Article contents
Phenotypic investigation of 4-nitrophenylacetyl- and 4-nitro-1H-imidazoyl-based compounds as antileishmanial agents
Published online by Cambridge University Press: 03 February 2022
Abstract
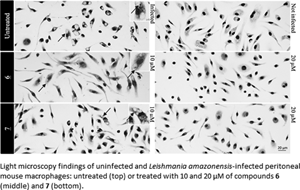
Cutaneous leishmaniasis (CL) is a spectrum of clinical manifestations characterized by severe skin ulcerations that leads to social stigma. There are limited treatment options for CL, and the available drugs are becoming less efficacious due to drug resistance. More efficacious and safer antileishmanial drugs are needed. In this study, the biological effect of seven synthetically accessible nitroaromatic compounds was evaluated in vitro against amastigotes of Leishmania amazonensis, followed by in vivo evaluation using mouse models of CL. Two compounds (6 and 7) were active against amastigotes in vitro [half-maximal effective concentration (EC50): 4.57 ± 0.08 and 9.19 ± 0.68 μm, respectively], with selectivity indexes >50, and the other compounds were not selective. In vivo, compounds 6 and 7 (10 mg kg−1, twice a day for 14 days) failed to reduce skin lesion sizes and parasite loads determined by light microscopy of lesion imprints and quantitative polymerase chain reaction. Nevertheless, the in vitro leishmanicidal efficacy sustained their use as templates for nitroimidazole-based antileishmanial drug discovery programmes focusing on analogues with more suitable properties.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 5
- Cited by





