlp-1 and flp-16 also promote CO2 attraction during starvation. Blue = excitatory activity, orange = inhibitory activity, grey = silencing of activity. Figure is adapted from Guillermin et al. (Reference Guillermin, Carrillo and Hallem2017) and Rengarajan et al. (Reference Rengarajan, Yankura, Guillermin, Fung and Hallem2019).
As is the case for some parasitic nematodes, CO2 responsiveness in C. elegans is subject to experience-dependent plasticity. In the case of C. elegans, one of the factors that influences CO2 responsiveness is the worm's nutritional status. Depriving adults of food suppresses CO2 avoidance behaviour (Bretscher et al., Reference Bretscher, Busch and de Bono2008; Hallem and Sternberg, Reference Hallem and Sternberg2008). Moreover, as C. elegans adults are starved, CO2 response shifts from repulsion to attraction (Fig. 3A) (Rengarajan et al., Reference Rengarajan, Yankura, Guillermin, Fung and Hallem2019). The effects of starvation can be reversed when the animals are re-exposed to food (Rengarajan et al., Reference Rengarajan, Yankura, Guillermin, Fung and Hallem2019). The shift from CO2 repulsion to CO2 attraction in starved worms may be beneficial for survival, since starved animals must find food in order to survive and bacterial food emits CO2. CO2 attraction by starved animals may also reflect an increased tolerance for risk taking; CO2 attraction is an inherently risky behaviour, since both predators and pathogens of C. elegans emit CO2 (Felix and Duveau, Reference Felix and Duveau2012; Brandt and Ringstad, Reference Brandt and Ringstad2015; Schulenburg and Felix, Reference Schulenburg and Felix2017).
CO2 responsiveness in C. elegans adults is also modulated by recently experienced environmental CO2 and oxygen (O2) levels, as well as immediate O2 context. For example, animals exposed to elevated CO2 levels (2.5% CO2) become robustly attracted to CO2 over the course of hours in a reversible manner (Fig. 3A) (Guillermin et al., Reference Guillermin, Carrillo and Hallem2017). Prior exposure to low O2 levels also suppresses CO2 avoidance in adults, an effect that depends on the hypoxia inducible factor gene hif-1 (Fig. 3B) (Bretscher et al., Reference Bretscher, Busch and de Bono2008). In addition, whether animals have been pre-exposed to low O2 affects their responsiveness to CO2 stimuli under some conditions (Fenk and de Bono, Reference Fenk and de Bono2017). CO2 responsiveness is also modulated by ambient O2 levels such that animals assayed under low O2 conditions are more strongly repelled by CO2 than animals assayed under high O2 conditions (Carrillo et al., Reference Carrillo, Guillermin, Rengarajan, Okubo and Hallem2013; Kodama-Namba et al., Reference Kodama-Namba, Fenk, Bretscher, Gross, Busch and de Bono2013). C. elegans prefers O2 concentrations below atmospheric (Gray et al., Reference Gray, Karow, Lu, Chang, Chang, Ellis, Marletta and Bargmann2004); however, when exposed to opposing gradients of O2 and CO2, the avoidance response to high CO2 dominates over the avoidance response to high O2 (Bretscher et al., Reference Bretscher, Busch and de Bono2008). In nature, C. elegans is found in rotting vegetation (Felix and Braendle, Reference Felix and Braendle2010; Schulenburg and Felix, Reference Schulenburg and Felix2017), where both O2 and CO2 concentrations fluctuate. Moreover, both O2- and CO2-sensing pathways control foraging behaviour (Bendesky et al., Reference Bendesky, Tsunozaki, Rockman, Kruglyak and Bargmann2011; Milward et al., Reference Milward, Busch, Murphy, de Bono and Olofsson2011; Juozaityte et al., Reference Juozaityte, Pladevall-Morera, Podolska, Norgaard, Neumann and Pocock2017). Thus, the interplay between O2- and CO2-evoked behaviours likely contributes to the ability of C. elegans to navigate the complex organic environments it inhabits.
CO2 responsiveness is also modulated by the presence or absence of food, and prior temperature experience (Bretscher et al., Reference Bretscher, Busch and de Bono2008, Reference Bretscher, Kodama-Namba, Busch, Murphy, Soltesz, Laurent and de Bono2011; Kodama-Namba et al., Reference Kodama-Namba, Fenk, Bretscher, Gross, Busch and de Bono2013). In the case of temperature-dependent modulation of CO2 responsiveness, animals cultivated at 22 °C show enhanced repulsion to 1% CO2 when assayed at 15 °C compared with 22 °C (Kodama-Namba et al., Reference Kodama-Namba, Fenk, Bretscher, Gross, Busch and de Bono2013), suggesting an interaction between recent temperature experience and CO2 sensing. The ecological significance of this interaction is not yet clear, but it suggests that CO2 preferences may vary on a diurnal cycle as the ambient temperature fluctuates. Thus, CO2-evoked behaviours are regulated by multiple sensory modalities, resulting in both context-dependent and experience-dependent responses to CO2.
CO2 can also elicit behavioural changes in C. elegans that are independent of changes in locomotion. For example, exposing C. elegans to high CO2 levels (5% CO2) inhibits egg-laying behaviour, at least transiently (Fenk and de Bono, Reference Fenk and de Bono2015). Modulation of egg-laying behaviour by high CO2 levels may prevent animals from exposing their progeny to unfamiliar environmental conditions. Feeding behaviour is also altered by CO2 such that brief exposure to high CO2 levels causes an acute reduction in pharyngeal pumping (Sharabi et al., Reference Sharabi, Hurwitz, Simon, Beitel, Morimoto, Rechavi, Sznajder and Gruenbaum2009). Well-fed animals stop pharyngeal pumping when exposed to a lower concentration of CO2 than starved animals, suggesting that this behavioural change is dependent on the nutritional state of the animal (Sharabi et al., Reference Sharabi, Hurwitz, Simon, Beitel, Morimoto, Rechavi, Sznajder and Gruenbaum2009).
Finally, C. elegans exhibits life-stage-specific responses to CO2. C. elegans dauers, which are developmentally arrested third-stage larvae that are similar to the iL3 and IJ stages of parasitic nematodes (Hotez et al., Reference Hotez, Hawdon and Schad1993; Viney et al., Reference Viney, Thompson and Crook2005; Crook, Reference Crook2014), are attracted to CO2 (Fig. 3A) (Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a). Dauer larvae form when food is limited or environmental conditions are otherwise unfavourable (Hu, Reference Hu2007), and CO2 attraction by dauers may enable them to locate bacterial food sources. In addition, dauers associate with invertebrates such as slugs, snails and isopods, which act as carriers that facilitate their dispersal to new niches (Baird, Reference Baird1999; Caswell-Chen et al., Reference Caswell-Chen, Chen, Lewis, Douhan, Nadler and Carey2005; Lee et al., Reference Lee, Choi, Lee, Kim, Hwang, Kim, Park, Paik and Lee2012; Schulenburg and Felix, Reference Schulenburg and Felix2017). Thus, CO2 attraction may also enable dauers to locate and associate with invertebrate carriers.
Effects of CO2 on C. elegans development and physiology
High levels of CO2 can also elicit physiological changes in C. elegans. Cultivating animals at CO2 levels above 9% CO2 decreases brood size and significantly slows the rate of embryonic development (Sharabi et al., Reference Sharabi, Hurwitz, Simon, Beitel, Morimoto, Rechavi, Sznajder and Gruenbaum2009). This developmental delay is not caused by any obvious reduction in the overall health of the embryos or the adults that develop from these embryos. In addition, chronic exposure (>4 days) to 19% CO2 causes defects in overall body muscle morphology, resulting in long-lasting movement defects (Sharabi et al., Reference Sharabi, Hurwitz, Simon, Beitel, Morimoto, Rechavi, Sznajder and Gruenbaum2009). Nevertheless, cultivation at 19% CO2 extends lifespan, an effect that appears to be independent of the inhibitory effect of CO2 on egg laying (Sharabi et al., Reference Sharabi, Hurwitz, Simon, Beitel, Morimoto, Rechavi, Sznajder and Gruenbaum2009). The lifespan extension induced by exposure to high CO2 also may be independent of the dietary restriction pathway, since eat-2 mutants, which show reduced pharyngeal pumping, show increased lifespan at high CO2 (Sharabi et al., Reference Sharabi, Hurwitz, Simon, Beitel, Morimoto, Rechavi, Sznajder and Gruenbaum2009). Thus, CO2 has diverse effects on C. elegans development, physiology and behaviour.
Behavioural and physiological responses of other free-living nematodes to CO2
Other free-living nematodes show diverse responses to CO2. For example, the free-living marine nematode Adoncholaimus thalassophygas is attracted to CO2 (Riemann and Schrage, Reference Riemann and Schrage1988). This effect is not due to a general decrease in the pH of the medium, since the addition of hydrochloric acid did not elicit a similar attractive response (Riemann and Schrage, Reference Riemann and Schrage1988). CO2 is emitted from bacteria present in decaying carcasses found in sediments and may act as a food signal for these nematodes. The necromenic nematode Pristionchus pacificus, which represents an evolutionary intermediate between free-living and parasitic nematodes, displays acute CO2 avoidance (Hallem and Sternberg, Reference Hallem and Sternberg2008). In contrast, the free-living nematodes Caenorhabditis briggsae, Caenorhabditis angaria and Panagrellus redivivus do not respond to CO2 in acute avoidance assays (Hallem and Sternberg, Reference Hallem and Sternberg2008). However, ambient CO2 concentrations play a prominent role in regulating reproduction in Panagrellus redivivus. Under low O2 conditions, the brood size of Panagrellus redivivus increases in response to an increase in CO2 concentration from 0 to 5% (Hansen and Buecher, Reference Hansen and Buecher1970). The effects of CO2 on nematode physiology also vary greatly across species. At the extreme, nematodes have been isolated from volcanic gas vents, where CO2 levels can reach 100%; these nematodes can survive under 100% CO2 conditions for at least 5 days (Pilz and Hohberg, Reference Pilz and Hohberg2015). Thus, CO2 has species-specific effects on nematode behaviour and physiology.
Cellular and molecular mechanisms of CO2 responsiveness in nematodes
Cellular mechanisms of CO2 responsiveness in C. elegans
The primary CO2-sensing neurons in C. elegans are the paired BAG neurons in the head. Ablation of the BAG neurons abolishes both CO2 avoidance in adults (Hallem and Sternberg, Reference Hallem and Sternberg2008) and CO2 attraction in dauers (Fig. 3C) (Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a). Exposing animals to varying concentrations of CO2 produces dose-dependent calcium activity in the BAG neurons in vivo (Fig. 3D) (Hallem et al., Reference Hallem, Spencer, McWhirter, Zeller, Henz, Ratsch, Miller, Horvitz, Sternberg and Ringstad2011b). In addition, isolated BAG neurons derived from C. elegans embryos respond to CO2 in vitro, suggesting that BAG neurons are intrinsically sensitive to CO2 (Smith et al., Reference Smith, Martinez-Velazquez and Ringstad2013). The calcium responses in isolated BAG neurons in culture are independent of carbonic anhydrase activity, indicating that these neurons can sense molecular CO2. Moreover, a majority of isolated BAG neurons do not respond to pH, although responses to low pH are detectable in some isolated BAG neurons (Smith et al., Reference Smith, Martinez-Velazquez and Ringstad2013). However, the role of BAG neurons is not limited to promoting CO2 responses. The BAG neurons also sense O2 (Zimmer et al., Reference Zimmer, Gray, Pokala, Chang, Karow, Marletta, Hudson, Morton, Chronis and Bargmann2009). Furthermore, they play a role in establishing food odour preferences and in foraging behaviour (Harris et al., Reference Harris, Shen, Ha, Donato, Wallis, Zhang and Zhang2014; Juozaityte et al., Reference Juozaityte, Pladevall-Morera, Podolska, Norgaard, Neumann and Pocock2017). In addition to BAG neurons, other sensory neurons including ASE, AFD, AWC, ASJ, ASK, ASH and ADL also exhibit CO2-evoked calcium activity and contribute to CO2 responsiveness (Bretscher et al., Reference Bretscher, Kodama-Namba, Busch, Murphy, Soltesz, Laurent and de Bono2011; Fenk and de Bono, Reference Fenk and de Bono2015). The interneurons AIY, RIG, RIA and AIZ act downstream of BAG neurons to mediate CO2-evoked behaviour (Kodama-Namba et al., Reference Kodama-Namba, Fenk, Bretscher, Gross, Busch and de Bono2013; Guillermin et al., Reference Guillermin, Carrillo and Hallem2017). The AIA interneurons also show CO2-evoked activity and are involved in CO2 responsiveness (Fenk and de Bono, Reference Fenk and de Bono2015). In well-fed adults, CO2 repulsion is correlated with activation of RIA, RIG and AIZ, and inhibition of AIY (Fig. 3E) (Guillermin et al., Reference Guillermin, Carrillo and Hallem2017).
Molecular mechanisms of CO2 responsiveness in C. elegans
The detection of CO2 by the BAG neurons requires the receptor-type guanylate cyclase GCY-9. Animals with loss-of-function mutations in gcy-9 are insensitive to CO2 in behavioural assays (Fig. 3C) (Hallem et al., Reference Hallem, Spencer, McWhirter, Zeller, Henz, Ratsch, Miller, Horvitz, Sternberg and Ringstad2011b). Moreover, CO2-evoked calcium transients in the BAG neurons require GCY-9, and ectopic expression of GCY-9 confers CO2 sensitivity to other sensory neurons (Hallem et al., Reference Hallem, Spencer, McWhirter, Zeller, Henz, Ratsch, Miller, Horvitz, Sternberg and Ringstad2011b; Brandt et al., Reference Brandt, Aziz-Zaman, Juozaityte, Martinez-Velazquez, Petersen, Pocock and Ringstad2012; Carrillo et al., Reference Carrillo, Guillermin, Rengarajan, Okubo and Hallem2013). The expression of GCY-9 in the BAG neurons requires the E26 transformation-specific (ETS)-domain transcription factor ETS-5, and ets-5 mutants fail to avoid CO2 (Guillermin et al., Reference Guillermin, Castelletto and Hallem2011; Brandt et al., Reference Brandt, Aziz-Zaman, Juozaityte, Martinez-Velazquez, Petersen, Pocock and Ringstad2012). Both ETS-5 and the SoxD transcription factor EGL-13 are also required more generally for normal differentiation of the BAG neurons (Guillermin et al., Reference Guillermin, Castelletto and Hallem2011; Brandt et al., Reference Brandt, Aziz-Zaman, Juozaityte, Martinez-Velazquez, Petersen, Pocock and Ringstad2012; Petersen et al., Reference Petersen, Romanos, Juozaityte, Riveiro, Hum, Traunmuller, Zimmer and Pocock2013). The Toll-like receptor TOL-1 is also required for normal BAG neuron differentiation, and tol-1 mutants are defective in pathogen avoidance behaviour as a result (Brandt and Ringstad, Reference Brandt and Ringstad2015).
In addition to GCY-9, the cGMP signalling pathway that mediates CO2 detection consists of the cGMP-gated cation channel TAX-2/TAX-4 (Bretscher et al., Reference Bretscher, Busch and de Bono2008; Hallem and Sternberg, Reference Hallem and Sternberg2008). Glutamate signalling and neuropeptide signalling are also required for BAG-mediated responses to CO2 (Guillermin et al., Reference Guillermin, Carrillo and Hallem2017). BAG neurons are glutamatergic (Serrano-Saiz et al., Reference Serrano-Saiz, Poole, Felton, Zhang, De La Cruz and Hobert2013), and well-fed adults lacking the vesicular glutamate transporter EAT-4 show neutral responses to CO2 (Guillermin et al., Reference Guillermin, Carrillo and Hallem2017). BAG neurons also release neuropeptides, and well-fed adults lacking the BAG-expressed FMRFamide-like neuropeptide FLP-17 do not respond to CO2 (Guillermin et al., Reference Guillermin, Carrillo and Hallem2017). In addition, several other signalling molecules, including the calcineurin subunits TAX-6 and CNB-1, the regulator of G-protein signalling RGS-3 and the nuclear hormone receptor NHR-49 are known to regulate CO2 response (Hallem and Sternberg, Reference Hallem and Sternberg2008). The microRNA mir-791 is also required for the normal CO2-evoked calcium activity of the BAG neurons (Drexel et al., Reference Drexel, Mahofsky, Latham, Zimmer and Cochella2016).
Mechanisms underlying the context-dependent modulation of CO2 responsiveness by O2
The extent to which CO2 responsiveness is regulated by ambient O2 levels depends on the neuropeptide Y receptor NPR-1 (McGrath et al., Reference McGrath, Rockman, Zimmer, Jang, Macosko, Kruglyak and Bargmann2009; Carrillo et al., Reference Carrillo, Guillermin, Rengarajan, Okubo and Hallem2013; Kodama-Namba et al., Reference Kodama-Namba, Fenk, Bretscher, Gross, Busch and de Bono2013). The laboratory wild-type (N2) strain of C. elegans contains a gain-of-function mutation in the npr-1 gene that confers CO2 avoidance on well-fed adults regardless of ambient O2 levels. However, animals containing loss-of-function (lf) mutations in npr-1 and animals carrying the natural variant of npr-1 avoid CO2 under low O2 conditions but do not respond to CO2 at normal atmospheric O2 levels (21% O2) (Carrillo et al., Reference Carrillo, Guillermin, Rengarajan, Okubo and Hallem2013; Kodama-Namba et al., Reference Kodama-Namba, Fenk, Bretscher, Gross, Busch and de Bono2013). The gain-of-function NPR-1 variant in N2 animals suppresses the activity of the O2-sensing URX neurons to promote CO2 avoidance regardless of ambient O2 levels. In animals containing an npr-1(lf) mutation or a natural variant of npr-1, the URX neurons are tonically active under high O2 conditions and inhibit CO2 avoidance at high O2. The RIA interneurons appear to act downstream of URX to partially mediate its effects on the CO2 circuit (Kodama-Namba et al., Reference Kodama-Namba, Fenk, Bretscher, Gross, Busch and de Bono2013). In addition, the neuroglobin gene glb-5 also acts via the URX neurons to modulate CO2 responsiveness as a function of ambient O2 levels (McGrath et al., Reference McGrath, Rockman, Zimmer, Jang, Macosko, Kruglyak and Bargmann2009; Kodama-Namba et al., Reference Kodama-Namba, Fenk, Bretscher, Gross, Busch and de Bono2013).
Mechanisms underlying the experience-dependent modulation of CO2 responsiveness
The mechanisms underlying experience-dependent modulation of CO2 responsiveness in C. elegans have been elucidated in some detail. The shift in CO2 response from repulsion to attraction when animals are moved from low CO2 to high CO2 cultivation conditions results from the differential activity of a single set of interneurons downstream of the BAG sensory neurons (Guillermin et al., Reference Guillermin, Carrillo and Hallem2017). In animals that were previously cultivated at low CO2, CO2 exposure inhibits the AIY interneurons and activates the RIA and RIG interneurons. In contrast, in animals that have been cultivated at high CO2, CO2 exposure activates AIY and inhibits RIA. Moreover, RIG is silenced such that it no longer responds to CO2 (Fig. 3E). Thus, CO2 response is not determined by whether an ‘attractive’ or ‘repulsive’ pathway is activated; rather, it is determined by experience-dependent modulation of interneuron activity in a single pathway (Guillermin et al., Reference Guillermin, Carrillo and Hallem2017). A number of neuropeptides also differentially modulate CO2 responsiveness in animals cultured under high vs low CO2 conditions (Fig. 3E) (Guillermin et al., Reference Guillermin, Carrillo and Hallem2017).
The shift from CO2 repulsion to CO2 attraction that occurs during starvation also arises due to the differential activities of the AIY and RIG interneurons (Rengarajan et al., Reference Rengarajan, Yankura, Guillermin, Fung and Hallem2019). In starved animals, RIG is silenced and AIY shows stochastic responses such that CO2 evokes activating and inhibiting responses with approximately equal frequency (Fig. 3E). At the molecular level, whether CO2 is attractive or repulsive is regulated by biogenic amine signalling. Dopamine promotes CO2 avoidance in well-fed animals by promoting activation of RIG and inhibition of AIY, while octopamine promotes CO2 attraction in starved animals by promoting activation of AIY (Fig. 3E) (Rengarajan et al., Reference Rengarajan, Yankura, Guillermin, Fung and Hallem2019). Thus, the CO2 circuit is modulated during starvation by opposing biogenic amine signals. Neuropeptide signalling also regulates CO2 responsiveness during starvation (Fig. 3E) (Rengarajan et al., Reference Rengarajan, Yankura, Guillermin, Fung and Hallem2019). Finally, CO2 attraction in dauer larvae is less well understood but is regulated at least in part by neuropeptide signalling (Lee et al., Reference Lee, Shih, Schaedel, Quintero-Cadena, Rogers and Sternberg2017).
Molecular and cellular mechanisms underlying other CO2-evoked behaviours
Some of the molecular and cellular mechanisms that mediate the effects of CO2 on other behaviours in C. elegans have also been elucidated. For instance, CO2-evoked activity in the AWC sensory neurons triggers a cGMP signalling pathway that ultimately inhibits the activity of the HSN neurons, resulting in the inhibition of egg-laying behaviour (Fenk and de Bono, Reference Fenk and de Bono2015). Antagonistic effects of the BAG neurons and the URX neurons regulate lifespan in C. elegans, resulting in increased longevity in BAG-ablated animals (Liu and Cai, Reference Liu and Cai2013). Mutations in the c-Jun N-terminal kinase (JNK) signalling pathway genes jnk-1 and kgb-2 suppress CO2-induced fertility defects, indicating that JNK signalling may be involved in regulating fertility in response to CO2 (Vadasz et al., Reference Vadasz, Dada, Briva, Helenius, Sharabi, Welch, Kelly, Grzesik, Budinger, Liu, Seeger, Beitel, Gruenbaum and Sznajder2012).
Unanswered questions regarding CO2 responsiveness in C. elegans
Although the mechanisms underlying CO2 responsiveness in C. elegans have been elucidated in appreciable detail, several questions remain unexplored. For example, more information is needed to fully understand how the differential flow of information from BAG neurons to downstream interneurons generates experience-dependent plasticity of CO2-evoked behaviour. One intriguing possibility is that the BAG neurons express or release different neurotransmitters or neuropeptides in response to CO2 under varying conditions. Consistent with this possibility, the BAG neurons modulate the expression of FLP-19 neuropeptides as a function of their CO2-evoked activity (Rojo Romanos et al., Reference Rojo Romanos, Petersen and Pocock2017). In addition, the interneurons that act downstream of other CO2-sensing neurons have not been identified. Finally, the CO2 microcircuit that drives CO2 attraction in dauers remains poorly understood, although it appears to involve dauer-specific, gap-junction-mediated signalling between the BAG neurons and the downstream AIB interneurons (Bhattacharya et al., Reference Bhattacharya, Aghayeva, Berghoff and Hobert2019). In future studies, it will be interesting to determine whether the same set of neurons or a distinct set of neurons promotes CO2 attraction in dauers. A better understanding of the neural circuits and signalling pathways that regulate CO2 responsiveness as a function of experience, context and life stage will provide important insights into how a single sensory cue can give rise to diverse behavioural responses in an ethologically-appropriate manner.
Mechanisms underlying CO2 responsiveness in other nematodes
The anatomy and function of nematode sensory neurons are generally conserved across species (Ashton et al., Reference Ashton, Bhopale, Fine and Schad1995, Reference Ashton, Li and Schad1999; Lopez et al., Reference Lopez, Boston, Ashton and Schad2000; Li et al., Reference Li, Ashton, Gamble and Schad2000a, Reference Li, Zhu, Boston, Ashton, Gamble and Schad2000b, Reference Li, Zhu, Ashton, Gamble and Schad2001; Bhopale et al., Reference Bhopale, Kupprion, Ashton, Boston and Schad2001; Forbes et al., Reference Forbes, Ashton, Boston, Zhu and Schad2004; Ketschek et al., Reference Ketschek, Joseph, Boston, Ashton and Schad2004; Ashton et al., Reference Ashton, Zhu, Boston, Lok and Schad2007; Bumbarger et al., Reference Bumbarger, Crum, Ellisman and Baldwin2007; Srinivasan et al., Reference Srinivasan, Durak and Sternberg2008; Bumbarger et al., Reference Bumbarger, Wijeratne, Carter, Crum, Ellisman and Baldwin2009; Zhu et al., Reference Zhu, Li, Nolan, Schad and Lok2011; Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a), making it possible to use knowledge of CO2 responsiveness in C. elegans as a starting point for launching investigations into the mechanisms of CO2 responsiveness in parasitic nematodes. In the case of both the necromenic nematode Pristionchus pacificus and the EPNs Heterorhabditis bacteriophora and Steinernema carpocapsae, BAG neurons were identified on the basis of conserved neuroanatomical position and shown to be required for behavioural responses to CO2 by laser ablation analyses (Hallem and Sternberg, Reference Hallem and Sternberg2008; Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a). BAG-ablated Pristionchus pacificus adults do not show acute CO2 avoidance, and BAG-ablated Heterorhabditis bacteriophora and Steinernema carpocapsae IJs do not show CO2 attraction (Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a). In addition, CO2-evoked jumping behaviour in Steinernema carpocapsae requires the BAG neurons (Hallem et al., Reference Hallem, Dillman, Hong, Zhang, Yano, DeMarco and Sternberg2011a). Thus, the neural circuits that mediate CO2 response are at least partly conserved across nematode species. However, the interneurons that operate downstream of BAG neurons to mediate CO2 responsiveness in other nematode species have not yet been identified. Moreover, nothing is currently known about the neural circuits and molecular signals that promote CO2 responsiveness in mammalian-parasitic nematodes. In future studies, it will also be interesting to determine whether similar or distinct mechanisms operate in C. elegans and parasitic nematodes to modulate CO2 responses depending on context, previous experience or life stage.
Directions for future research
A major focus going forward will be on elucidating the cellular and molecular mechanisms underlying CO2 responsiveness in mammalian-parasitic nematodes. The identification of the neural mechanisms that drive or regulate the CO2 responses of mammalian-parasitic nematodes both inside and outside the host could lead to the identification of new drug targets or new strategies for nematode control. Until recently, investigations into the mechanisms underlying sensory behaviours in parasitic nematodes were limited to laser ablation analysis due to the dearth of resources and tools required for the genetic manipulation of these parasites. Laser ablation analysis has been used to establish the function of a number of different sensory neurons in mammalian-parasitic nematodes, including Strongyloides stercoralis, hookworms and Haemonchus contortus (Ashton et al., Reference Ashton, Bhopale, Holt, Smith and Schad1998; Lopez et al., Reference Lopez, Boston, Ashton and Schad2000; Li et al., Reference Li, Zhu, Boston, Ashton, Gamble and Schad2000b; Bhopale et al., Reference Bhopale, Kupprion, Ashton, Boston and Schad2001; Forbes et al., Reference Forbes, Ashton, Boston, Zhu and Schad2004; Ketschek et al., Reference Ketschek, Joseph, Boston, Ashton and Schad2004; Nolan et al., Reference Nolan, Brenes, Ashton, Zhu, Forbes, Boston and Schad2004; Ashton et al., Reference Ashton, Zhu, Boston, Lok and Schad2007). However, several recent advances have facilitated the study of gene function and the genetic basis of sensory behaviours in these parasites. High-quality genome sequences of several nematode species have been identified and are readily accessible (Mitreva et al., Reference Mitreva, Zarlenga, McCarter and Jasmer2007; Brindley et al., Reference Brindley, Mitreva, Ghedin and Lustigman2009; Hunt et al., Reference Hunt, Tsai, Coghlan, Reid, Holroyd, Foth, Tracey, Cotton, Stanley, Beasley, Bennett, Brooks, Harsha, Kajitani, Kulkarni, Harbecke, Nagayasu, Nichol, Ogura, Quail, Randle, Xia, Brattig, Soblik, Ribeiro, Sanchez-Flores, Hayashi, Itoh, Denver, Grant, Stoltzfus, Lok, Murayama, Wastling, Streit, Kikuchi, Viney and Berriman2016; Howe et al., Reference Howe, Bolt, Shafie, Kersey and Berriman2017; International Helminth Genomes, 2019). Transcriptomic data are also now available for many parasitic nematode species (Jex et al., Reference Jex, Gasser and Schwarz2019), which may significantly advance the study of gene expression and the identification of novel signalling pathways that drive sensory behaviours.
The most genetically tractable parasitic nematodes are Strongyloides stercoralis and Strongyloides ratti. Strongyloides stercoralis and Strongyloides ratti are more readily amenable to genetic manipulation than other parasitic nematodes because they can undergo one free-living generation (Viney, Reference Viney1999, Reference Viney2006; Lok, Reference Lok2007). Foreign DNA can be introduced by gonadal microinjection into free-living adults using techniques based on those originally developed for C. elegans (Evans, Reference Evans2006). Most other mammalian-parasitic nematodes lack a free-living generation, which makes it difficult to introduce foreign DNA into these worms. Strongyloides stercoralis is a human parasite that infects approximately 370 million people worldwide (Page et al., Reference Page, Judd and Bradbury2018) and is therefore of direct interest as a human pathogen; additionally, Strongyloides stercoralis is of interest as a model for other human-parasitic nematodes such as hookworms that cannot be genetically manipulated.
Transgenic nematodes can be generated by introducing plasmid DNA containing exogenous genes; these genes are then expressed as extrachromosomal arrays in the F1 progeny of the microinjected adults (Lok and Massey, Reference Lok and Massey2002; Li et al., Reference Li, Massey, Nolan, Schad, Kraus, Sundaram and Lok2006, Reference Li, Shao, Junio, Nolan, Massey, Pearce, Viney and Lok2011; Junio et al., Reference Junio, Li, Massey, Nolan, Todd Lamitina, Sundaram and Lok2008; Lok and Artis, Reference Lok and Artis2008; Lok, Reference Lok2012; Shao et al., Reference Shao, Li, Nolan, Massey, Pearce and Lok2012; Lok et al., Reference Lok, Shao, Massey and Li2017; Shao et al., Reference Shao, Li and Lok2017). This technique can be potentially used to express any gene of choice, including those required for genetic ablation or silencing of neurons (Schiavo et al., Reference Schiavo, Benfenati, Poulain, Rossetto, Polverino de Laureto, DasGupta and Montecucco1992; Qi et al., Reference Qi, Garren, Shu, Tsien and Jin2012; Williams et al., Reference Williams, Bejjani, Ramirez, Coakley, Kim, Lee, Wen, Samuel, Lu, Hilliard and Hammarlund2013; Pokala et al., Reference Pokala, Liu, Gordus and Bargmann2014) and those required for monitoring neuronal activity (Nagai et al., Reference Nagai, Yamada, Tominaga, Ichikawa and Miyawaki2004; Chen et al., Reference Chen, Wardill, Sun, Pulver, Renninger, Baohan, Schreiter, Kerr, Orger, Jayaraman, Looger, Svoboda and Kim2013; Dana et al., Reference Dana, Mohar, Sun, Narayan, Gordus, Hasseman, Tsegaye, Holt, Hu, Walpita, Patel, Macklin, Bargmann, Ahrens, Schreiter, Jayaraman, Looger, Svoboda and Kim2016). The targeted expression of exogenous genes in Strongyloides has been aided by the identification of several promoters that drive expression in single cells or subsets of cells (Junio et al., Reference Junio, Li, Massey, Nolan, Todd Lamitina, Sundaram and Lok2008; Stoltzfus et al., Reference Stoltzfus, Massey, Nolan, Griffith and Lok2012; Bryant et al., Reference Bryant, Ruiz, Gang, Castelletto, Lopez and Hallem2018). However, whereas extrachromosomal arrays in C. elegans are stably expressed across generations, extrachromosomal arrays in Strongyloides are silenced after the F1 generation by as-yet-unknown mechanisms (Junio et al., Reference Junio, Li, Massey, Nolan, Todd Lamitina, Sundaram and Lok2008; Li et al., Reference Li, Shao, Junio, Nolan, Massey, Pearce, Viney and Lok2011). Persistent expression across generations can be achieved in Strongyloides by methods that promote genomic integration of transgenes, such as transposon-mediated random integration (Shao et al., Reference Shao, Li, Nolan, Massey, Pearce and Lok2012; Lok, Reference Lok2013) and CRISPR/Cas9-mediated targeted integration (Gang et al., Reference Gang, Castelletto, Bryant, Yang, Mancuso, Lopez, Pellegrini and Hallem2017).
Methods for disrupting gene function are also now available for Strongyloides stercoralis and Strongyloides ratti. The recent development of an approach for CRISPR/Cas9-mediated targeted gene disruption in these species provided the first insights into the genetic mechanisms that drive sensory behaviours (Fig. 4) (Gang et al., Reference Gang, Castelletto, Bryant, Yang, Mancuso, Lopez, Pellegrini and Hallem2017; Lok et al., Reference Lok, Shao, Massey and Li2017; Bryant et al., Reference Bryant, Ruiz, Gang, Castelletto, Lopez and Hallem2018). For example, knockout of the gene encoding the cyclic-nucleotide-gated channel subunit TAX-4 severely disrupts the thermosensory behaviour of Strongyloides stercoralis infective larvae (Bryant et al., Reference Bryant, Ruiz, Gang, Castelletto, Lopez and Hallem2018). RNA interference (RNAi) has also now been successfully applied to Strongyloides ratti. RNAi approaches using both dsRNA and siRNA have been used to study the effects of transcriptional knockdown of genes in several parasitic nematode species, although with varying efficacy (Geldhof et al., Reference Geldhof, Murray, Couthier, Gilleard, McLauchlan, Knox and Britton2006; Kotze and Bagnall, Reference Kotze and Bagnall2006; Visser et al., Reference Visser, Geldhof, de Maere, Knox, Vercruysse and Claerebout2006; Kang and Hong, Reference Kang and Hong2008; Lendner et al., Reference Lendner, Doligalska, Lucius and Hartmann2008; Viney and Thompson, Reference Viney and Thompson2008; Samarasinghe et al., Reference Samarasinghe, Knox and Britton2011; Britton et al., Reference Britton, Samarasinghe and Knox2012; Zawadzki et al., Reference Zawadzki, Kotze, Fritz, Johnson, Hemsworth, Hines and Behm2012; Tzelos, Reference Tzelos2014). In the case of Strongyloides ratti, a recent study demonstrated the first successful knockdown of multiple mRNAs using an siRNA approach (Dulovic and Streit, Reference Dulovic and Streit2019). In addition, chemical mutagenesis has been used to perform unbiased forward genetic screens to generate dominant non-targeted mutations in Strongyloides ratti iL3s, although mapping the locations of these mutations has not been possible yet (Viney et al., Reference Viney, Green, Brooks and Grant2002; Guo et al., Reference Guo, Chang, Dieterich and Streit2015).

Fig. 4. Targeted mutagenesis in Strongyloides stercoralis. (A) Strategy for CRISPR/Cas9-mediated targeted mutagenesis in Strongyloides stercoralis. Plasmid vectors encoding Cas9, the single guide RNA (sgRNA) for the gene of interest and a repair template for homology-directed repair encoding an mRFPmars reporter are introduced into Strongyloides stercoralis free-living adult females (P 0) by gonadal microinjection. The iL3 progeny (F 1) from microinjected females are screened for mRFPmars expression, indicative of a possible disruption of the gene of interest. iL3s are then tested in single-worm chemotaxis assays and genotyped post hoc for homozygous disruption of the gene of interest. Figure is adapted from Gang et al. (Reference Gang, Castelletto, Bryant, Yang, Mancuso, Lopez, Pellegrini and Hallem2017).
Using a combination of the above approaches, it should be possible to identify the neural mechanisms and molecular pathways that are involved in driving behavioural and physiological responses of Strongyloides stercoralis to CO2. For example, it will be interesting to determine whether the BAG neurons, which sense CO2 and promote behavioural responses to CO2 in C. elegans, play a similar role in Strongyloides stercoralis. It will also be important to elucidate the neural circuitry that operates downstream of the CO2-sensing neurons to mediate or modulate CO2-evoked behaviours in Strongyloides stercoralis. An intriguing possibility is that while sensory neuron function may be generally conserved across species, interneuron function may be less well conserved and may instead reflect species-specific behavioural and physiological responses to CO2. In addition, through the systematic screening of candidate genes known to be involved in CO2 responsiveness in C. elegans, it might be possible to uncover molecular signals that regulate parasite–host interactions or that are required for successful parasitism. In the long run, a better understanding of the molecular and cellular bases of CO2-evoked behaviours in parasitic nematodes may lead to new avenues for nematode control. It may also shed light on some of the unique sensory mechanisms that operate in parasitic nematodes to shape parasite-specific behavioural responses.
Acknowledgments
We thank Astra Bryant, Michelle Castelletto, Elisa Rojas-Palato, Felicitas Ruiz and Breanna Walsh for their insightful comments on the manuscript.
Financial support
This work was supported by the National Institutes of Health (1DP2DC014596 and 1R01DC017959 to E.A.H, and 1F32AI147617 to N.B.). E.A.H. is also supported by a Burroughs Wellcome Fund Investigators in the Pathogenesis of Disease Award and a Howard Hughes Medical Institute Faculty Scholar Award.









