INTRODUCTION
Chagas disease (CD), caused by the kinetoplastid haemoflagellate Trypanosoma cruzi, is a parasitic infection naturally transmitted in 21 Latin American countries by the contaminated feces of blood-sucking bugs (i.e. triatomines). In the endemic area, CD provokes more than 7000 deaths per year and maintains over 25 million people at risk for the infection (WHO, 2015). Moreover, its emerging character in non-endemic areas due to population mobility and alternative routes of transmission (e.g. blood transfusion, organ donation, mother-to-child and through contaminated food) (Schmunis and Yadon, Reference Schmunis and Yadon2010) has contributed to the spreading of an illness that currently affects about 7 million people worldwide (WHO, 2015).
As one of the 17 tropical diseases defined by the WHO as neglected (WHO, 2015), strategies of research and development are focused on the finding of a suitable CD chemotherapy (Zingales et al. Reference Zingales, Miles, Moraes, Luquetti, Guhl, Schijman and Ribeiro2014). Many efforts have been made by institutions in order to develop new compounds potentially applicable to the treatment of CD, since the available chemotherapy relies on two old nitroheterocyclic drugs: benznidazole (BZ), the first-line treatment in most countries, and nifurtimox (NX) (WHO, 2015). Although these medicines are currently accepted to treat the acute and the early-chronic disease, both show limited effectiveness in long-term chronic infections (Urbina, Reference Urbina2015) and also exhibit undesirable side effects (Castro et al. Reference Castro, Montalto de Mecca and Bartel2006).
Concerning experimental chemotherapy research, in a previous study we proposed the 1,2-disubstituted 5-nitroindazolinone scaffold as prototype of antichagasic drug (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012). Concretely, the 2-benzyl-1-propyl (22) and the 2-benzyl-1-butyl (24) derivatives (Fig. 1) achieved outstanding activity over the replicative stages of T. cruzi (i.e. epimastigotes and amastigotes) without toxicity on macrophages, what led to great selectivity on CL Brener strain (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012; Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014).

Fig. 1. Chemical structures of the prototypes 2-benzyl-5-nitro-1-propylindazolin-3-one (22) and 2-benzyl-1-butyl-5-nitroindazolin-3-one (24).
Trypanosoma cruzi populations have been classified into six discrete typing units (DTUs TcI–TcVI) according to molecular genetics, eco-epidemiological features and pathogenicity (Zingales et al. Reference Zingales, Miles, Moraes, Luquetti, Guhl, Schijman and Ribeiro2014). Moreover, susceptibility to reference drugs also varies among T. cruzi strains, with CL Brener and Tulahuen (TcVI) known as drug-sensitive, Y (TcII) as drug-moderately resistant and Colombian (TcI) as drug-resistant strain (Soeiro et al. Reference Soeiro, de Souza, da Silva, Batista, Batista, Pavão, Araújo, Aiub, da Silva, Lionel, Britto, Kim, Sulikowski and Hargrove2013).
In this context, the Parasitology Department of UCM (Madrid, Spain), the Medicinal Chemistry Institute of CSIC (Madrid, Spain) and the Cellular Biology Laboratory of IOC/Fiocruz (Rio de Janeiro, Brazil) have worked in collaboration to further explore the trypanocidal spectrum of these candidates, following routinely screening procedures standardized by these laboratories. Consequently, the aims of the present study were: (i) to analyse the trypanocidal profile of prototypes 22 and 24 on other T. cruzi strains belonging to DTUs involved in human infection; (ii) to evaluate their activity in an in vitro golden model that uses primary cultures of cardiac cells as mammalian hosts since heart is one of the main targets for CD infection and inflammation; and (iii) to confirm their lack of toxicity over different mammalian cell cultures.
MATERIALS AND METHODS
Ethics
All procedures involving mice were carried out in accordance with the guidelines established by the Fiocruz Committee of Ethics for the Use of Animals (CEUA LW16/14).
Compounds
The synthesis of the two 5-nitroindazole derivatives assayed in the present work (Fig. 1) was previously described (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012). The numbering of compounds used in this reference has been followed in the present paper. For all the in vitro assays, stock solutions of 22 and 24 were prepared in dimethyl sulfoxide and extemporaneously added to the cultures in a final concentration of the solvent non-toxic itself (<1%, v/v). BZ (Laboratório Farmacêutico do Estado de Pernambuco – LAFEPE, Brazil) was assayed as a reference drug.
Mammalian cell cultures
Primary cultures of embryonic cardiomyocytes (CM) were obtained from Swiss mice as previously described (Meirelles et al. Reference Meirelles, de Araújo-Jorge, Miranda, de Souza and Barbosa1986). After their purification, CM cultures were sustained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 2·5 mm CaCl2, 1 mm L-glutamine, 5% heat-inactivated fetal bovine serum (FBS) (30 min, 56 °C) and 2% chicken embryo extract. CM cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2.
Murine L929 fibroblasts were grown in plastic culture flasks (75 cm2) using either Minimal Essential Medium (MEM) without phenol-red and supplemented as reported (Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014) (MEMS) or RPMI-1640 without phenol-red supplemented with 10% heat-inactivated FBS and 2 mm glutamine (RPMIS). L929 cultures were maintained in a humidified 5% CO2 atmosphere at 37 °C and subpassaged once a week. A 0·03% ethylenediaminetetraacetic acid (EDTA) and 0·05% trypsin in phosphate-buffered saline (PBS) solution was used for cell detachment.
Parasites
The Y strain of T. cruzi, originally isolated from an acute human case (Silva and Nussenzweig, Reference Silva and Nussenzweig1953) and the Tulahuen strain, stably transfected with the Escherichia coli β-galactosidase gene lacZ (Buckner et al. Reference Buckner, Verlinde, La Flamme and Van Voorhis1996) were used throughout the experiments.
Regarding the Y strain, epimastigotes were maintained at 28 °C in supplemented liver infusion tryptose (LIT) medium as described in (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012) and the axenic cultures continuously maintained in logarithmic growth by weekly passages. Bloodstream trypomastigotes (BT) of this strain were obtained by heart puncture from infected Swiss mice at the parasitaemia peak day and after their purification, resuspended in RPMI medium supplemented with 5% heat-inactivated FBS.
Concerning Tulahuen parasites, tissue culture-derived trypomastigotes (TCT) of this β-galactosidase-transfected strain were harvested in the supernatant of L929 cultures previously infected with invasive forms of T. cruzi and maintained in RPMIS at 37 °C in a humidified 5% CO2 atmosphere (Romanha et al. Reference Romanha, Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010).
Epimastigote susceptibility assay
The activity on epimastigotes was evaluated by applying the resazurin assay previously standardized by Rolón et al. (Reference Rolón, Vega, Escario and Gómez-Barrio2006). Log-phase epimastigotes in LIT medium were seeded in the culture tubes at a density of 3 × 106 parasites mL−1 and maintained at 28 °C overnight to allow homogeneous growth. Afterwards, cultures were distributed in 96-well microplates (200 µL perwell) and incubated within the compounds for 48 h at 28 °C. Growth, medium and drug controls were also included in each assay and concentrations tested in triplicate. Finally, 20 μ L of a resazurin solution in 1% PBS (3 mm, pH 7) was added per well and the plates maintained at 28 °C for another 5 h. Fluorescence intensity was measured with excitation (535 nm) and emission (590 nm) wavelengths (alamarBlue® Assay, U.S. Patent No. 5 501 959) and the results represent the percentage of epimastigote growth inhibition (%EGI). For each compound, the concentration that inhibits 50% of epimastigote growth (IC50) was estimated by plotting the concentrations tested vs the %EGI. Selectivity indexes (SI) and potencies relative to BZ (RP) were also estimated.
Cytotoxicity assays
In order to detect any potential toxicity towards the host cell, cultures of CM and L929 were incubated within these compounds and the metabolic cell function was measured in the presence of resazurin-based indicators (PrestoBlue® and Resazurin sodium salt, respectively).
According to this, 100 µL of DMEM containing 6 × 104 CM per well were seeded in 96-well microplates previously coated with gelatin and incubated overnight at 37 °C in a humidified 5% CO2 atmosphere. Afterwards, the medium was replaced by solutions of each compound in fresh DMEM and the plates were incubated either 24 or 48 h in the conditions aforementioned. Each concentration was evaluated in triplicate and controls of cellular growth were included in all the plates. Once the incubation concluded, both cell morphology and contractibility were examined by light microscopy and cellular viability evaluated by adding the redox indicator PrestoBlue® according to the manufacturer's instructions. After 5 h of incubation at 37 °C in a humidified 5% CO2 atmosphere, the absorbance was read at 570 and 600 nm and the results were expressed as the percentage of cytotoxicity on CM (%CCM) (Romanha et al. Reference Romanha, Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010).
Moreover, the unspecific cytotoxicity over L929 was assayed in 96-well plates by seeding either 10 × 103 or 15 × 103 cells in 100 µL of MEM per well. In order to allow cell attachment, the plates were incubated for 3 h at 37 °C in a humidified 5% CO2 atmosphere and then, the medium was replaced by 200 µL of compounds diluted in fresh MEM. Each concentration was tested in triplicate. Growth, medium and drug controls were also included in each plate. Fibroblasts were exposed to the compounds for 48, 72 and 96 h at 37 °C with 5% CO2. Afterwards, 20 µL of a resazurin in 1% PBS solution (2 mm, pH 7) was added to each well and the plates were returned to the incubator for another 3 h. Finally, fluorescence intensity was read at 535 nm (excitation) and 590 nm (emission) and the results were expressed as %CL929 (Fonseca-Berzal et al. Reference Fonseca-Berzal, Palmeiro-Roldán, Escario, Torrado, Arán, Torrado-Santiago and Gómez-Barrio2015).
For both assays, the concentration that inhibits 50% of cellular growth (LC50) was estimated by plotting drug concentrations vs %C.
Intracellular amastigote susceptibility assays
The activity on intracellular amastigotes was evaluated by infecting either L929 fibroblasts with TCT (Tulahuen strain) or CM with BT (Y strain) in a 10:1 ratio (parasite:cell).
For the first bioassay, 100 µL of RPMIS containing 4000 L929 cells per well was seeded in 96-well tissue culture plates and maintained for 24 h at 37 °C in a humidified 5% CO2 atmosphere. Afterwards, cells were incubated within TCT for another 2 h and then, non-penetrated parasites were discarded replacing the culture medium by fresh RPMIS. In order to establish the infection, the plates were maintained during 48 h at 37 °C and 5% CO2 and then, the medium was replaced by solutions of each compound in fresh RPMIS. Each concentration was evaluated in triplicate. Controls of infection and cell growth were also included and the plates were incubated for 96 h at the same conditions of temperature and humidity. After this period, 50 µL of 500 µ m chlorophenol red glycoside in 0·5% Nonidet P40 was added to each well and the plates were incubated for 18 h at 37 °C. Finally, the absorbance was read at 570 nm and the results were expressed as the percentage of amastigote growth inhibition (%AGI) (Romanha et al. Reference Romanha, Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010).
To assay these compounds over Y strain amastigotes, 100 000 CM per well were seeded in 24-well tissue culture plates provided with round coverslips previously coated with gelatin and then, maintained in DMEM overnight at 37 °C in a humidified 5% CO2 atmosphere. After 24 h of parasite–host cell interaction, the infected cultures were washed to remove non-internalized trypomastigotes and then, incubated for another 48 h within the compounds diluted in fresh DMEM. Next, the cultures were fixed with Bouin's fixative and stained with Giemsa. The mean number of infected CM and the mean number of parasites per infected CM were scored in 400 host cells by duplicate. Only parasites with characteristic nuclei and kinetoplast were counted, since the irregular ones were considered as parasites undergoing death. Finally, activity results were estimated by calculating the inhibition of the endocytic index (EI) (da Silva et al. Reference da Silva, Batista, Mota, de Souza, Stephens, Som, Boykin and Soeiro2007).
In each assay, compounds IC50 and IC90 (concentration that inhibits 50 and 90% of amastigote proliferation or EI, respectively) were estimated by plotting concentrations vs %AGI or %EI. SI over the respective host cell and RP were also calculated.
All the in vitro assays were run in the same conditions at least twice separately. For each assay, the results are expressed as the mean value of activity ± standard deviation (s.d.).
Statistical analysis
SPSS Statistics software (version 20, IBM) was used for the statistical analysis. The non-parametric Kruskal–Wallis test was applied to compare compounds activity between the different strains, as well as with that of BZ. P < 0·05 was considered statistically significant.
RESULTS
Toxicity on mammalian host cells
Since both CM and L929 were used as T. cruzi host cell, the potential toxicity of 22 and 24 over these mammalian cells was assessed in vitro. As Fig. 2 reflects, no toxicity was perceived on the treated CM, and therefore, percentages of cell viability greater than 85% were registered in all the cases. In fact, both derivatives achieved LC50 values higher than 100 µ m after the incubation times assayed (i.e. 24 and 48 h). Moreover, the light microscopy analysis revealed neither alteration in cell morphology nor affectation in contractibility as a treatment consequence.

Fig. 2. Toxic effect of the 5-nitroindazolinones over primary cultures of CM after 24 h (A) and 48 h (B) of treatment.
Nevertheless, both compounds achieved a different cytotoxic profile on L929 fibroblasts. After 48 h of drug–cell contact, concentrations higher than 33·3 µ m led to a pronounced loss of cellular viability that varied in a time-dependent manner. Likewise, BZ induced higher toxicity on this mammalian cell line, showing a response similar to that of derivative 22 (Fig. 3 and Table 1). However, at the highest concentration tested and after completing the three incubation times assayed on L929, no differences were detected between the toxic profile of 5-nitroindazolinones and BZ (P > 0·05).

Fig. 3. Toxic effect of the 5-nitroindazolinones over L929 cells after 48 h (A), 72 h (B) and 96 h (C) of treatment.
Table 1. Toxic effect of derivatives 22 and 24 on L929 cells

* Significant differences (P < 0·05) compared with BZ (Kruskal–Wallis test).
a Results expressed as the mean value of cytotoxicity (%C) at the highest concentration tested (100 µ m) ± standard deviation (s.d.) of three independent experiments (n = 3).
b The concentration causing 50% of cellular lethality (LC50) was estimated by plotting drug concentrations vs %C. The results are expressed as the mean ± s.d. of three independent experiments (n = 3).
Activity on epimastigotes (Y strain)
As a primary screening, the inhibitory effect of derivatives 22 and 24 was evaluated on axenic cultures of epimastigotes. Both compounds displayed a better trypanocidal profile compared with that of BZ (Fig. 4). They obtained improved IC50 values and remarkable SI on the extracellular parasite (Table 2), significantly better in the case of derivative 22 (P < 0·05). Otherwise, the potency of these molecules could not be determined at this level, since their IC90 were reached at concentrations superior than the highest one assayed.
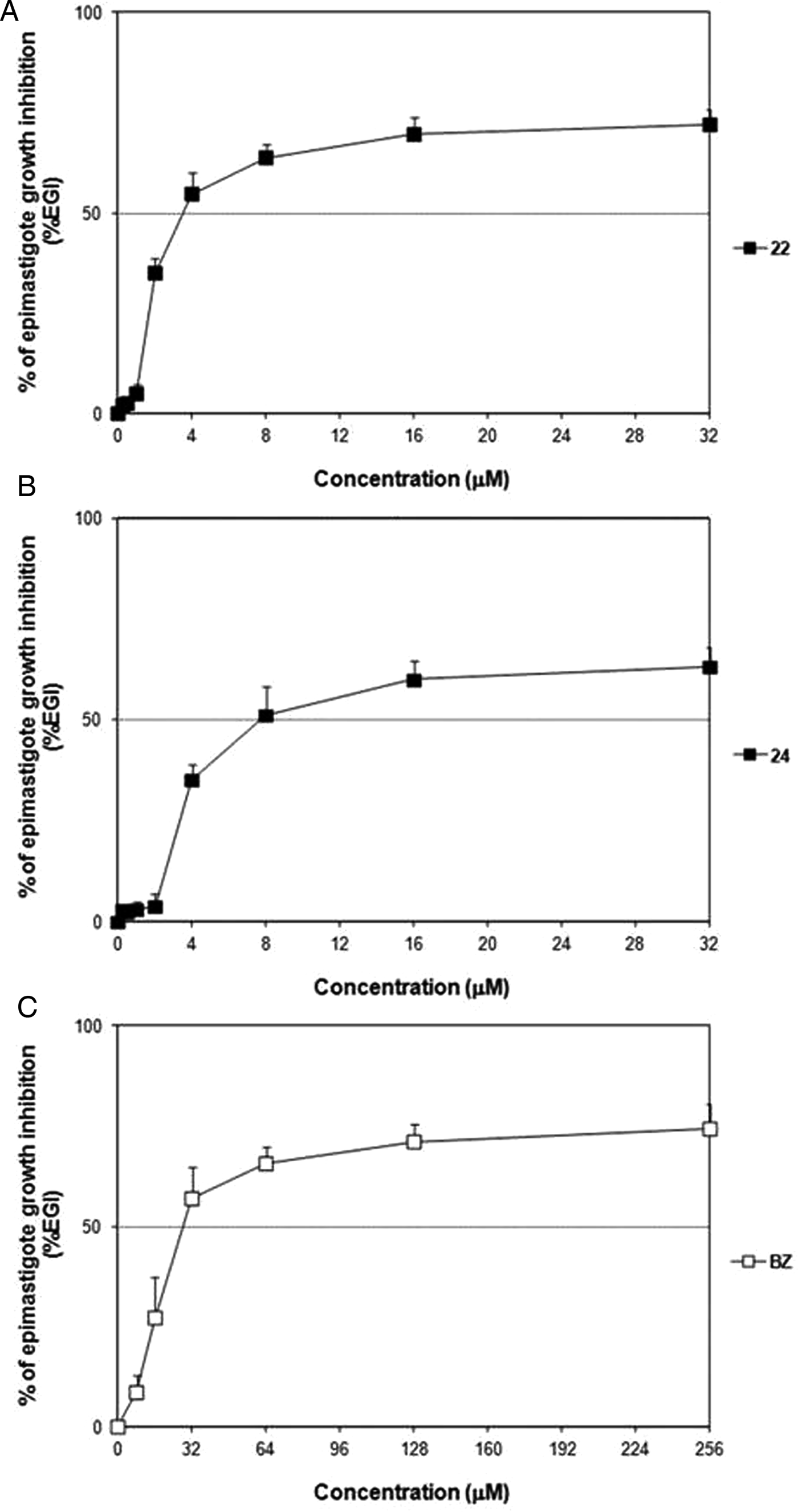
Fig. 4. Dose–response curves of 22 (A), 24 (B) and BZ (C) on epimastigotes (Y strain).
Table 2. Trypanocidal activity of 22 and 24 on Y strain epimastigotes expressed as IC50, IC90 and SI

* Significant differences (P < 0·05) compared with BZ (Kruskal–Wallis test).
** Significant differences (P < 0·05) compared with the activity on CL-B5 epimastigotes (see Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012) (Kruskal–Wallis test).
a The concentration causing 50 and 90% of EGI (IC50 and IC90, respectively) was estimated by plotting drug concentrations vs %EGI. The results are expressed as the mean ± s.d. of three independent experiments (n = 3).
b After 48 h of drug treatment.
c Selectivity indexes are defined as SI = LC50 (48 h CM cells)/IC50 Y epimastigotes.
Activity on intracellular amastigotes
The inhibitory effect of 22 and 24 was further evaluated over Tulahuen and Y strain amastigotes grown in L929 and CM cultures, respectively. According to the results of cytotoxicity (Table 1), concentrations higher than 50 µ m were not tested at this level. Although both compounds displayed slightly lower dose–response curves upon Tulahuen parasites compared with that of BZ (Fig. 5 and Table 3), they reached outstanding SI on the intracellular stage and were as potent (P > 0·05) as the reference drug (Table 3).
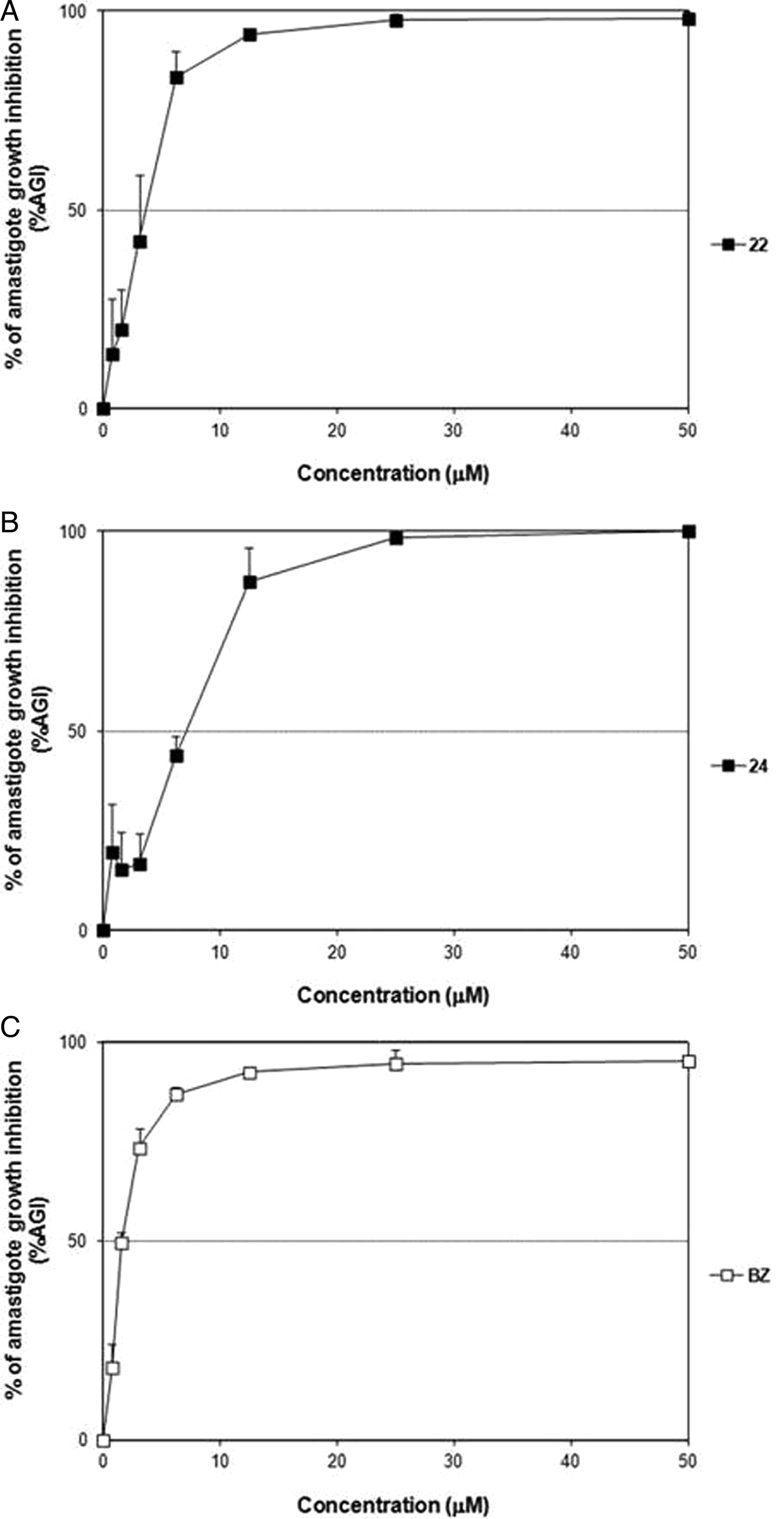
Fig. 5. Dose–response curves of 22 (A), 24 (B) and BZ (C) on intracellular amastigotes (Tulahuen strain) grown in L929 cultures.
Table 3. Trypanocidal activity of 22 and 24 on intracellular amastigotes of Tulahuen strain expressed as IC50, IC90 and SI. Compounds RP were also estimated

* Significant differences (P < 0·05) compared with BZ (Kruskal–Wallis test).
** Significant differences (P < 0·05) compared with the activity on CL-B5 amastigotes (see Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014) (Kruskal–Wallis test).
a The concentration causing 50 and 90% of amastigote growth inhibition (IC50 and IC90, respectively) was estimated by plotting drug concentrations vs %AGI. The results are expressed as the mean ± s.d. of three independent experiments (n = 3).
b After 96 h of drug treatment (see Table 1).
c Selectivity indexes are defined as SI = LC50 (96 h L929 cells)/IC50 Tulahuen amastigotes.
d Relative potencies are defined as RP = IC90 BZ/IC90 tested compound.
Regarding the activity upon Y strain amastigotes, although compound 22 achieved similar IC50 values for both the strains (P > 0·05), it accomplished a better IC90 after a 2-day treatment of Y-infected cells and a nearly suppression of the EI at 25 µ m (%EI = 99·43 ± 0·32%) (Fig. 6), showing a trypanocidal profile similar to that of BZ (Table 4). Otherwise, derivative 24 was not only less effective but also with lower potency on this latter strain (Table 4). However, at 25 µ m it obtained a considerable reduction in both the percentage of infected cells and the mean number of amastigotes per infected cell, as the inhibition in the EI reflects (%EI ca. 71%) (Figs 6 and 7).
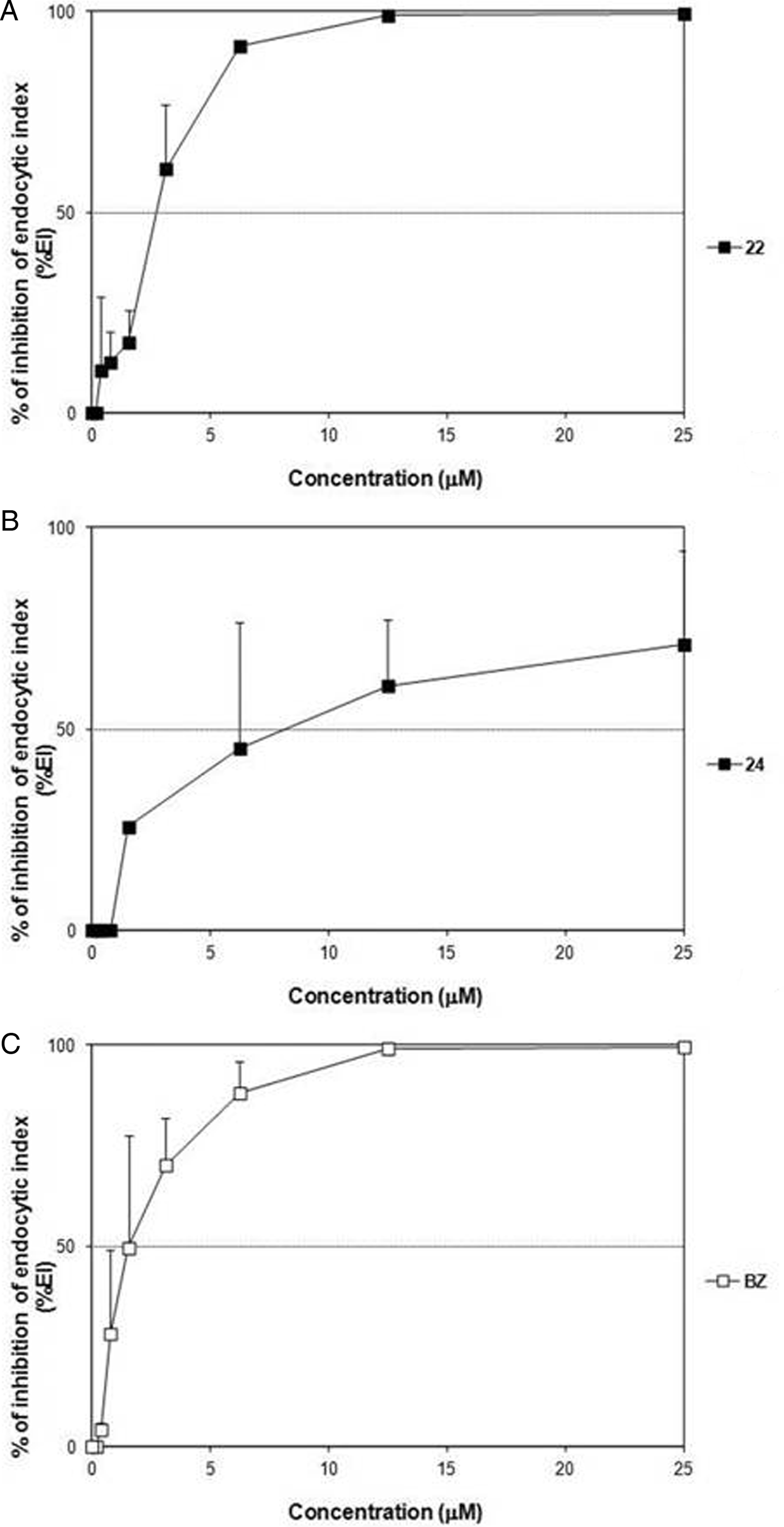
Fig. 6. Dose–response curves of 22 (A), 24 (B) and BZ (C) on intracellular amastigotes (Y strain) grown in CM cultures.
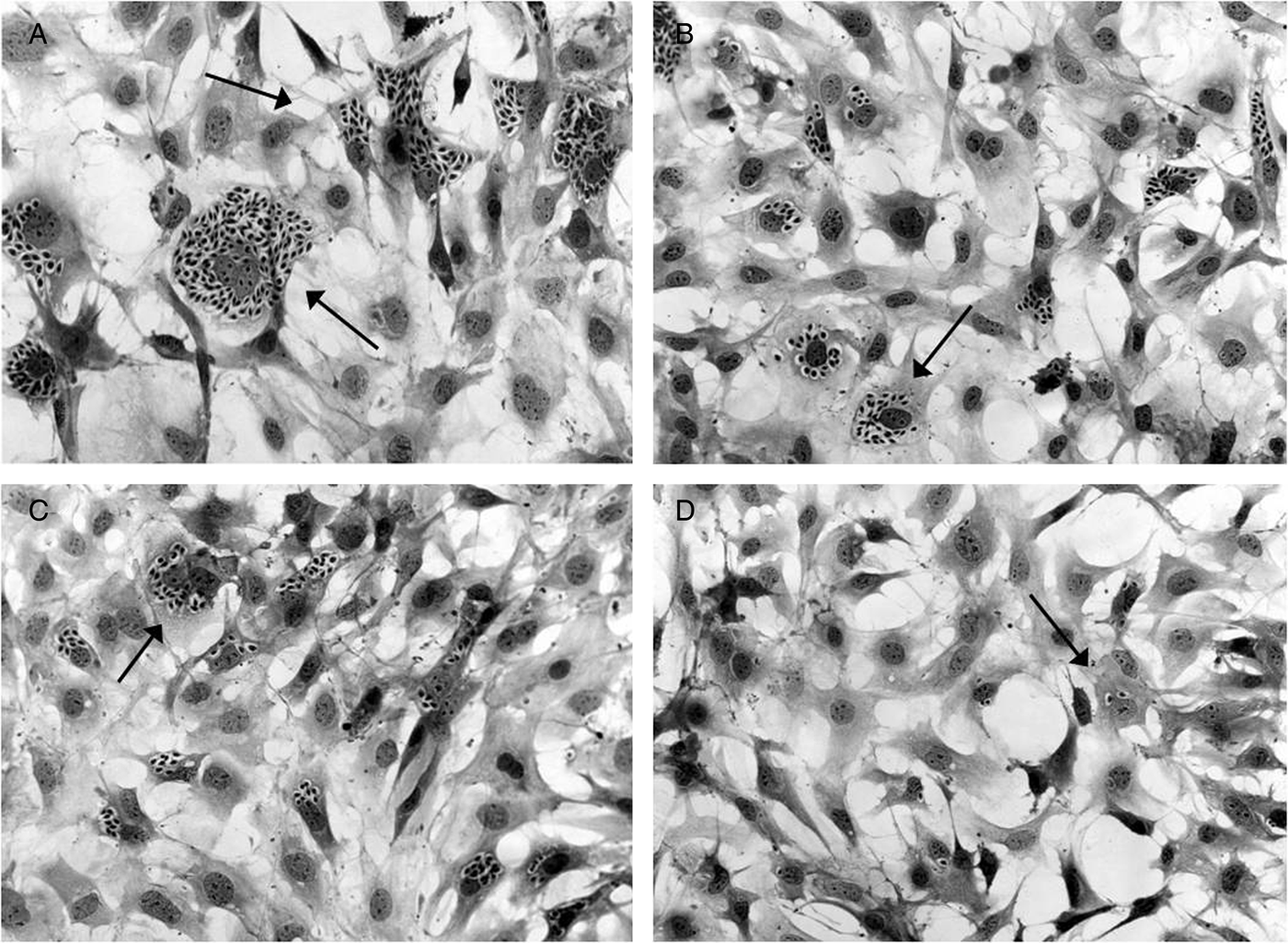
Fig. 7. Light microscopy analysis of CM infected with Y strain and treated for 48 h with 6·25 µ m (B), 12·50 µ m (C) and 25 µ m (D) of derivative 24, as well as untreated cells (A). The black arrows indicate intracellular amastigotes.
Table 4. Trypanocidal activity of 22 and 24 on intracellular amastigotes of Y strain expressed as IC50, IC90 and SI. Compounds RP were also estimated

* Significant differences (P < 0·05) compared with BZ (Kruskal–Wallis test).
** Significant differences (P < 0·05) compared with the activity on CL-B5 (see Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014) and Tulahuen amastigotes (Kruskal–Wallis test).
a The concentration causing 50 and 90% of EI inhibition (IC50 and IC90, respectively) was estimated by plotting drug concentrations vs %EI. The results are expressed as the mean ±s.d. of two independent experiments (n = 2).
b After 48 h of drug treatment.
c Selectivity indexes are defined as SI = LC50 (48 h CM cells)/IC50 Y amastigotes.
d Relative potencies are defined as RP = IC90 BZ/IC90 tested compound.
DISCUSSION
The selection of diverse T. cruzi strains and clones for in vitro drug screening is an important point to consider in the early research of putative candidates for CD chemotherapy (Zingales et al. Reference Zingales, Miles, Moraes, Luquetti, Guhl, Schijman and Ribeiro2014). In fact, the different effectiveness that BZ and NX display among divergent strains is one of the aspects on which the current Chagas therapy failure resides (Urbina, Reference Urbina2015). Although a clear correlation between T. cruzi genetic variability and its presumable response to drugs has not been evidenced (Moraes et al. Reference Moraes, Giardini, Kim, Franco, Araujo-Junior, Schenkman, Chatelain and Freitas-Junior2014), strategies for drug screening should focus its performance on parasite DTUs more often associated with human infection (DTUs TcI, TcII, TcV and TcVI) (Zingales et al. Reference Zingales, Miles, Moraes, Luquetti, Guhl, Schijman and Ribeiro2014).
In previous studies, indazole-based compounds have demonstrated great effectiveness on different T. cruzi strains (reviewed in Aguilera-Venegas et al. Reference Aguilera-Venegas, Olea-Azar, Arán and Speisky2013). Concretely, several series of 5-nitroindazole derivatives have achieved important outcomes exhibiting notable anti-T. cruzi activity (Arán et al. Reference Arán, Ochoa, Boiani, Buccino, Cerecetto, Gerpe, González, Montero, Nogal, Gómez-Barrio, Azqueta, López de Ceráin, Piro and Castellano2005; Boiani et al. Reference Boiani, Gerpe, Arán, Torres de Ortiz, Serna, Vera de Bilbao, Sanabria, Yaluff, Nakayama, Rojas de Arias, Maya, Morello, Cerecetto and González2009; Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012; Muro et al. Reference Muro, Reviriego, Navarro, Marín, Ramírez-Macías, Rosales, Sánchez-Moreno and Arán2014), being some of these molecules proposed to trigger reactive oxygen species (ROS)-based mechanisms of action (Folch-Cano et al. Reference Folch-Cano, Olea-Azar, Arán and Díaz-Urrutia2010).
In this framework, we have formerly identified the 5-nitroindazolinones 22 and 24 as promising antichagasic candidates, since the great activity they achieved on epimastigotes (IC50 = 0·93 and 1·17 µ m, respectively) and intracellular amastigotes (IC50 < 1 and 3·71 µ m, respectively) of T. cruzi CL Brener (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012; Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014). The results of this primary screening on a β-galactosidase-transfected clone (Buckner et al. Reference Buckner, Verlinde, La Flamme and Van Voorhis1996) with a biological behaviour similar to that of the parental CL strain (TcVI) (Le-Senne et al. Reference Le-Senne, Muelas-Serrano, Fernández-Portillo, Escario and Gómez-Barrio2002), prompted us to perform a deeper study of their broad activity on other T. cruzi strains (Zingales et al. Reference Zingales, Miles, Moraes, Luquetti, Guhl, Schijman and Ribeiro2014). Consequently, further screening procedures were carried out on intracellular forms of Tulahuen strain to confirm their activity on TcVI parasites, as well as over both extracellular and intracellular stages of T. cruzi Y, representative strain of TcII.
Moreover, an analysis of compounds relative cytotoxicity was simultaneously conducted. Although both candidates did not exert any toxic effect on J774 macrophages after 24 h of drug exposure (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012), it is important to verify this fact over those mammalian cells hosting the parasite in subsequent trypanocidal assays (Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014). In addition, either CM or fibroblasts are preferred as host cell for such an experimental model, since both are T. cruzi target cells more strictly involved in the pathology of the disease and only some parasite isolates concentrate infection in the mononuclear phagocytic system (Teixeira et al. Reference Teixeira, Nascimento and Sturm2006). Moreover, heart is an important target for infection and inflammation in CD pathology and then, pre-clinical studies using these cells are desirable to exclude potential drug candidates with cardiotoxicity characteristics (da Silva et al. Reference da Silva, Batista, Mota, de Souza, Stephens, Som, Boykin and Soeiro2007). According to this, we tested compounds 22 and 24 over uninfected L929 fibroblasts and primary cultures of CM in order to rule out the toxic effects. Neither compound 22 nor 24 resulted in toxicity on CM after completing 48 h of incubation (Fig. 2). Otherwise, high concentrations of both derivatives seemed to induce a loss of L929 viability that increased in a time-dependent manner, showing a behaviour similar to that of BZ (P > 0·05) (Fig. 3 and Table 1). It could happen as a result of the different nature of both mammalian cell cultures (i.e. primary cultures and continuous cell line, respectively) (Duran-Rehbein et al. Reference Duran-Rehbein, Vargas-Zambrano, Cuéllar, Puerta and González2014), having obtained a similar pattern with the reference drug (Figs 2 and 3). In fact, differences related to susceptibility and sensitivity using the same drug have been also reported according to the mammalian cell type and the respective viability assay employed (Zwolak, Reference Zwolak2014; Emter and Natsch, Reference Emter and Natsch2015; Xu et al. Reference Xu, McCanna and Sivak2015). Herein, the noticed differences in drug sensitivity detected among fibroblasts and cardiac cells may arise due to the different cytotoxic assays performed (Resazurin sodium salt × PrestoBlue®) and from the dissimilar impact susceptibility of each target cell (fibroblasts vs cardiac cells).
Several studies have reported no apparent DTU association regarding epimastigote susceptibility to both reference drugs (Boiani et al. Reference Boiani, Boiani, Denicola, Torres de Ortiz, Serna, Vera de Bilbao, Sanabria, Yaluff, Nakayama, Rojas de Arias, Vega, Rolón, Gómez-Barrio, Cerecetto and González2006; Moreno et al. Reference Moreno, D’ávila, Silva, Galvão, Macedo, Chiari, Gontijo and Zingales2010; Zingales et al. Reference Zingales, Miles, Moraes, Luquetti, Guhl, Schijman and Ribeiro2014) agreeing this statement with the results we achieved with BZ on CL-B5 (IC50 = 27·32 µ m) (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012) and Y strain (IC50 = 28·00 µ m) (P > 0·05). The variation in glutathione content (free or mostly conjugated as trypanothione) found among T. cruzi strains has been also proposed as an explanation of their different susceptibility to both NX and BZ (Repetto et al. Reference Repetto, Opazo, Maya, Agosin and Morello1996), since these reduced thiols play an important role in the free radical detoxification mechanisms of trypanosomatids (Irigoin et al. Reference Irigoin, Cibils, Comini, Wilkinson, Flohe and Radi2008). Moreover, no relevant differences in glutathione content were formerly established among Tulahuen, CL and Y epimastigotes (Moncada et al. Reference Moncada, Repetto, Aldunate, Letelier and Morello1989) presuming a comparable response of these strains to BZ, what correlates with the similar profiles herein obtained (Table 2). Although the epimastigote is not the clinically relevant form of T. cruzi, the easy maintenance this model offers (Muelas et al. Reference Muelas, Di Maio, Cerecetto, Seoane, Ochoa, Escario and Gómez-Barrio2001) and the identification of an intracellular epimastigote-like form as intermediate stage within the mammalian host (Faucher et al. Reference Faucher, Baltz and Petry1995), support the performance of pre-screening models on this stage of T. cruzi. Additionally, a correlation between both the activity on epimastigotes and amastigotes occurs (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012; Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014). However, compounds IC50 on these both stages usually differ in several orders of magnitude (Moreno et al. Reference Moreno, D’ávila, Silva, Galvão, Macedo, Chiari, Gontijo and Zingales2010). Undoubtedly, posterior inhibition assays on infective forms (i.e. trypomastigotes and amastigotes) must be carried out in vitro before advancing compounds to in vivo assays (Romanha et al. Reference Romanha, Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010), since different drug susceptibility can be registered among different stages from the same T. cruzi stock (Moraes et al. Reference Moraes, Giardini, Kim, Franco, Araujo-Junior, Schenkman, Chatelain and Freitas-Junior2014).
According to these facts, we evaluated the effectiveness of derivatives 22 and 24 upon the three main forms of T. cruzi Y strain, which is routinely used in our laboratories for in vitro and in vivo drug screening models (Castillo-Garit, et al. Reference Castillo-Garit, del Toro-Cortés, Kouznetsov, Puentes, Romero Bohórquez, Vega, Rolón, Escario, Gómez-Barrio, Marrero-Ponce, Torrens and Abad2012; da Silva et al. Reference da Silva, Batista, Oliveira, de Souza, Hammer, da Silva, Daliry, Araújo, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2012; Araújo et al. Reference Araújo, da Silva, Batista, da Silva, Batista, Aiub, da Silva, Araújo-Lima, Banerjee, Farahat, Stephens, Kumar, Boykin and Soeiro2014; Fonseca-Berzal et al. Reference Fonseca-Berzal, Palmeiro-Roldán, Escario, Torrado, Arán, Torrado-Santiago and Gómez-Barrio2015). The trypanocidal profile both prototypes accomplished on this strain was quite similar to that of over CL-B5 parasites (Vega et al. Reference Vega, Rolón, Montero-Torres, Fonseca-Berzal, Escario, Gómez-Barrio, Gálvez, Marrero-Ponce and Arán2012; Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014). However, our compounds displayed lower activity towards epimastigotes of Y strain (P < 0·05), unlike BZ (Table 2). This fact may be connected with the moderately resistance to nitroderivative drugs attributed to T. cruzi Y (Romanha et al. Reference Romanha, Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010), contrasting with the susceptible CL Brener (Le-Senne et al. Reference Le-Senne, Muelas-Serrano, Fernández-Portillo, Escario and Gómez-Barrio2002). Moreover, 22 and 24 seemed to be more selective than BZ on extracellular epimastigotes (SI22 > 28·17, SI24 > 12·62 and SIBZ > 3·57), since they got better effectiveness on this stage too (Table 2).
Following the criteria proposed by DNDi (Don and Ioset, Reference Don and Ioset2014), compounds that obtain IC50 values inferior than 10 µ m on intracellular amastigotes of TcVI or TcII are identified as putative hits for CD chemotherapy, likewise our two derivatives. These compounds formerly displayed such an outstanding activity on CL-B5 (Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014) now confirmed in the phenotypic assay on Tulahuen (P > 0·05) (Table 3), both TcVI strains. Besides, derivatives 22 and 24 also fulfilled it over Y amastigotes (TcII) grown in cardiac cells in vitro (Table 4). In fact, both compounds proved to be 10-fold more active on the Y strain than toxic to mammalian cells (Don and Ioset, Reference Don and Ioset2014), successfully overpassing this preliminary hit stage (SI22 > 35·71 and SI24 > 11·08).
Nevertheless, both nitroheterocycles not only displayed great activity on T. cruzi models, but also were considered as suitable templates for the design of novel anti-Trypanosoma brucei agents (Arán et al. Reference Arán, Kaiser and Dardonville2012). However, the activity they achieved against blood trypomastigotes of both African (Arán et al. Reference Arán, Kaiser and Dardonville2012) and American trypanosomes (data not shown) was lower than the obtained on the multiplying stages of T. cruzi. In the present study, our compounds achieved an improved trypanocidal profile on intracellular TcII parasites, likewise BZ (Tables 2 and 4). The activity displayed by the reference drug on T. cruzi Y, could be again related to variations in thiol concentration among different stages of a unique strain (Maya et al. Reference Maya, Repetto, Agosin, Ojeda, Téllez, Gaule and Morello1997). According to this, the higher the content of reduced thiols (i.e. epimastigotes > trypomastigotes > amastigotes), the less susceptible to BZ (IC50 value on epimastigotes > trypomastigotes > amastigotes). Actually, in a previous in vivo study we found that 22 and 24 did not entirely suppress parasitaemia through the acute disease in mice, but obtained a considerably reduction in BT levels after concluding a 5-day treatment (Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014). The activity on BT was higher for derivative 24 either in vivo (Fonseca-Berzal et al. Reference Fonseca-Berzal, Escario, Arán and Gómez-Barrio2014) or in vitro models (data not shown), which conversely was the less active derivative on intracellular T. cruzi.
The results compiled in the present study corroborate these two 5-nitroindazolinone derivatives as putative antichagasic prototypes. Both compounds bore out remarkable effectiveness on the clinically relevant stage of TcII and TcVI strains with a lack of toxicity on diverse host cells that resulted in great selectivity on T. cruzi. Concretely, the 2-benzyl-5-nitro-1-propylindazolin-3-one (22) showed trypanocidal and cytotoxic profiles similar to those of the reference drug BZ. Further investigation directed to explore the mechanism of action triggered by these compounds is currently underway.
ACKNOWLEDGEMENTS
The authors thank the Programme for Technological Development in Tools for Health (PDTIS-Fiocruz) for the facilities. C.F.-B., J.A.E., V.J.A and A.G.-B. acknowledge M.N.C.S for kindly accepting C.F.-B. predoctoral stay in LBC/IOC/Fiocruz.
FINANCIAL SUPPORT
The present study was supported by grants from Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Oswaldo Cruz, PDTIS, PROEP/CNPq/Fiocruz, CAPES. M.N.C.S. is research fellows of CNPq and CNE researchers. This work was also supported by the UCM-BSCH Research Group ‘Terapia Antiparasitaria’ (ref. no. 911120). Research by C.F.-B. was supported by a PICATA predoctoral fellowship and a PICATA predoctoral mobility grant of the Moncloa Campus of International Excellence (UCM-UPM & CSIC).
CONFLICT OF INTEREST
None.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.















