Article contents
Early immune responses and parasite tissue distribution in mice experimentally infected with oocysts of either archetypal or non-archetypal genotypes of Toxoplasma gondii
Published online by Cambridge University Press: 14 December 2020
Abstract
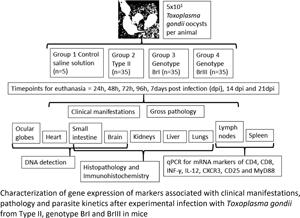
In most of the world Toxoplasma gondii is comprised of archetypal types (types I, II and III); however, South America displays several non-archetypal strains. This study used an experimental mouse model to characterize the immune response and parasite kinetics following infection with different parasite genotypes. An oral inoculation of 50 oocysts per mouse from T. gondii M4 type II (archetypal, avirulent), BrI or BrIII (non-archetypal, virulent and intermediate virulent, respectively) for groups (G)2, G3 and G4, respectively was used. The levels of mRNA expression of cytokines, immune compounds, cell surface markers and receptor adapters [interferon gamma (IFNγ), interleukin (IL)-12, CD8, CD4, CD25, CXCR3 and MyD88] were quantified by SYBR green reverse transcription-quantitative polymerase chain reaction. Lesions were characterized by histology and detection by immunohistochemistry established distribution of parasites. Infection in G2 mice was mild and characterized by an early MyD88-dependent pathway. In G3, there were high levels of expression of pro-inflammatory cytokines IFNγ and IL-12 in the mice showing severe clinical symptoms at 8–11 days post infection (dpi), combined with the upregulation of CD25, abundant tachyzoites and tissue lesions in livers, lungs and intestines. Significant longer expression of IFNγ and IL-12 genes, with other Th1-balanced immune responses, such as increased levels of CXCR3 and MyD88 in G4, resulted in survival of mice and chronic toxoplasmosis, with the occurrence of tissue cysts in brain and lungs, at 14 and 21 dpi. Different immune responses and kinetics of gene expression appear to be elicited by the different strains and non-archetypal parasites demonstrated higher virulence.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s) 2020. Published by Cambridge University Press
References
- 7
- Cited by





