Introduction
Trypanosoma cruzi is the aetiological agent of Chagas disease, a vector-borne disease transmitted by the bite of a triatomine bug, which affects 8 million people worldwide (Perez-Molina and Molina, Reference Perez-Molina and Molina2018). In Brazil, vector transmission was drastically reduced by governmental policies of insect control, which were directed to Triatoma infestans (Abad-Franch et al., Reference Abad-Franch, Diotaiuti, Gurgel-Goncalves and Gurtler2013). Nevertheless, other triatomine species are known to transmit T. cruzi (Noireau et al., Reference Noireau, Diosque and Jansen2009; Bern et al., Reference Bern, Kjos, Yabsley and Montgomery2011; Waleckx et al., Reference Waleckx, Gourbiere and Dumonteil2015), and several others are on the merge of becoming competent vectors, to sum up, vector domiciliation is also an emerging threat (Salazar et al., Reference Salazar, Castillo-Neyra, Tustin, Borrini-Mayori, Naquira and Levy2015). The comprehension of the molecular mechanism of parasite–insect vector interaction can help to envision vector transmission control strategies.
Few studies have been performed on the characterization of T. cruzi molecules involved in the parasite adhesion, interaction and colonization of the insect. After a triatomine blood meal, replicative non-infective epimastigotes adhere and proliferate in the midgut, and subsequently migrate to the posterior midgut, where metacyclogenesis occurs, then non-proliferative metacyclic trypomastigotes can infect a mammalian host (Azambuja et al., Reference Azambuja, Ratcliffe and Garcia2005). Glycoinositolphospholipids (GIPLs) are the major molecules expressed on the epimastigote cell surface. These molecules are involved in the parasite adhesion to the luminal surface of the insect midgut and may be one of the determinants of the infection in the vector (Nogueira et al., Reference Nogueira, Gonzalez, Gomes, de Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007). In addition, Gp72, cruzipain and calpain-like molecules may also be involved in this part of the parasite life cycle (de Jesus et al., Reference de Jesus, Cooper, Espinosa, Gomes, Garcia, Paul and Cross1993; Basombrio et al., Reference Basombrio, Gomez, Padilla, Ciaccio, Nozaki and Cross2002; Ennes-Vidal et al., Reference Ennes-Vidal, Menna-Barreto, Santos, Branquinha and d'Avila-Levy2011; Uehara et al., Reference Uehara, Moreira, Oliveira, Azambuja, Lima, Britto, Santos, Branquinha and d'Avila-Levy2012).
Peptidases, a class of hydrolytic enzymes responsible for breaking peptide bonds, has attracted the attention of distinct research groups because of their role in several crucial steps of the life cycle of the trypanosomatid parasites (Alvarez et al., Reference Alvarez, Niemirowicz and Cazzulo2012; Santos et al., Reference Santos, Sodre, Valle, Silva, Abi-Chacra, Silva, Souza-Goncalves, Sangenito, Goncalves, Souza, Palmeira, d'Avila-Levy, Kneipp, Kellett, McCann and Branquinha2012; Branquinha et al., Reference Branquinha, Marinho, Sangenito, Oliveira, Goncalves, Ennes-Vidal, d'Avila-Levy and Santos2013, Reference Branquinha, Oliveira, Sangenito, Sodre, Kneipp, d'Avila-Levy and Santos2015; d'Avila-Levy et al., Reference d'Avila-Levy, Altoe, Uehara, Branquinha and Santos2014). The most abundant and extensively studied metallopeptidase in trypanosomatids is gp63, which was first identified and described as an immunodominant protein from several Leishmania spp. (d'Avila-Levy et al., Reference d'Avila-Levy, Altoe, Uehara, Branquinha and Santos2014), and it is a well-characterized virulence factor in Leishmania spp. (Olivier et al., Reference Olivier, Atayde, Isnard, Hassani and Shio2012), which also takes a role in insect adhesion (Soares et al., Reference Soares, Altoe, Ennes-Vidal, da Costa, Rangel, de Souza, da Silva, Volf and d'Avila-Levy2017). Due to the strong recognition of gp63 and cruzipain by the sera from infected patients in the early 80s, the role of these molecules has been extensively investigated in leishmaniasis and Chagas disease, respectively. The biochemical characterization revealed their hydrolytic activity, which guided the peptidase research in each disease-causing parasite. For instance, in T. cruzi from the total of indexed articles in the Pubmed (http://www.ncbi.nlm.nih.gov/pubmed/) that are retrieved by the search string ‘peptidase’ and its synonyms, 50% accounts for articles related to cruzipain and its synonyms. In Leishmania spp., 37% of the scientific literature linked to peptidase accounts for gp63. Besides the historical discovery of each molecule, a routine zymography, which is the most popular technique for peptidase screening, reveals an abundant metallopeptidase in Leishmania spp. cellular extracts against an abundant cysteine peptidase in T. cruzi cellular extracts (Chaudhuri and Chang, Reference Chaudhuri and Chang1988; Cazzulo et al., Reference Cazzulo, Cazzulo Franke, Martinez and Franke de Cazzulo1990). In this sense, it is not clear whether gp63 homologues in T. cruzi have a secondary role for parasite virulence or if cruzipain leaded research to the detriment of gp63. Genes encoding gp63 have been extensively amplified in the T. cruzi genome, and an indirect correlation between gene expansion and proteolytic activity in trypanosomatids is clearly found (d'Avila-Levy et al., Reference d'Avila-Levy, Altoe, Uehara, Branquinha and Santos2014). Nevertheless, T. cruzi gp63 homologues involvement in mammalian host cells invasion have been demonstrated by two independent research groups (Cuevas et al., Reference Cuevas, Cazzulo and Sanchez2003; Kulkarni et al., Reference Kulkarni, Olson, Engman and McGwire2009).
Here, we have assayed the role of T. cruzi metallo-dependent enzymes, particularly Tcgp63-I, on the T. cruzi–R. prolixus vector interaction. The influence of ion chelators on parasite viability, the capacity of adhesion to the vector midgut and expression of Tcgp63-I was analysed. Also, Tcgp63-I levels were evaluated after the protozoa colonization of R. prolixus. Collectively, the results indicate the participation of metallo-dependent enzymes and Tcgp63-I on the interaction with the insect vector and add new insights into the functional role of Tcgp63-I in T. cruzi epimastigotes.
Materials and methods
Abbreviations, chemicals and buffer composition
BHI – 3.7% brain heart infusion medium; BSA – bovine serum albumin; Cha – cyclohexylalanine; CHAPS – 3-((3-cholamidopropyl)dimethylammonio)-1-propanesulphonate; Dpa – N-3-(2, 4-dinitrophenyl)-L-2,3-diaminopropionyl; DMSO – dimethyl sulphoxide; DTT – dithiothreitol; E-64 – trans-epoxysuccinyl-L-leucylamido(4-guanidino)butane; EDTA – ethylenediaminetetraacetic acid; EGTA – ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid; FBS – heat-inactivated fetal bovine serum; MCA – (7-methoxycoumarin-4-yl)acetyl; Nva – norvaline; PBS – phosphate buffer saline, 150 mm NaCl, 20 mm phosphate buffer, pH 7.2; PMSF – phenylmethylsulphonyl fluoride; RSB – ‘Rhodnius saline buffer’ (160 mm KCl, 100 mm D-glucose, 4.2 mm NaHCO3, pH 7.8); SDS-PAGE – sodium dodecyl sulphate-polyacrylamide gel electrophoresis; SDS-PAGE sample buffer (62 mm Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue and 1 mm β-mercaptoethanol); TBST – Tris buffer saline (150 mm NaCl; 10 mm Tris, pH 7.4 containing 0.05% Tween 20); TLCK – Nα-tosyl- L-lysine chloromethyl ketone hydrochloride; White's solution-HgCl2 0.25 g, NaCl 6.5 g, HCl 1.25 mL, 250 mL of ethanol, H2O qsp 1000 mL.
Parasite strains and cultivation
The following T. cruzi isolates were obtained from the Coleção de Protozoários da Fundação Oswaldo Cruz (COLPROT-FIOCRUZ, http://colprot.fiocruz.br): Dm28c (COLPROT 010), G (COLPROT 216), Y (COLPROT 106) and CL Brener (COLPROT 005). The epimastigote forms were grown in BHI medium, containing 0.002% hemin, supplemented with 10% FBS, at 28 °C for 4 days, to reach late-log growth phase.
Effect of divalent metal chelators on parasite growth and viability
To this set of experiments, 2 × 107 T. cruzi Dm28c epimastigotes were collected from late-log growth cultures by centrifugation (1500 × g for 5 min at 4 °C), washed three times in PBS and incubated either in RSB for 1 h, or in BHI medium for 24–96 h in the presence of divalent metal chelators (EDTA, EGTA and phenanthroline) at concentrations ranging from 0.5 to 100 µ m. Control parasites were incubated with no compound. Parasites treated with the highest DMSO dose used to dissolve phenanthroline were assessed in parallel for the solvent control. Afterwards, the percentage of viable parasites was estimated by trypan blue dye exclusion and counting of actively motile parasites in a Neubauer chamber. Cell viability was expressed as percentage in relation to control cells, assigned as 100%. The 50% inhibitory concentration (IC50), i.e. the minimum drug concentration that caused a 50% reduction in survival/viability in comparison with that in identical cultures without the compound, was evaluated after 1 h of exposure. These values were determined by linear regression analysis by plotting the number of viable epimastigotes vs log drug concentration.
Parasite treatment prior to insect interaction and flow cytometry analyses
Trypanosoma cruzi Dm28c parasites (2 × 107 cells in 100 µL RSB) were pretreated or not for 1 h with different modulators: (1) divalent metal chelators (phenanthroline, EDTA and EGTA) at concentrations ranging from 0.2 to 1 µ m; (2) anti-Tcgp63-I antibodies (kindly provided by Dr Daniel O. Sánchez, Universidad Nacional de General San Martín, Argentina) at 1:500 or 1:1000 (a condition that did not promote parasite agglutination or affected parasite motility) or an IgG negative control (rabbit pre-immune serum) at 1:1000. After each treatment, parasites were washed three times with RSB (1500 × g for 5 min at 4 °C) prior to the following experiments.
Insects
Rhodnius prolixus were reared and maintained as previously described (Azambuja and Garcia, Reference Azambuja, Garcia, Crampton, Beard and Loius1997) by the insectary of the Laboratório de Bioquímica e Fisiologia de Insetos, Instituto Oswaldo Cruz, FIOCRUZ. Briefly, fifth-instars larvae were randomly chosen, starved for 30 days after the last ecdysis and then allowed to feed on defibrinated rabbit blood through a membrane feeder. Ten days after feeding, the insects were dissected, the posterior midguts (small intestine) removed, longitudinally sectioned and washed three times in RSB to expose their luminal surfaces, as previously described (Gonzalez et al., Reference Gonzalez, Silva, Albuquerque-Cunha, Nogueira, Mattos, Castro, Azambuja and Garcia2011). Then, they were processed as described below.
Ex vivo interaction between R. prolixus dissected midgut and T. cruzi
Tissue fragments from individual intestines were placed into 1.5 mL microtubes containing the treated parasites as described above, and then, incubated for 15 min at room temperature, under gentle shaking. Four dissected midguts were assayed per treatment. Afterwards, the explanted midguts were spread onto glass slides and the numbers of attached parasites per 100 randomly chosen epithelial cells in 10 different fields of each midgut explanted were quantified by counting under the light microscope (Gonzalez et al., Reference Gonzalez, Silva, Albuquerque-Cunha, Nogueira, Mattos, Castro, Azambuja and Garcia2011). Results are shown as the mean ± standard error of the mean of two experiments.
In vivo colonization of R. prolixus by T. cruzi and parasite re-isolation
After a starvation period of 30 days, fifth-instars larvae were fed through a membrane feeder on defibrinated rabbit blood containing 9 × 106 epimastigotes mL−1. In the 30th and 32nd days from feeding, the insects were washed and incubated in White's solution for 90 min (Bronfen et al., Reference Bronfen, de Assis Rocha, Machado, Perillo, Romanha and Chiari1989) and subsequently washed abundantly in RSB. Afterwards, the rectal ampule was explanted through abdominal compression and homogenized in RSB. Feces content was analysed by optical microscopy. Then, the gut content from 10–15 insects were incubated in 25 cm3 flask culture containing BHI medium supplemented with streptomycin (100 µg mL−1), penicillin (10 µg mL−1), ampicillin (100 µg mL−1), kanamycin (20 µg mL−1) and gentamicin (140 µg mL−1). The culture was checked daily for parasite, fungi and bacteria growth, and when required the following combined approaches were performed to eliminate contamination: centrifugation at low or high g force followed by inoculation in W tubes.
Flow cytometry analysis
T rypanosoma cruzi epimastigotes (Dm28c) (3 × 106 cells) were incubated or not with 0.5 µ m phenanthroline for 1 h or 24 h at 28 °C. Then, cells were washed three times in PBS and the cellular viability was confirmed by motility and lack of staining after challenging with Trypan blue. Treated cells were further monitored by assessing its growth in phenanthroline-free medium, after cellular normalization, the growth was similar to non-treated parasites (data not shown). Thereafter, cells were fixed for 30 min in 1% paraformaldehyde in PBS at room temperature, followed by extensive washing. Then, cells were incubated for 1 h at room temperature with anti-Tcgp63-I antibody (Cuevas et al., Reference Cuevas, Cazzulo and Sanchez2003) at 1:500 dilution, respectively. Cells were then incubated for an additional hour with 1:500 dilution of ALEXA 488- labelled goat anti-rabbit immunoglobulin G. Finally, cells were washed three times in PBS and analysed in a flow cytometry EPICS ELITE (Coulter Electronics, Hialeah, FL, USA) equipped with 15 mW argon laser emitting at 488 nm. In addition, the exposition of gp63 homologues in several T. cruzi isolates (G, Dm28c, Y and CL Brener) was compared by the analysis of the mean fluorescence intensity after anti-Tcgp63-I antibody incubation. Non-treated cells, those treated with the pre-immune serum and those treated with the secondary antibody alone were run in parallel as controls. Each experimental population was then mapped by using a two-parameter histogram of forward-angle light scatter vs side scatter. The mapped population (n = 10 000) was then analysed for log green fluorescence by using a single-parameter histogram.
Western blotting analysis
Epimastigotes (1 × 109 parasites) from Dm28c were collected by centrifugation at 3000 × g for 10 min at 4 °C, washed three times with cold PBS and lysed by six freezing and thawing cycles in liquid nitrogen at room temperature in a Tris-HCl 40 mm (pH 6.8) buffer containing 4% CHAPS, 0.5 mm TLCK, 1 mm PMSF and 100 µ m E-64, which were included to inhibit cruzipain and other cysteine peptidases and allow the analysis of minority peptidases. Then, cellular debris was removed by centrifugation at 16 000 × g for 10 min at 4 °C and the protein concentration of the supernatants was determined using BSA as standard (Lowry et al., Reference Lowry, Rosebrough, Farr and Randall1951). This cellular extract was used for western blotting analysis, zymography and in solution proteolytic assays. For western blotting analysis, 10 µg of the parasite extract was added to reducing SDS-PAGE sample buffer 1×, boiled for 5 min, and resolved by electrophoresis on a 10% SDS-PAGE under reducing conditions, as previously described (d'Avila-Levy et al., Reference d'Avila-Levy, Santos, Marinho, Dias, Lopes, Santos and Branquinha2006b). Following electrophoresis, the membrane was blocked overnight in 10% skim milk dissolved in TBST. Subsequently, the membrane was incubated for 2 h with the rabbit anti-Tcgp63-I at 1:100 dilution. The secondary antibody used was peroxidase-conjugated goat anti-rabbit IgG at 1:25 000 (Pierce) followed by chemiluminescence immunodetection after reaction with ECL reagents.
Zymography analysis
To this set of experiments, soluble extracts (10 µg) from Dm28c were separated by 12% SDS-PAGE co-polymerized with 0.1% gelatin (Heussen and Dowdle, Reference Heussen and Dowdle1980). Samples were diluted (v/v) in SDS-PAGE sample buffer 4× without reducing agent and loaded onto gels. Electrophoresis was carried out under constant current (25 mA) at 4 °C. The gels were then soaked in 2.5% Triton X-100 under agitation for 1 h at room temperature for SDS removal and enzyme renaturation. The effect of pH on the proteolytic activity was determined by incubating the gels for 24 h at 37 °C in 50 mm phosphate buffer (pH 5.5 and 7.4) and 100 mm glycine-NaOH buffer pH 10.0 containing or not 2 mm DTT. Then, the gels were stained with 0.2% Coomassie Brilliant Blue R-250 in methanol:acetic acid:water (40:10:50) and destained in the same solution without the dye. The gels were scanned with the Image Scanner III (GE HealthCare) and analysed by the Image Master 2D Elite software (GE HealthCare). To determine the peptidase class, the lysates were pre-incubated for 30 min with the following inhibitors for 30 min at room temperature: pepstatin A (1 µ m) for aspartic-peptidases, phenanthroline (10 mm) for metallo-peptidases, PMSF (1 mm) for serine-peptidases and E-64 (10 µ m) for cysteine-peptidases. These inhibitors were also included in the pH 10.0 buffer in which the zymograms were incubated overnight.
Enzyme assay in solution
Peptidase activity was measured continuously using the fluorogenic peptide substrate MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 at 37 °C in 0.1 M glycine-NaOH buffer containing 1 mm CaCl2 pH 10.0. The assays were performed in black round bottom 96-microwell plates in the Spectra Max Gemini spectrofluorimeter (Molecular Devices), with excitation wavelength at 328 nm and emission at 393 nm. The reaction started by the addition of 10 µ m substrate to the parasite extract (10 µg) in a total volume of 100 µL. The inhibition studies were performed by pre-incubation for 30 min at room temperature with the following inhibitors: 1 µ m pepstatin A, 10 mm phenanthroline, 1 mm PMSF or 10 µ m E-64. The assays were controlled for self-liberation of the fluorophore over the same time interval. All results were performed three times in triplicate, and are expressed as mean ± standard deviation.
Statistical analysis
The data were analysed statistically using Student's t test or ANOVA using Prisma 6 GraphPad software. P values of 0.05 or less were considered statistically significant.
Results
Effects of divalent metal chelators on parasite viability
T rypanosoma cruzi epimastigotes were incubated in the presence of EDTA, EGTA and phenanthroline for 1 h (Fig. 1). Concentration of 1 µ m for EDTA and EGTA, and 0.5 µ m for phenanthroline did not affect parasite viability compared to DMSO-treated controls under the assayed conditions (P > 0.05). The IC50 of EDTA, EGTA and phenanthroline was 7, 8 and 10 µ m, respectively (Fig. 1).
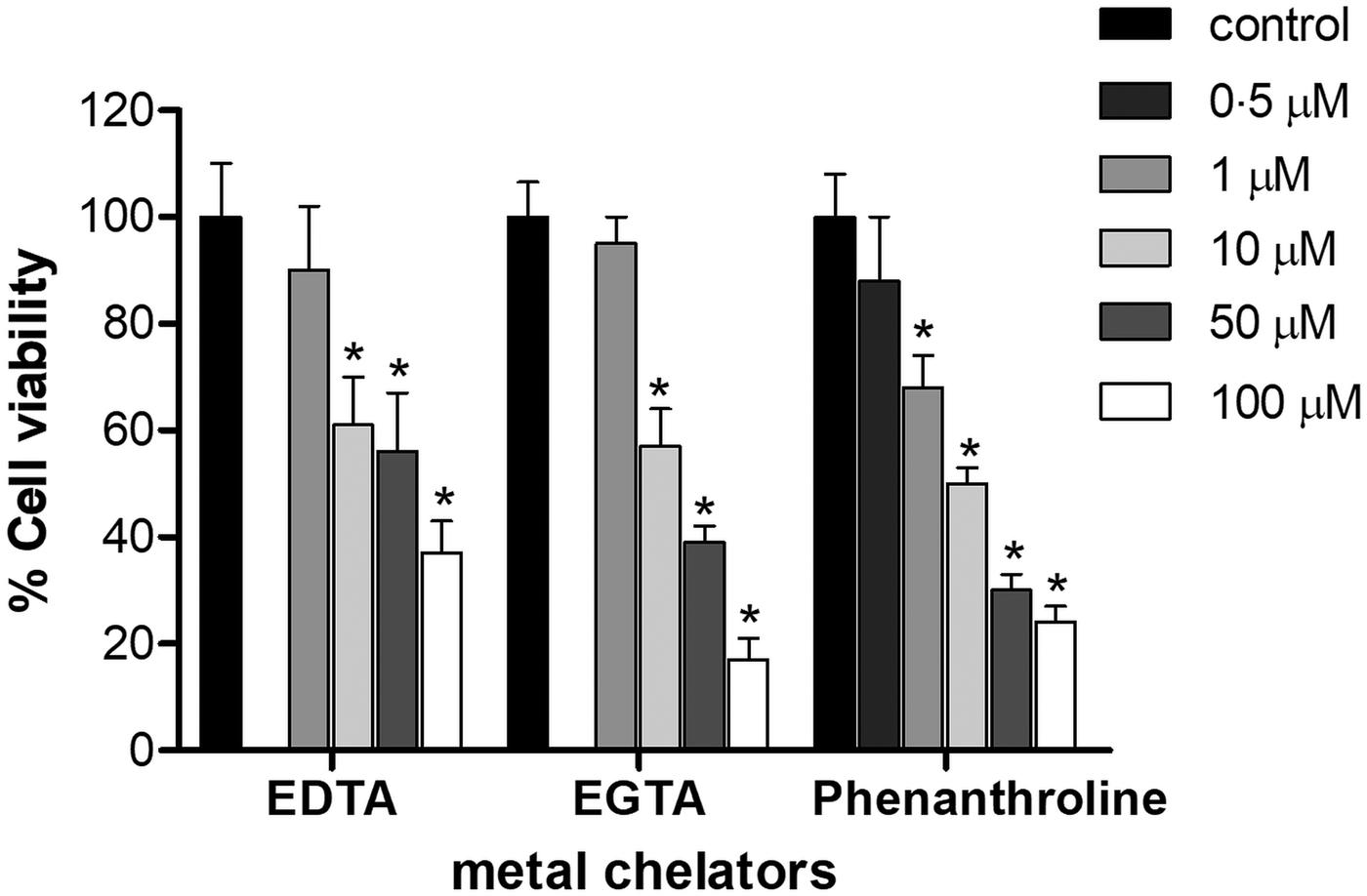
Fig. 1. Effect of metal chelators on the viability of T. cruzi Dm28c epimastigotes. Parasites (2 × 107 cells) were cultured for 1 h in the presence or absence of different concentration of either EDTA, EGTA or phenanthroline (0–100 µ m). The results correspond to the mean ± s.e.m. of three independent experiments performed in triplicate. An asterisk denotes values significantly different from control (untreated cells) using Student's t test (*P < 0.05).
Effect of divalent metal chelators and anti-Tcgp63-I antibodies on T. cruzi–R. prolixus interaction
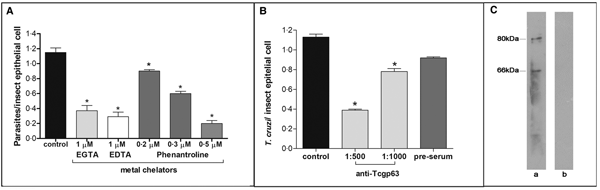
In this set of experiments, each divalent metal chelator was incubated with T. cruzi epimastigotes followed by their exposure to dissected posterior midgut of the insect. The metal chelators strongly impaired the parasite-gut binding by approximately 68, 75 and 83% for EDTA (1 µ m), EGTA (1 µ m) and phenanthroline (0.5 µ m), respectively, in comparison to control as shown in Fig. 2A. A distinguished feature of phenanthroline is that it chelates Zn2+ more avidly than EDTA and EGTA. This ion is crucial for maintaining leishmanial gp63 active site conformation and cannot be efficiently replaced by other divalent ions (Bouvier et al., Reference Bouvier, Bordier, Vogel, Reichelt and Etges1989). Considering the structural similarities between T. cruzi and leishmanial gp63 molecules (El-Sayed and Donelson, Reference El-Sayed and Donelson1997; Kulkarni et al., Reference Kulkarni, Olson, Engman and McGwire2009; Abad-Franch et al., Reference Abad-Franch, Diotaiuti, Gurgel-Goncalves and Gurtler2013), we evaluated the dose-dependent effect of phenanthroline on the proportion of parasite adhesion to the midgut. In phenanthroline doses ranging from 0.2 to 0.5 µ m, the adhesion of epimastigotes was reduced in the range of 39–83% in relation to the control (Fig. 2A), which supports the hypothesis that metallo-dependent enzymes play a role in the adhesion to R. prolixus. Supporting this hypothesis, the pre-treatment of parasites with anti-Tcgp63-I antibodies considerably reduced the interaction process, in relation to the control. The inhibition was 31 and 65% as antibody concentration rose from 1:1000 to 1:500, respectively (Fig. 2B). The antibody concentrations used did not promote parasite agglutination (data not shown). Parasites treated with the pre-immune serum adhered to the midguts at a rate similar to that of the control (Fig. 2B). Considering that the antibody was raised against T. cruzi CL Brener strain, we performed Western blotting against Dm28c epimastigote extracts, which revealed two reactive polypeptides migrating at 80 and 66 kDa (Fig. 2C).
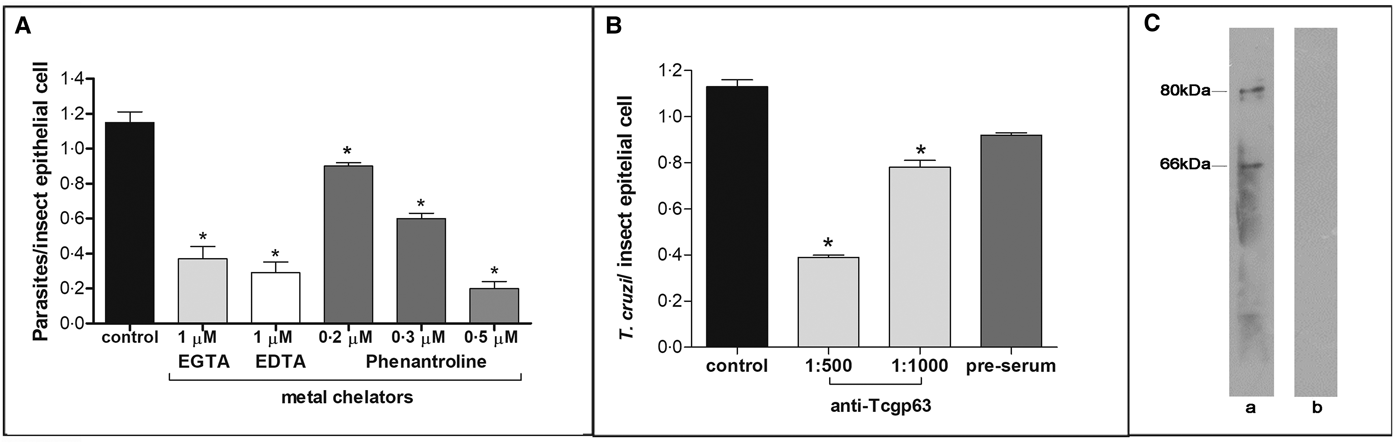
Fig. 2. Effect of divalent metal chelators and anti-Tcgp63-I antibodies on T. cruzi–R. prolixus interaction. Dm28c epimastigotes (2 × 107 cells) were treated for 1 h at 27 °C with 1 µ m EGTA, EDTA or phenanthroline at 0.2, 0.3 or 0.5 µ m (A). Alternatively, the parasites were treated for 60 min at room temperature with anti-Tcgp63-I antibodies at 1:500 and 1:1000 dilution, or pre-immune serum at 1:500 (B). Parasite viability was not affected by the treatments used in this set of experiments. Following interaction for 15 min with the insect gut, the number of adhered parasites/insect gut epithelial cells was estimated by randomly counting at least 100 epithelial cells in quadruplicate in 10 random fields. The results are shown as the mean ± s.e.m. of two independent experiments. An asterisk denotes significantly different from control (untreated cells) using Student's t test (*P < 0.001). Lastly, western blotting analyses identified two reactive bands against T. cruzi Dm28c epimastigotes (a), while no reactive molecules were recognized by the pre-immune serum (b) (C).
Modulation of Tcgp63-I levels after different stimulus
Aiming to determine the effect of insect passage on the expression levels of Tcgp63-I, we re-isolated parasites after 30 days from the initial infection. The rectal ampoules were analysed for parasite presence and either epimastigotes or trypomastigotes were visualized after insect abdominal compression (data not shown). Then, pure cultures were established as described in Materials and methods, and recently isolated from insect colonization or multiple passages parasites were incubated with anti-Tcgp63-I antibodies and analysed by flow cytometry (Fig. 3). Recently isolated parasites presented a significant increase in the mean of fluorescence intensity (MFI) levels of Tcgp63-I molecules in comparison to culture-adapted parasites (inset of Fig. 3).

Fig. 3. Tcgp63-I expression in T. cruzi Dm28c is enhanced after passage in R. prolixus. Cells were re-isolated in culture after insect colonization, as described in materials and methods. Long-term culture-adapted parasites (white) and recently isolated parasites (grey) were incubated in the presence of anti-Tcgp63-I antibody at 1:500 dilution and analysed by flow cytometry. Representative data of the analysis of 10 000 cells from one of three experiments are shown. The inset shows the mean of fluorescence intensity (MFI) levels. The asterisk indicates different values for MFI level between control and trypomastigotes re-isolated (*P < 0.05).
Aiming to evaluate if phenanthroline would promote an imbalance in the exposition of Tcgp63-I from T. cruzi, parasites were treated with phenanthroline at 0.5 µ m for 1 h and assayed by flow cytometry using anti-Tcgp63-I. No statistical significance between treated and non-treated parasites was detected (data not shown). Therefore, we exposed the parasite for longer periods (up to 96 h) with phenanthroline at lower concentrations (0.25 µ m). Subsequently, we compared Tcgp63-I levels between treated parasites and control cells. Under the assayed conditions, no relevant change in FL-1 peak was detected (data not shown).
Evaluation of Tcgp63-I levels in different T. cruzi strains
The adhesion rate to R. prolixus dissected midguts of different T. cruzi strains was previously determined by our research group (Uehara et al., Reference Uehara, Moreira, Oliveira, Azambuja, Lima, Britto, Santos, Branquinha and d'Avila-Levy2012). To this end, we assayed Tcgp63-I levels in these strains, and no direct correlation was found between adhesion rate and anti-Tcgp63-I binding, since the G strain presented the strongest antibody recognition as assessed by MFI, and this strain presented the lowest binding rate among Dm28c, CL Brener and Y isolates (Uehara et al., Reference Uehara, Moreira, Oliveira, Azambuja, Lima, Britto, Santos, Branquinha and d'Avila-Levy2012). In addition, no statistical difference in MFI was observed among these three isolates, while they clearly presented different binding rates to R. prolixus explanted midguts (Uehara et al., Reference Uehara, Moreira, Oliveira, Azambuja, Lima, Britto, Santos, Branquinha and d'Avila-Levy2012) (Supplementary Material Fig. S1).
Evaluation of enzyme activity
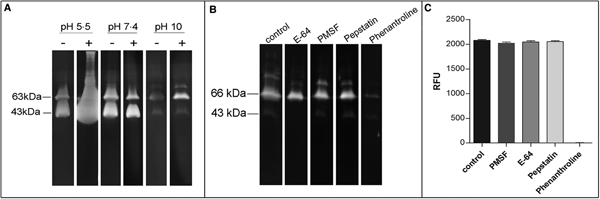
In order to confirm the presence of active metallopeptidases in T. cruzi extracts, we analysed gelatin degradation in zymography and cleavage of a fluorogenic peptide substrate by a parasite extract obtained under cysteine peptidase inhibition. Using zymography assay, we observed degradation haloes even after a strict protocol for cysteine peptidase inhibition in a wide pH range (5.5–10.0), with maximum activity observed at pH 5.5. The presence of DTT in the reaction buffer enhanced the proteolytic activity (Fig. 4A). Zymography analysis revealed two major bands migrating at approximately 43 and 63 kDa, which were strongly inhibited by phenanthroline (a reversible inhibitor of metal-dependent peptidases) (Fig. 4B). Densitometric analysis showed that the proteolytic activity is reduced by ~87% compared to control. PMSF (a reversible inhibitor of serine peptidases), E-64 (an irreversible inhibitor of cysteine peptidases) or pepstatin (a reversible inhibitor of aspartic peptidases) did not affect the proteolytic activity (data not shown). These results confirm the presence of active metallopeptidases in T. cruzi epimastigote extracts. We have also assayed the soluble extract with a fluorogenic substrate generally used for metallo-type peptidase characterization in the absence or presence of the same specific peptidase inhibitors described above. The substrate was readily hydrolysed by the soluble extract, and this proteolytic activity was completely inhibited only in the presence of phenanthroline, corroborating our previous findings (Fig. 4C).

Fig. 4. Effect of peptidase inhibitors, DTT and pH on the proteolytic activity of soluble extracts from T. cruzi Dm28c epimastigotes. Gelatin-SDS-PAGE were incubated in different buffers supplemented (+) or not (−) with DTT 2 mm at 37 °C for 24 h (A). To determine the enzymatic class, zymograms were incubated for 24 h in 100 mm glycine-NaOH buffer pH 10.0 in the absence (control) or presence of the following proteolytic inhibitors: 10 µ m E-64, 1 mm PMSF, 1 µ m pepstatin, or 10 mm phenanthroline (B). The numbers on the left indicate apparent molecular masses of the active bands expressed in kDa. To provide additional evidence on the enzyme class characterization, in solution assays were performed using the fluorogenic substrate MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2 in 100 mm glycine-NaOH buffer pH 10.0, in the absence (control) or presence of the same inhibitors described above. Enzyme activity was expressed as micromoles of MCA per minute per micrograms of protein (C).
Discussion
Parasite peptidases are known to be involved in many cellular events, including interaction with both mammalian hosts and insect vectors (Garcia et al., Reference Garcia, Genta, Azambuja and Schaub2010; Isnard et al., Reference Isnard, Shio and Olivier2012; Santiago et al., Reference Santiago, de Araujo, Motta, Praca, Charneau, Bastos and Santana2017; Siqueira-Neto et al., Reference Siqueira-Neto, Debnath, McCall, Bernatchez, Ndao, Reed and Rosenthal2018). In T. cruzi, metallopeptidases have been less studied and only in the last decade gp63 homologues, orthologues and pseudogenes were described (Kulkarni et al., Reference Kulkarni, Olson, Engman and McGwire2009; Alvarez et al., Reference Alvarez, Niemirowicz and Cazzulo2012). To this end, the functional role of T. cruzi gp63 molecules is poor explored (d'Avila-Levy et al., Reference d'Avila-Levy, Altoe, Uehara, Branquinha and Santos2014). The high heterogeneity of T. cruzi GP63 genes creates a challenge to the study of this gene family. The first challenge in studying multigenic families is to classify the genes under study. Cuevas and colleagues (Cuevas et al., Reference Cuevas, Cazzulo and Sanchez2003) analysed the T. cruzi genome and identified several groups of genes that belong to the GP63 family, with multiple members in each. In this sense, GP63 genes were grouped in Tcgp63-I and II, which are present as high-copy-number genes, as well as Tcgp63-III, which are pseudogenes (Cuevas et al., Reference Cuevas, Cazzulo and Sanchez2003). At least four GP63 mRNA, which belong to Tcgp63-I, are developmentally regulated in the different parasite stages, being more abundant in amastigotes than in epimastigotes or trypomastigotes (Grandgenett et al., Reference Grandgenett, Coughlin, Kirchhoff and Donelson2000). Although Tcgp63-II genes are more abundant in the genome than Tcgp63-I, only the latter is detectable at the protein level and presents expressive mRNA levels in all developmental stages of CL Brener strain. Tcgp63-I is a proteolytically active enzyme bound to the membrane by a GPI anchor and with a possible role in the infection of host cells, since antibodies raised against a synthetic peptide derived from Tcgp63-I sequence partially blocked invasion of Vero cells by trypomastigotes (Cuevas et al., Reference Cuevas, Cazzulo and Sanchez2003).
Here, we demonstrated that protein homologues to Tcgp63-I are present in epimastigote extracts of T. cruzi clone Dm28. CL-Brener, to which the antibody was raised, belongs to discrete typing unit (DTU) VI, while the clone Dm28c to DTU I. The distinct T. cruzi DTUs present a previously underestimated genome complexity (Callejas-Hernandez et al., Reference Callejas-Hernandez, Rastrojo, Poveda, Girones and Fresno2018) that is reflected in significant phenotypic variation and different behaviour both in vitro and in vivo in terms of pathophysiology, virulence, tropism and immunological responses (Rassi et al., Reference Rassi, Rassi and Marcondes de Rezende2012). In addition, we have also detected and partially characterized active metallopeptidases in this parasite strain, which are more active at acidic pH, are stimulated by DTT and inhibited by phenanthroline, characteristics shared by several metallopeptidases in trypanosomatids, including gp63 (d'Avila-Levy et al., Reference d'Avila-Levy, Souza, Gomes, Vermelho and Branquinha2003; Santos et al., Reference Santos, Branquinha and d'Avila-Levy2006). A 65-kDa metallo-dependent enzyme has been previously detected in T. cruzi Dm28c, and parasite treatment with phenanthroline strongly inhibited metacyclogenesis (Bonaldo et al., Reference Bonaldo, d'Escoffier, Salles and Goldenberg1991). Our data also demonstrated a clear impact on epimastigote proliferation caused by phenanthroline exposure, which is in accordance with previous report (Santos et al., Reference Santos, Sodre, Valle, Silva, Abi-Chacra, Silva, Souza-Goncalves, Sangenito, Goncalves, Souza, Palmeira, d'Avila-Levy, Kneipp, Kellett, McCann and Branquinha2012).
The exposure of parasites to the pressure of peptidase inhibitors influenced the protein content of the inhibited molecule or other abundant peptidases (Sangenito et al., Reference Sangenito, Ennes-Vidal, Marinho, Da Mota, Santos, d'Avila-Levy and Branquinha2009; Santos et al., Reference Santos, Marinho, Altoe, Vitorio, Alves, Britto, Motta, Branquinha, Santos and d'Avila-Levy2009). Here, the zinc-chelator phenanthroline, even after 24 h exposure, did not promote any change in either cruzipain or Tcgp63-I levels. In addition, the possible role of Tcgp63-I or metallo-dependent enzymes on the interaction of T. cruzi with the midgut of triatomines has not been assessed up to now, although Tcgp63-I has a surface location and expression in epimastigotes, which is conceivable with a role in this part of the parasite life cycle (Cuevas et al., Reference Cuevas, Cazzulo and Sanchez2003). In this sense, the present study investigated the effect of metal chelators as well as the effect of the antibody raised by Cuevas et al. (Reference Cuevas, Cazzulo and Sanchez2003) on the interaction of T. cruzi with R. prolixus, and both treatments considerably reduced the interaction of the parasite with the insect explanted guts.
The use of divalent metal chelators as an indirect approach to access gp63 functions in the insect interaction has been previously applied by our research group, for instance while assessing the interaction of Leishmania infantum and L. braziliensis with insect cell lines, as well as the live insect vectors, Lutzomyia intermedia and Lu. longipalpis, respectively (Soares et al., Reference Soares, Altoe, Ennes-Vidal, da Costa, Rangel, de Souza, da Silva, Volf and d'Avila-Levy2017), and the interaction of Angomonas deanei, Crithidia fasciculata, Herpetomonas spp., Leptomonas spp. with Aedes aegypti or Aedes albopictus and Phytomonas spp. with Oncopeltus fasciatus (d'Avila-Levy et al., Reference d'Avila-Levy, Altoe, Uehara, Branquinha and Santos2006a, Reference d'Avila-Levy, Santos, Marinho, Dias, Lopes, Santos and Branquinhab; Nogueira de Melo et al., Reference Nogueira de Melo, d'Avila-Levy, Dias, Armada, Silva, Lopes, Santos, Branquinha and Vermelho2006; Pereira et al., Reference Pereira, Bernardo, Dias Junior, Silva, Romanos, d'Avila-Levy, Branquinha and Santos2009; Pereira et al., Reference Pereira, Dias, Elias, d'Avila-Levy, Silva, Santos-Mallet, Branquinha and Santos2010). Either model insect hosts or the natural pairs were analysed (d'Avila-Levy et al., Reference d'Avila-Levy, Altoe, Uehara, Branquinha and Santos2006a, Reference d'Avila-Levy, Santos, Marinho, Dias, Lopes, Santos and Branquinhab; Soares et al., Reference Soares, Altoe, Ennes-Vidal, da Costa, Rangel, de Souza, da Silva, Volf and d'Avila-Levy2017). Although phenanthroline can act on any metal-dependent cellular process, the bulk of evidence supports the role of metallopeptidases, particularly Tcgp63-I. Although it is still uncertain the mechanism of action of these molecules, a proteolytic independent role of gp63 has been demonstrated and an insect gut receptor was recognized by gp63 molecules in other trypanosomatids (d'Avila-Levy et al., Reference d'Avila-Levy, Santos, Marinho, Dias, Lopes, Santos and Branquinha2006b).
To adapt and survive into extreme distinct environments like mammalian hosts and insects, a myriad of parasite proteins plays a concerted action. In T. cruzi, cruzipain, calpain-like molecules, GIPLs and GP72 seems to be involved in T. cruzi interaction with the invertebrate host (de Jesus et al., Reference de Jesus, Cooper, Espinosa, Gomes, Garcia, Paul and Cross1993; Basombrio et al., Reference Basombrio, Gomez, Padilla, Ciaccio, Nozaki and Cross2002; Nogueira et al., Reference Nogueira, Gonzalez, Gomes, de Souza, Garcia, Azambuja, Nohara, Almeida, Zingales and Colli2007; Ennes-Vidal et al., Reference Ennes-Vidal, Menna-Barreto, Santos, Branquinha and d'Avila-Levy2011). The molecular interactions that take place in the insect midgut that allows T. cruzi epimastigote binding and multiplication with further migration to the rectum and metacyclogenesis are certainly depend on multiple molecules. The study of isolated molecules helps but is insufficient to fully elucidate the functional impact of the complex structures that can be formed and are upon the influence of the microenvironment of the insect midgut. Interestingly, T. cruzi re-isolated after insect colonization presented an enhanced exposure of Tcgp63-I. Roberts et al. (Reference Roberts, Wilson and Donelson1995) first described that passage of L. chagasi attenuated parasites through mice stimulated the expression of a surface 59-kDa glycoprotein (Roberts et al., Reference Roberts, Wilson and Donelson1995). Furthermore, Sadlova et al. (Reference Sadlova, Volf, Victoir, Dujardin and Votypka2006) showed experimentally that attenuated L. major parasites presented low enzymatic activity of gp63. However, these parasites presented a drastic enhancement in the proteolytic activity after passages through mice (Sadlova et al., Reference Sadlova, Volf, Victoir, Dujardin and Votypka2006).
In conclusion, we provided for the first time evidence on the participation of Tcgp63-I and metallo-dependent enzymes on T. cruzi interaction with the insect vector, and demonstrated that the challenge that the parasite deals during insect vector colonization induces the enhanced production of Tcgp63-I. Although, the precise molecular mechanism underlying the roles of these molecules in the vector interaction still needs further research, it sheds some light on a neglected interface: the parasite–insect interaction.
Author ORCIDs
Claudia M. d'Avila-Levy, 0000-0001-8042-4695.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182019000441
Acknowledgements
The authors are grateful to Dr Daniel Sanchez from Institute of Biotechnological Research of National University of General San Martin (Buenos Aires, Argentina) for kindly providing the anti-Tcgp63-I antibody. We thank the research group of Laboratório de Bioquímica e Fisiologia de Insetos (IOC-FIOCRUZ) for the R. prolixus supplies.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa do Rio de Janeiro (FAPERJ) and Fundação Oswaldo Cruz (Fiocruz).
Conflict of interest
None.
Ethical standards
Not applicable.