No CrossRef data available.
Article contents
Phylogeny, species delimitation and ecological and morphological diversity of Characithecium (Monogenoidea: Dactylogyridae)
Published online by Cambridge University Press: 03 March 2022
Abstract
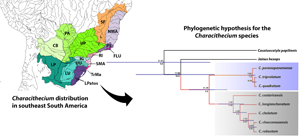
Characithecium (Monogenoidea, Dactylogyridae) is a genus containing nine species that live on the gills of a characid clade containing genera Astyanax, Andromakhe, Psalidodon and Oligosarcus (Characiformes, Characidae) in South and Central America. Earlier studies suggest a tight coevolutionary history between these parasites and their hosts mainly due to the phylogenetic proximity between these genera of fish. Hence, this study explores phylogenetic relationships, species limits and extrinsic factors (geography and ecology) explaining parasite prevalence. To understand the evolutionary history of the genus, we constructed a time-calibrated phylogenetic hypothesis, which includes eight of the nine known species of Characithecium sampled from a broad spectrum of host species. The phylogeny supports the monophyly of Characithecium, with its most recent common ancestor dating from the Miocene. Using generalized mixed-yule coalescent and Bayesian Poisson tree process methods, species delimitation analyses suggested fewer species than the proposed delimitation based on morphology alone, recovering four and six entities, respectively. The results indicate that species of Characithecium have wider geographical and host distribution and higher prevalence on Oligosarcus species compared to Astyanax and Psalidodon. Correlation between parasite prevalence and biotic and abiotic traits, based on generalized linear models, indicates that the frequency of occurrence of different species of Characithecium is associated with distinct factors, such as host genus, high altitudes, rivers and streams, and different ecoregions. Our results suggest that species of Characithecium are highly opportunistic, exploring resources in different manner as our data reveal the ability of these parasites to explore a diverse environment of variable biotic (e.g. hosts) and abiotic features.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press




