Article contents
Establishment of suitable reference genes for studying relative gene expression during the transition from trophozoites to cyst-like stages and first evidences of stress-induced expression of meiotic genes in Trichomonas vaginalis
Published online by Cambridge University Press: 08 April 2021
Abstract
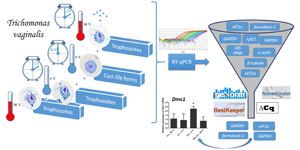
Trichomonas vaginalis is a parasite of the human urogenital tract and the causative agent of trichomoniasis, a sexually transmitted disease of worldwide importance. This parasite is usually found as a motile flagellated trophozoite. However, when subjected to stressful microenvironmental conditions, T. vaginalis trophozoites can differentiate into peculiar cyst-like stages, which exhibit notable physiological resistance to unfavourable conditions. Although well documented in morphological and proteomic terms, patterns of gene expression changes involved in the cellular differentiation into cyst-like stages are mostly unknown. The real-time reverse transcription polymerase chain reaction (RT-qPCR) is recognized as a sensitive and accurate method for quantification of gene expression, providing fluorescence-based data that are proportional to the amount of a target RNA. However, the reliability of relative expression studies depends on the validation of suitable reference genes, which RNAs exhibit a minimum of variation between tested conditions. Here, we attempt to determine suitable reference genes to be used as controls of invariant expression during cold-induced in vitro differentiation of T. vaginalis trophozoites into cyst-like forms. Furthermore, we reveal that the mRNA from the meiotic recombinase Dmc1 is upregulated during this process, indicating that cryptic sexual events may take place in cyst-like stages of T. vaginalis.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
Footnotes
Present address: Laboratório de Biologia Molecular e Doenças Endêmicas, Instituto Oswaldo Cruz, FIOCRUZ, Rio de Janeiro/RJ, Brazil.
References
- 1
- Cited by





