Article contents
Efficacy of flukicides on Fasciola gigantica, a food-borne zoonotic helminth affecting livestock in Bangladesh
Published online by Cambridge University Press: 10 May 2022
Abstract
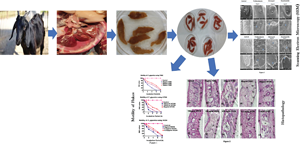
Fasciola gigantica, the causative agent of tropical fasciolosis, is a food-borne zoonotic trematode that affects around 80% livestock of Bangladesh. Triclabendazole (TCBZ), nitroxynil (NTON) and oxyclozanide (OCZN) are frequently used against fascioliasis; however, the current status of potency of these flukicides was unknown. In this study, in vitro efficacy of TCBZ, NTON and OCZN at various concentrations on F. gigantica has been evaluated by relative motility (RM), morphological distortions of apical cone through an inverted microscope, architectural and ultra-structural changes through histopathological and scanning electron microscopy (SEM). It is observed that TCBZ, NTON and OCZN at higher concentrations significantly (P < 0.05) reduced RM of the flukes compared to untreated control. NTON at 150 μg mL−1 was the most potent to reduce the motility within 4 h whereas TCBZ and OCZN were much delayed. Histopathological changes showed swollen, extensive cracking, numerous vacuoles and splitting of the tegument surrounding the spines; spine dislodged from its socket in treated flukes compared to untreated worms. Histopathological changes were more conspicuous at higher doses of TCBZ, NTON and OCZN. SEM has shown the disruption of the apical cone, apart from swelling of the tegument on the ventral surface corrugation and disruption of the ventral apical cone. All these changes indicate that NTON is the most potent in killing flukes in vitro among the tested flukicides and suggest the presence of TCBZ-resistant fluke populations in Bangladesh. It is imperative to explore the in vivo effects of these flukicides and subsequently their molecular mechanisms.
Keywords
- Type
- Research Article
- Information
- Parasitology , Volume 149 , Special Issue 10: Foodborne Trematodes – time to rise from neglected status , September 2022 , pp. 1339 - 1348
- Copyright
- Copyright © Department of Parasitology, Bangladesh Agricultural University, 2022. Published by Cambridge University Press
References
- 2
- Cited by





