Introduction
Heart failure (HF) occurs when the heart is unable to pump sufficiently to match blood flow to requirement. Signs and symptoms include shortness of breath, excessive tiredness and leg swelling, resulting in decreased exercise capacity and impaired quality of life.
HF is one of the leading causes of hospitalisation, morbidity and mortality worldwide, and incidence is increasing. Although medical advances have improved HF survival, mortality rates remain high( Reference Mozaffarian, Benjamin and Go 1 ), with a 10-year survival rate of about 25 %. Therefore, feasible preventive and treatment measures are of considerable clinical and public health importance, yet few exist. In this context, nutritional modification represents an attractive strategy.
CHD, hypertension, atrial fibrillation, valvular heart disease, excess alcohol use, infection and idiopathic cardiomyopathy represent the most common causes of HF. Hypertension, obesity, dyslipidaemia, insulin resistance/diabetes and systemic inflammation are associated with HF incidence/severity. Nutritional factors are major contributors to these risk factors and to CVD itself( Reference Mozaffarian, Benjamin and Go 1 ). Multiple landmark trials have documented the profound effect of nutritional intake on CVD incidence/severity including the Diet And Reinfarction Trial (DART)( Reference Burr, Fehily and Gilbert 2 ), Dietary Approaches to Stop Hypertension (DASH)( Reference Appel, Moore and Obarzanek 3 ) and Prevención con Dieta Mediterránea (PREDIMED)( Reference Estruch, Ros and Salas-Salvadó 4 ).
Lifestyle modifications (exercise, smoking cessation) form the cornerstone of early HF treatment combined with medications (for example, angiotensin-converting enzyme inhibitors, beta blockers, diuretics) as well as implanted devices (for example, pacemakers). Regarding HF, most research efforts have focused on pharmacology and devices, with little attention paid to nutrition( Reference Rich and Hauptman 5 ), limiting the understanding of nutritional factors in HF pathogenesis/treatment( Reference Rich and Hauptman 5 – Reference Van Horn and Yancy 7 ). However, nutritional modification has a relatively low risk:cost ratio and represents an attractive strategy to reduce HF incidence/severity. Dietary guidelines regarding HF are typically modest and unspecific with a focus on Na and fluid restrictions. However, targeted nutritional modification may have superior effects in HF prevention/treatment. The aim of the present review is to summarise current evidence regarding dietary patterns/components and HF.
Methods
References were identified by searches of MEDLINE, CINAHL, Embase and online Cochrane databases up to October 2017. Table 1 details multiple specific keyword searches used, each in conjunction with ‘heart failure’. Only papers published in English were included and their bibliographies were searched for further references.
Table 1 Keyword searches
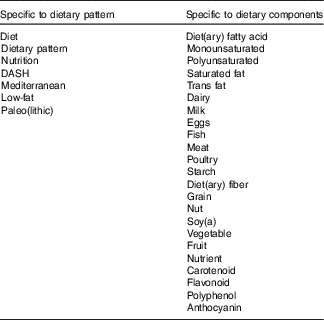
DASH, Dietary Approaches to Stop Hypertension.
Studies were excluded if they reported solely on pharmaceutical intervention or animal model research. Further, salt/Na or fluid restriction, micronutrient supplementation, alcohol and over-/undernutrition (i.e. obesity or malnutrition/cardiac cachexia) are important and major topics themselves and are outside the scope of the present review. These topics have been omitted as the focus here is on dietary pattern and food components as opposed to individual nutrients. All references specific to HF were included, while references related to CVD in general or non-HF CVD were not included.
Results
Evidence linking specific lifestyles with heart failure
Several epidemiological studies in the last decade have demonstrated an inverse association between healthy lifestyle and decreased HF risk. In these studies, a healthy lifestyle (regular physical activity, healthy dietary pattern, normal BMI, no/moderate alcohol, not smoking) was associated with 45–81 % decreased HF incidence( Reference Djoussé, Driver and Gaziano 8 – Reference Larsson, Tectomidis and Gigante 16 ). These studies demonstrate that greater adherence to healthy behaviours was associated with a graded reduction in HF incidence (Table 2), suggesting a major role for lifestyle factors, including diet, in primary prevention of HF. Because these studies included several lifestyle factors, the specific effect of nutrition cannot be elucidated.
Table 2 Prospective studies of lifestyle and heart failure (HF) risk

AA, African-American; AHEI, Alternative Healthy Eating Index; BP, blood pressure; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction; mph, miles per h, PA, physical activity.
Additionally, there have been several randomised controlled trials that focused on educational interventions to improve nutritional knowledge and compliance with dietary recommendations in HF. These trials noted increased nutritional knowledge, higher compliance with dietary guidelines, increased exercise tolerance( Reference Abshire, Xu and Baptiste 17 ) and even decreased HF hospitalisation/death( Reference Ferrante, Varini and Macchia 18 ). These educational trials are important because they suggest that nutritional intervention in HF is not only possible but also has broad clinical benefit.
Evidence linking specific dietary patterns with heart failure
Dietary patterns involve complex relationships between components of dietary intake, not just a single nutrient. Overall diet assessment represents a broad picture of food and nutrient consumption and has been suggested to be more predictive of disease risk than individual foods/nutrients( Reference Hu 19 ). Studies of dietary patterns/components regarding HF incidence/severity are detailed in Tables 2–6, including prospective studies (Table 2), cross-sectional studies (Table 3), longitudinal studies (Table 4), nutritional biomarker studies (Table 5) and nutritional intervention trials (Table 6).
Table 3 Cross-sectional studies of dietary patterns/food and heart failure (HF) outcome
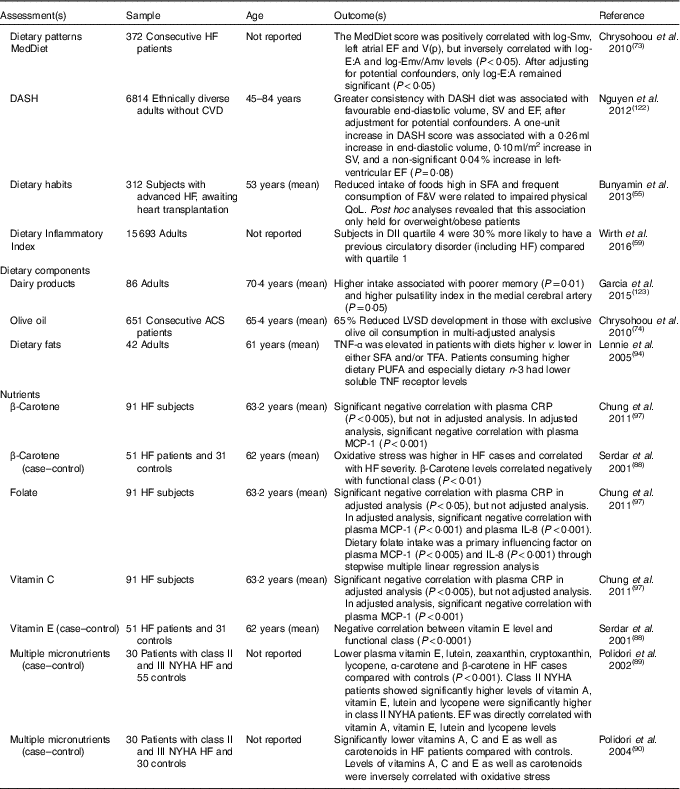
ACS, acute coronary syndrome; Amv, mitral velocity A wave; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; DII, dietary inflammatory index; E:A, ratio of peak velocity flow in early diastole to peak velocity flow in late diastole; EF, ejection fraction; Emv, mitral velocity E wave; F&V, fruit and vegetables; LVSD, left ventricular systolic dysfunction; MCP-1, monocyte chemoattractant protein-1; MedDiet, Mediterranean diet; NYHA, New York Heart Association; QoL, quality of life; Smv, skeletal muscle ventricles; SV, stroke volume; TFA, trans-fatty acids; V(p), velocity of flow progression.
Table 4 Longitudinal studies of dietary patterns/food and heart failure (HF) risk and/or outcome
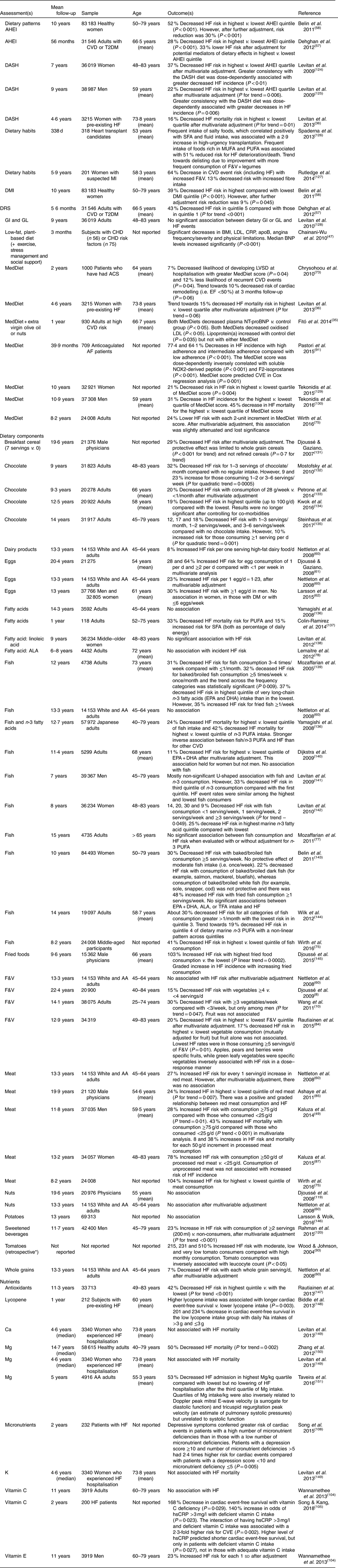
AA, African-American; ACS, acute coronary syndrome; AF, atrial fibrillation; AHEI, Alternate Healthy Eating Index; ALA, α-linolenic acid; BNP, brain natriuretic peptide; CRP, C-reactive protein; CVE, cardiovascular events; DASH, Dietary Approaches to Stop Hypertension; DMI, Dietary Modification Index; DRS, Diet Risk Score; EF, ejection fraction; F&V, fruit and vegetables; GI, glycaemic index; GL, glycaemic load; hsCRP, high-sensitivity CRP; LVSD, left ventricular systolic dysfunction; MedDiet, Mediterranean diet; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; T2DM, type 2 diabetes; TFA, trans-fatty acids.
* Retrospective study.
Table 5 Nutritional biomarker studies and heart failure (HF) risk and/outcome*
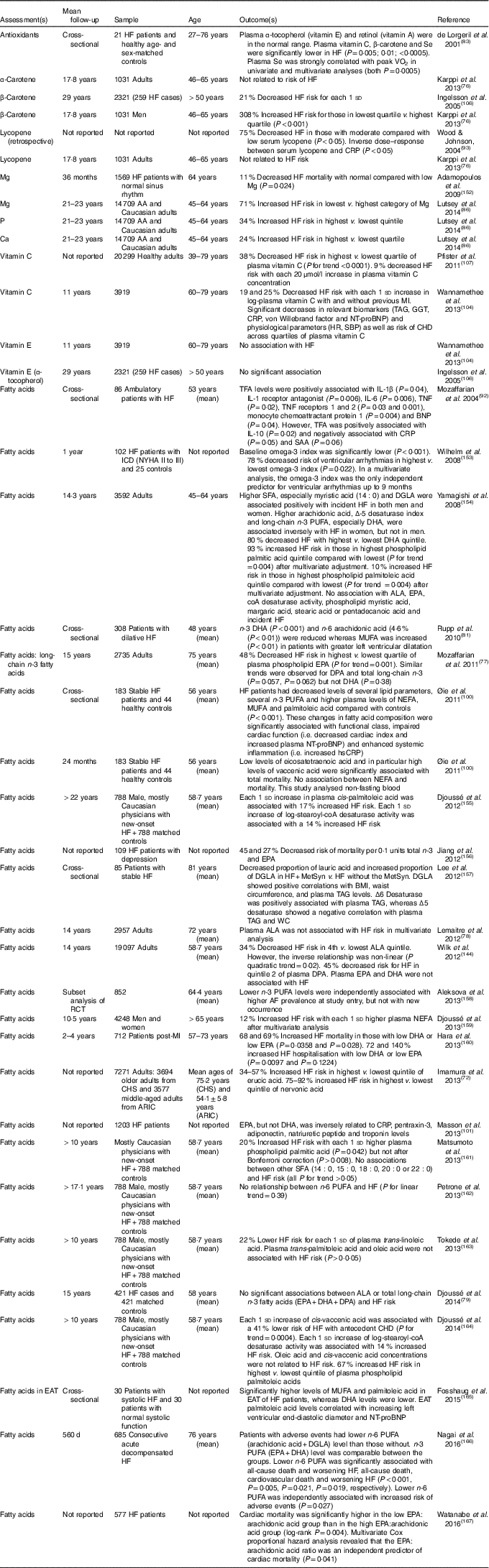
AA, African-American; ARIC, Atherosclerosis Risk in Communities Study; ALA, α-linolenic acid; BNP, B-type natriuretic peptide; CHS, Cardiovascular Health Study; CRP, C-reactive protein; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; EAT, epicardial adipose tissue; GGT, γ-glutamyl transferase; HR, hazard ratio; hsCRP, high-sensitivity CRP; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; MetSyn, metabolic syndrome; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RCT, randomised controlled trial; SAA, serum amyloid A; SBP, systolic blood pressure; TFA, trans-fatty acid; WC, waist circumference.
* Except were specified, all studies were prospective cohort studies.
Table 6 Intervention trials of diet and heart failure (HF) risk and/outcome
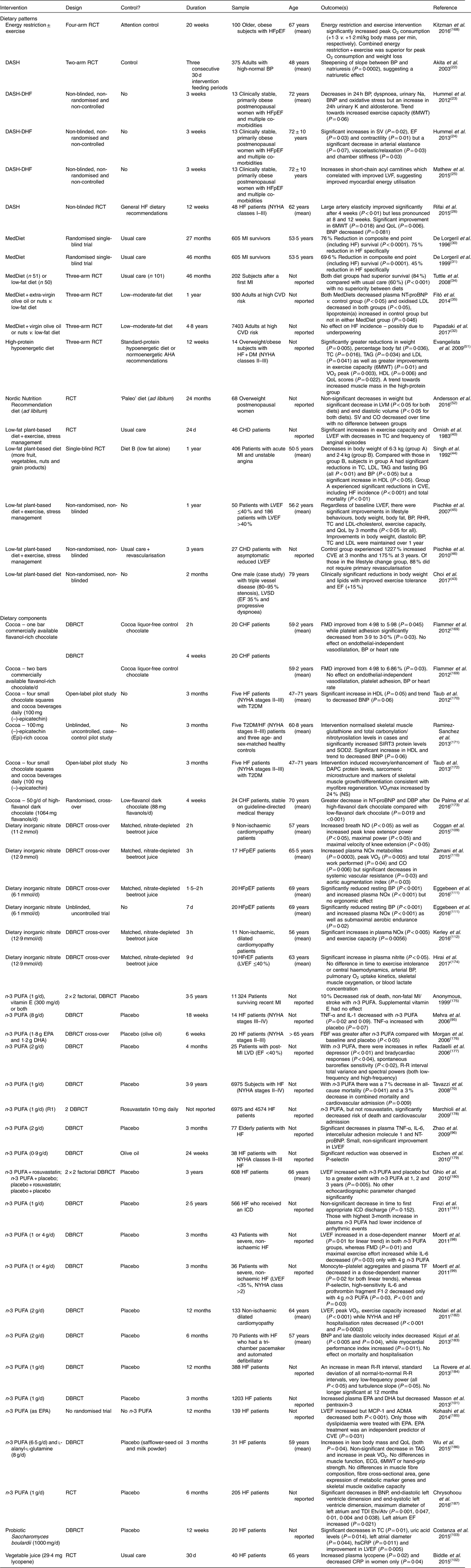
6MWT, 6-minute walk test; ADMA, asymmetric dimethylarginine; AHA, American Heart Association; Atv, peak late diastolic tissue velocity; BG, blood glucose; BNP, B-type natriuretic peptide; BP, blood pressure; CO, cardiac output; CHF, congestive HF; CRP, C-reactive protein; CVE, cardiovascular events; DAPC, dystrophin-associated protein complex; DASH, Dietary Approaches to Stop Hypertension; DASH-DHF, Dietary Approaches to Stop Hypertension in Diastolic Heart Failure (50 mmol or 1150 mg Na per 2100 kcal (8786 kJ)); DBRCT, double-blind, randomised controlled trial; DM, diabetes mellitus; ECG, electrocardiogram; EF, ejection fraction; Etv, peak early diastolic tissue velocity; FBF, forearm blood flow; FMD, flow-mediated dilation; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; hsCRP, high-sensitivity C-reactive protein; ICD, implantable cardioverter-defibrillator; LVD, left ventricular dysfunction; LVEF, left ventricular ejection fraction; LVF, left ventricular function; LVM, left ventricular mass; MCP-1, monocyte chemoattractant protein-1; MedDiet, Mediterranean diet; MI, myocardial infarction; NOx, NO metabolites; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; QoL, quality of life; R-R interval, cycle length variability; RCT, randomised controlled trial; RHR, resting heart rate; SIRT-3, sirtuin-3; SOD2, superoxide dismutase-2; SV, stroke volume; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TDI, tissue Doppler imaging.
Dietary Approaches to Stop Hypertension (DASH) diet
The DASH diet was designed to prevent and treat hypertension( Reference Appel, Moore and Obarzanek 3 , Reference Sacks, Obarzanek and Windhauser 20 ). Based on early studies of lower blood pressure in vegetarians, ‘the diet design goals were to create patterns that would have the blood pressure lowering benefits of a vegetarian diet, yet contain enough animal products to make them palatable to non-vegetarians’( Reference Sacks, Obarzanek and Windhauser 20 ). As such, it is a carbohydrate-rich and low-fat diet that emphasises consumption of fruits, vegetables, whole grains, nuts, fish, poultry and low-fat dairy products, and minimises consumption of red meat, sugar and processed foods.
Tables 3 and 4 detail studies of DASH and HF incidence, and Tables 3, 4 and 6 describe studies of DASH and HF severity. A 2013 systematic review and meta-analysis of observational prospective studies including >144 000 adults reported that a DASH-like diet was associated with significant reductions in CVD incidence, including CHD and stroke (19–21 %) but the greatest risk reduction was against HF (29 %)( Reference Salehi-Abargouei, Maghsoudi and Shirani 21 ). Although plausible and consistent, these studies are limited by their observational nature.
However, a preliminary intervention trial was published in 2003( Reference Akita, Sacks and Svetkey 22 ) with follow-up from the pilot Dietary Approaches to Stop Hypertension in Diastolic Heart Failure (DASH-DHF) study in 2012( Reference Hummel, Seymour and Brook 23 – Reference Mathew, Seymour and Byun 25 ) (Table 6). Importantly, all foods were prepared and served under observation by dietitians in a metabolic kitchen in DASH-DHF. This pilot study was conducted among a small group of primarily obese, postmenopausal, women. Further, there was a mean weight loss of 1·7 kg, which may explain most/some benefit. Nevertheless, improvements in cardiac function after 21 d infer a remarkable response. A subsequent randomised, non-blinded trial reported vascular, ergonomic and quality-of-life benefit without weight change( Reference Rifai, Pisano and Hayden 26 ).
A limitation of both trials is the lack of investigator blinding. Nevertheless, the DASH diet seems promising for preventing and treating HF. Based on consistent evidence of benefit in CVD, including HF, the DASH diet has been called ‘an optimal dietary plan for symptomatic HF’( Reference Rifai and Silver 27 ) and was formally adopted into the 2013 American College of Cardiology/American Heart Association CVD risk prevention guidelines (strong recommendation: 2013: level 1A)( Reference Eckel and Jakicic Ard 28 ).
Mediterranean diet
Dietary patterns in the Mediterranean region are often characterised by a high intake of vegetables, fruits, nuts, whole grains and extra-virgin olive oil (EVOO) with a moderate intake of fish and sometimes wine (with meals), and a low intake of dairy products, poultry, red/processed meats and free sugars( Reference Trichopoulou, Bamia and Trichopoulos 29 ).
One of the first reports of the Mediterranean diet (MedDiet) was published in 1996 from the Lyon Diet Heart Study. This was a randomised, single-blind trial to test the hypothesis that a MedDiet would reduce CVD complications in survivors of a first acute myocardial infarction. In the usual-care group, there were eight cases of non-fatal HF in 303 subjects (1·35 %), while there were two cases in 302 subjects following a MedDiet (0·33 %) after 27 months( Reference de Lorgeril, Salen and Martin 30 ). An extended follow-up of this study demonstrated that the MedDiet significantly reduced the risk of a composite endpoint that included HF by 67 % (P=0·0001)( Reference de Lorgeril, Salen and Martin 31 ). These preliminary observations were followed by several cross-sectional and prospective studies (Tables 2–6).
There is only a single randomised, controlled trial assessing primary prevention of HF with the MedDiet( Reference Papadaki, Martínez-González and Alonso-Gómez 32 ). This was a secondary analysis of the PREDIMED trial, which differs from the Lyon Heart study in that it compares a MedDiet supplemented with either EVOO or nuts with a low-fat diet. PREDIMED involved 7403 adults and 6·8 years of follow-up. Although there was no protective association of the MedDiet (with EVOO or nuts) on HF incidence, HF incidence was 22–32 % lower in point estimates throughout the trial for the MedDiet+EVOO intervention. Further exploratory analysis regarding a composite outcome of total CVD events (including HF) demonstrated 38 and 23 % reduced HF incidence associated with the MedDiet+EVOO and MedDiet+nuts interventions. The lack of protective effect may be partially explained by the low HF incidence, short follow-up and the moderate–high baseline adherence to the MedDiet( Reference Papadaki, Martínez-González and Alonso-Gómez 32 ). A 2016 systematic review and meta-analysis involving 10 950 participants comparing randomised controlled trials of Mediterranean with control diets suggested that the MedDiet was associated with a 70 % decreased HF incidence( Reference Liyanage, Ninomiya and Wang 33 ).
There are currently no intervention studies of the MedDiet in HF. However, a 2008 report based on a controlled, clinical trial of the MedDiet v. a low-fat diet v. usual care noted improved event-free survival with both the MedDiet (84 %) and low-fat diet (84 %) than usual-care controls (60 %) (log-rank P<0·001). It is noteworthy that both diets were low in saturated fat (≤7 % energy) and dietary cholesterol (≤200 mg/d)( Reference Tuttle, Shuler and Packard 34 ). Further, a 2014 biochemical analysis of the PREDIMED trial suggested that the MedDiet could decrease important HF biomarkers, even in the absence of weight loss( Reference Fitó, Estruch and Salas-Salvadó 35 ).
Dietary Approaches to Stop Hypertension (DASH) v. Mediterranean diet
Both patterns are largely plant-based, centred on fruits, vegetables and whole grains and low in red/processed meat and refined carbohydrates. A 2013 prospective study explored the effect of Mediterranean and DASH diet scores on mortality rates in women with pre-existing HF (n 3215; aged 50–79 years). The highest v. lowest diet score for the DASH diet was associated with 16 % significantly lower mortality (P trend=0·01) and a trend towards 15 % reduced mortality with the MedDiet (P trend=0·08)( Reference Levitan, Lewis and Tinker 36 ). A 16 % mortality reduction after HF hospitalisation eclipses any currently available or previously investigated therapy( Reference Van Horn and Yancy 7 ).
The Rice Diet
In 2014, two articles recounted Dr Walter Kempner and his ‘Rice Diet’( Reference Estes and Kerivan 37 , Reference Klemmer, Grim and Luft 38 ). The Rice Diet Program was founded in 1939 and the diet consisted of white rice, sugar, fruit, fruit juices, supplemental Fe and vitamins (including thiamine) and provided about 2000 kcal (8370 kJ), 20 g protein, about 2–3 % fat, 1000 ml liquid and 150–250 mg Na daily. Blood pressure and related symptoms reportedly began to decline rapidly. If results were good, after several months small amounts of lean meat and vegetables were added. A 1949 editorial stated ‘Kempner’s own therapeutic results are little short of miraculous…practically speaking, there is probably no more effective diet for obese decompensated cardiac patients’( Reference Loofbourow, Galbraith and Palmer 39 ).
A low-Na, low-fat, largely plant-based diet is the basis for multiple CVD dietary regimens. The Rice Diet has never been subjected to a randomised controlled trial but it is interesting to note that results of the Rice Diet seem to have first been published in 1946, predating other CVD dietary regimens by about 50 years.
Low-fat, plant-based diet
Low-fat diets have been and remain the cornerstone of cardiovascular dietary advice. A low-fat, plant-based diet, as part of an overall healthful lifestyle programme, remains the only dietary pattern to be objectively proven to reverse CHD( Reference Ornish, Scherwitz and Doody 40 – Reference Esselstyn 42 ). To date, a low-fat, plant-based diet has not been subjected to a trial specific to HF incidence or outcome. Nevertheless, the remarkable effect sizes reported in these trials is worthy of further investigation in CVD, including HF.
The PREDIMED trial used a low-fat diet in the control group. Both intervention diets (i.e. MedDiet+EVOO or nuts) displayed significant decreases in multiple non-HF, CVD endpoints. However, the authors acknowledge that ‘changes in total fat were small’ in the low-fat group. Because the participants in the ‘low-fat’ diet group did not follow a low-fat diet, this trial cannot conclude a superiority of the MedDiet over a low-fat diet.
A recent case report demonstrated the effects of a plant-based diet in a 79-year-old male with documented triple vessel disease (80–95 % stenosis) and left ventricular systolic dysfunction (ejection fraction=35 %) in the context of progressive dyspnoea. In the study, 2 months of a low-fat, plant-based diet led to clinically significant reductions in body weight and lipids with improved exercise tolerance and ejection fraction (+15 %)( Reference Choi, Allen and McDonnough 43 ).
Further, several relevant clinical trials are detailed in Table 6 ( Reference Singh, Rastogi and Verma 44 – Reference Chainani-Wu, Weidner and Purnell 47 ).
A 2005 review suggested that certain lifestyle measures, including plant-based diets (moderate to low in bioavailable phosphate), might modulate parathyroid hormone secretion and reduce left ventricular hypertrophy as well as HF risk( Reference McCarty 48 ). Similarly, a 2014 editorial regarding a prospective study of processed/unprocessed red meat consumption and HF risk( Reference Kaluza, Akesson and Wolk 49 ) suggested that plant-rich diets could lower HF incidence and severity( Reference Weiss 50 ). Taken together, these reports suggest that not only are comprehensive lifestyle changes potentially effective but are also achievable and sustainable, even for those with severe disease.
High-protein diets
Two small trials have demonstrated benefit of high-protein diets in HF, perhaps due to energy restriction( Reference Evangelista, Heber and Li 51 , Reference Andersson, Mellberg and Otten 52 ) (Table 6). Interestingly, patients in the high-protein group were encouraged to increase plant sources of protein as opposed to animal sources. This pilot study did not report actual dietary intake but it is likely that consumption of nuts, seeds, legumes and soya products increased( Reference Evangelista, Heber and Li 51 ).
Conversely, excess dietary protein may be harmful to HF. One pilot study reported a decrease in stroke volume and cardiac output with either high-protein Nordic recommendations or the Paleolithic diet(52). Further, protein-bound uraemic toxins (PBUT) are derived from the colonic microbiota metabolism of dietary amino acids. Evidence linking PBUT to adverse CVD outcomes has been accumulating. Recent reviews on PBUT suggest that a low-protein diet may reduce PBUT with beneficial effects on CVD( Reference Lekawanvijit and Krum 53 , Reference Lekawanvijit 54 ). However, there is a lack of interventional evidence. Nevertheless, some dietary patterns discussed above could be considered low–moderate protein. More research in this area is warranted regarding high-protein diets but caution is warranted regarding HF patients with advanced disease and co-morbidities (for example, renal disease).
Studies based on overall diet quality
One spurious cross-sectional study among subjects with advanced HF unexpectedly reported that dietary habits typically considered healthy (reduced intake of foods high in saturated fats and frequent consumption of fruits and vegetables) were related to impaired quality of life( Reference Bunyamin, Spaderna and Weidner 55 ). However, this study contrasts with other evidence (Tables 2–6) and as such may represent reverse causation whereby a HF diagnosis may lead to healthier dietary habits as opposed to improved diet causing severe HF.
There are several other spurious reports of dietary pattern and HF incidence/outcome. The Alternate Healthy Eating Index (AHEI), a nine-component index, was designed to assess food choices and macronutrient sources associated with reduced chronic disease risk( Reference McCullough, Feskanich and Stampfer 56 ). The nine components include vegetables, fruit, nuts and soya protein, cereal fibre, multivitamin use, low in trans-fat and alcohol, as well as high PUFA:SFA and white meat:red meat ratios. A healthy lifestyle, including a high AHEI score, was associated with a 77 % reduction in HF incidence( Reference Agha, Loucks and Tinker 13 ). Further, two prospective studies demonstrated that a higher AHEI score was associated with reduced HF incidence (Table 4). One of these studies was a prospective analysis of two combined trials of anti-hypertensive medication( Reference Dehghan, Mente and Teo 57 ). A protective effect of higher AHEI score was observed regardless of receipt of proven medications or presence of co-morbidities/risk factors, suggesting that diet can be protective in the absence of pharmacology but can also act synergistically( Reference Dehghan, Mente and Teo 57 ). An additive benefit of nutrition to pharmacology was reported in one the first reports of the MedDiet and CVD( Reference de Lorgeril, Salen and Martin 31 ).
Several reports have associated higher overall diet quality with decreased HF incidence, including the Dietary Modification Index, Diet Risk Score and Dietary Inflammatory Index.
The Diet Risk Score is based on food items that are considered predictive (meat, salty snacks and fried foods) or protective (fruits and green leafy vegetables, other cooked vegetables and other raw vegetables) of CVD/HF( Reference Dehghan, Mente and Teo 57 ). The Dietary Modification Index score is based on percentage of total energy intake from fat, vegetables and fruit servings, grain servings, percentage of energy intake from saturated fat, percentage of energy intake from trans-fat and dietary cholesterol intake( Reference Belin, Greenland and Allison 58 ). The Dietary Inflammatory Index was developed to characterise dietary intake from maximally anti- to pro-inflammatory( Reference Wirth, Shivappa and Hurley 59 ). Scores on these diet quality scales have been inversely associated with HF incidence (Tables 3–6).
Evidence linking dietary components with heart failure
As mentioned above, the effect of a single food or nutrient may be confounded by dietary habits and patterns( Reference Hu 19 ). However, studies of single foods/nutrients can provide additional information. Further, nutritional research has traditionally focused on single foods/nutrients. Evidence for specific foods and dietary components regarding HF incidence/severity is detailed in Tables 2–6.
Briefly, fruit, vegetable, whole grain and chocolate consumption was inversely associated with HF incidence/severity, while processed and/or red meat and dairy were positively associated. Although two early prospective studies reported a positive association between egg consumption and HF incidence( Reference Nettleton, Steffen and Loehr 60 , Reference Djoussé and Gaziano 61 ), a subsequent prospective study reported no association in women or diabetics and a positive association in men, but only with >6 eggs weekly( Reference Larsson, Åkesson and Wolk 62 ). Nevertheless, a 2017 meta-analysis reported an elevated risk of incident HF associated with frequent egg consumption( Reference Khawaja, Singh and Luni 63 ).
Interestingly, any protective effect of fish may be influenced by its preparation (fried v. non-fried) and type (i.e. oily v. non-oily)( Reference Mozaffarian, Gottdiener and Siscovick 64 ). Increased consumption of baked or broiled fish has been inversely associated with HF risk, whereas higher consumption of fried fish was associated with a higher risk of incident HF. Further, fried fish has been associated with reduced ejection fraction, lower cardiac output and higher systemic vascular resistance in older adults( Reference Mozaffarian, Gottdiener and Siscovick 64 ). There are three meta-analyses of fish intake and HF risk. Two meta-analysis reported an inverse association between HF risk and oily fish consumption( Reference Djoussé, Akinkuolie and Wu 65 , Reference Li, Zhou and Pei 66 ). However, a third meta-analysis reported no significant association, except for a positive association with fried fish( Reference Hou, Li and Zhou 67 ). The seemingly inconsistent observations may be partially related to toxins (for example, Hg). Alternatively, it is possible that dietary displacement may explain the inconsistencies. For example, if consumed in place of red/processed meat or eggs, fish may appear protective; however, if consumed in place of vegetables or whole grains, fish may not be protective. There are no randomised trials of fish consumption in HF.
One potentially important aspect of fish is n-3 fatty acids. Several small trials of n-3 supplementation in HF are detailed in Table 6. A recent meta-analysis of randomised controlled trials supported selected benefit of n-3 in HF( Reference Wang, Xiong and Huang 68 ). A 2017 science advisory from the American Heart Association regarding n-3, based mostly on secondary prevention trials in those at high risk of CVD, suggested that those with recent myocardial infarction or current HF may benefit from supplementation( Reference Siscovick, Barringer and Fretts 69 ). This HF recommendation is based on a single, large randomised, double-blind, placebo-controlled trial examining the effect of n-3 supplementation in patients with chronic HF( Reference Tavazzi, Maggioni and Marchioli 70 ). Interestingly, in an earlier trial, dietary intervention was superior to usual care but a low-fat diet was as effective as the MedDiet, despite containing less n-3( Reference Tuttle, Shuler and Packard 34 ), suggesting that n-3 intake may not be as beneficial in the context of a low-fat, plant-based diet.
Several findings from large, observational studies of nutrition and HF deserve discussion. In one prospective one study, processed red meat was associated with HF incidence and mortality, while unprocessed red meat was not( Reference Kaluza, Akesson and Wolk 49 ). This null finding regarding unprocessed red meat should be interpreted with caution because the difference between the groups with the highest and lowest consumption was <1 serving/d. An accompanying editorial suggested ‘one possibility is that high intakes of red and other meat might displace micronutrient-rich plant foods from the diet and thus lead to micronutrient deficiencies that promote HF’ and concluded by stating ‘there is an urgent need to educate patients and the general public about the adverse health effects of red and processed red meat consumption’( Reference Weiss 50 ).
A recent dose–response meta-analysis of >1 million participants reported no association between increasing dietary Mg intake and total CVD risk. However, there was a significant 31 % reduction in HF risk( Reference Fang, Wang and Han 71 ). Rich dietary sources of Mg include vegetables, legumes and nuts.
Evidence from specific studies of dietary patterns linking dietary components with heart failure
Several studies of dietary patterns discussed above also assessed the effects of individual foods and/or food components.
Multivariate analysis of the Women’s Health Initiative Observational Study suggested a protective association of a diet low in cholesterol (P=0·001) and high in fibre (P=0·026) regarding HF( Reference Belin, Greenland and Allison 58 ).
A prospective analysis of the AHEI and HF incidence from two combined pharmacology trials was introduced above( Reference Dehghan, Mente and Teo 57 ). The authors further analysed each component of the AHEI, noting that all types of vegetables, green leafy vegetables, other raw vegetables, fruit, soya protein and nuts were inversely associated with HF incidence, while meat, poultry and eggs were positively associated with increased risk. There was no association with fish( Reference Dehghan, Mente and Teo 57 ).
A 2013 analysis of two prospective cohort studies demonstrated a positive association between plasma long-chain MUFA and HF incidence. Multiple foods related to plasma long-chain MUFA include fish, salad oils, poultry, processed meats, mustard seeds/oil and mixed meals (for example, pizza, meat sandwiches)( Reference Imamura, Lemaitre and King 72 ).
In one prospective study, a greater MedDiet score was associated with decreased risk of CVD incidence/recurrence. Of the overall dietary pattern, only consumption of vegetables, salads and nuts was associated with lower risk of recurrent cardiac events (including HF)( Reference Chrysohoou, Panagiotakos and Aggelopoulos 73 ). A cross-sectional study by the same group reported that fish, olive oil, pasta and moderate alcohol were associated with improved echocardiography parameters( Reference Chrysohoou, Kastorini and Panagiotakos 74 ). In contrast, the MedDiet was not associated with HF risk in multivariate analysis in a separate prospective study( Reference Wirth, di Giuseppe and Boeing 75 ). However, when dairy products were excluded, a significant protective association was observed. Further, only moderate alcohol (moderate v. low/high intakes) and fish were significantly associated with decreased HF incidence, while meat was the only factor significantly positively associated with HF incidence( Reference Wirth, di Giuseppe and Boeing 75 ).
A prospective study of Mediterranean and DASH diet scores on mortality in women with HF was discussed above. Interestingly, the specific diet score components inversely associated with HF mortality were vegetables, nuts and whole grains( Reference Levitan, Lewis and Tinker 36 ). This is consistent with a 2009 prospective study in which moderate consumption of alcohol, low consumption of meat/meat products, and high consumption of vegetables, fruits, nuts, olive oil and legumes were the MedDiet components inversely associated with all-cause mortality( Reference Trichopoulou, Bamia and Trichopoulos 29 ).
Biomarker studies
Nutritional studies generally suffer from a lack of objective measure of dietary intake. Several nutrients can be measured in serum or plasma. Multiple studies have assessed blood nutrient levels and HF incidence/severity (Table 4). The Lyon Diet Heart study was briefly introduced above (MedDiet section). This secondary prevention trial noted striking decreases in CVD, including HF, in conjunction with increased plasma vitamins C and E as well as increased plasma n-3 and decreased n-6 fatty acids (linoleic and arachidonic acids)( Reference de Lorgeril, Salen and Martin 30 ). Multiple additional studies demonstrate an inverse association between blood antioxidant level (β-carotene, lycopene, vitamin C) and HF incidence/severity, although this is not fully consistent( Reference Karppi, Kurl and Mäkikallio 76 ). Further, there is evidence that plasma micronutrient level is lower in more severe HF but also that ejection fraction correlates with multiple micronutrients. Similarly, serum/plasma trans-fatty acids and SFA were positively associated with HF incidence/severity, while long-chain PUFA were negatively associated.
Most of these studies relied on a single measurement; however, levels can change over time. Nevertheless, several studies reported modest correlations (r 0·2–0·6) between plasma fatty acids measured in samples from studies of HF incidence collected 6–15 years apart( Reference Mozaffarian, Lemaitre and King 77 – Reference Djoussé, Petrone and Weir 79 ), suggesting that measurement of fatty acids at a single time point may be sufficient. In fact, the proportion of long-chain n-3 fatty acids in erythrocytes has been proposed as a HF risk factor( Reference von Schacky 80 , Reference Rupp, Rupp and Alter 81 ).
Although it is attractive to assume that serum and plasma reflect dietary intake alone, additional factors may be present in HF such as increased requirements, impaired intestinal absorption, alteration in renal reabsorption, impaired cellular regulation and increased losses secondary to increased oxidant stress and/or medications, for example, diuretics( Reference Michowitz, Dishy and Zaidenstein 82 ). One study demonstrated decreased plasma vitamin C in HF patients compared with controls despite similar intake( Reference de Lorgeril, Salen and Accominotti 83 ). Regardless of the cause(s) of altered blood nutrients in HF, it is likely that dietary modification regarding these nutrients will prove beneficial to preventing/treating HF.
Potential mechanisms
Although existing research is limited, dietary patterns associated with decreased HF incidence/severity have several common features, i.e. high in micronutrients, antioxidants, nitrate and fibre but low in animal protein/fat as well as saturated/trans-fatty acids, Na and bioavailable phosphate( Reference Kaluza, Akesson and Wolk 49 , Reference Weiss 50 , Reference Nettleton, Steffen and Loehr 60 , Reference Wirth, di Giuseppe and Boeing 75 , Reference Rautiainen, Levitan and Mittleman 84 – Reference Kaluza, Åkesson and Wolk 87 ). It is likely that these features contribute to decreased oxidative stress( Reference Serdar, Yesilbursa and Serdar 88 – Reference Pastori, Carnevale and Bartimoccia 91 ) and inflammation( Reference Chainani-Wu, Weidner and Purnell 47 , Reference Mozaffarian, Rimm and King 92 – Reference Wannamethee, Bruckdorfer and Shaper 104 ) but higher antioxidant defence( Reference de Lorgeril, Salen and Accominotti 83 , Reference Serdar, Yesilbursa and Serdar 88 – Reference Pastori, Carnevale and Bartimoccia 91 , Reference Wannamethee, Bruckdorfer and Shaper 104 – Reference Song, Moser and Kang 108 ) and NO bioavailability( Reference Coggan, Leibowitz and Spearie 109 – Reference Kerley, O’Neill and Reddy Bijjam 112 ) (see Fig. 1).
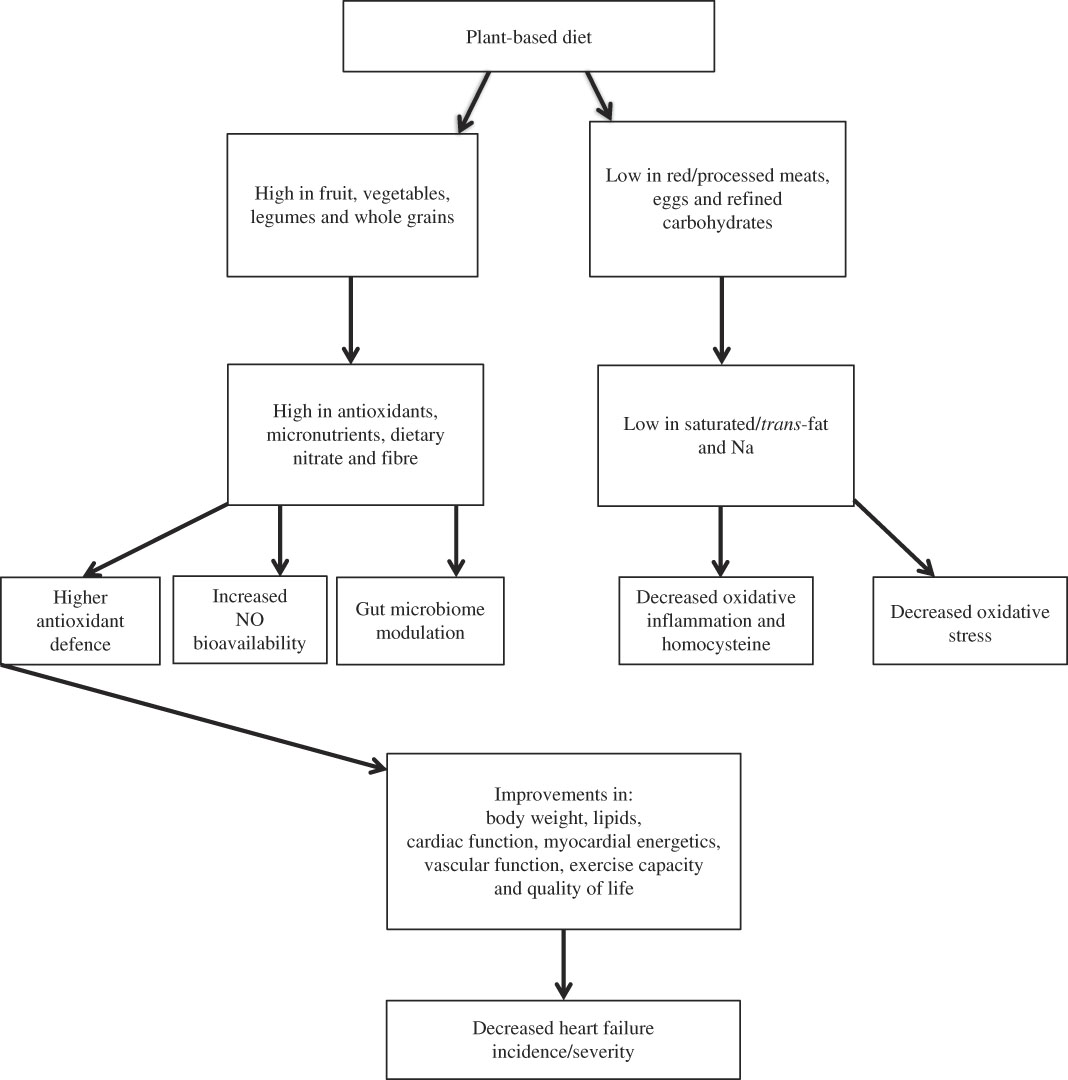
Fig. 1 Cardioprotective effects of a plant-based diet.
Another potential mechanism is dietary modulation of the gut microbiome. A series of well-conducted human studies demonstrated that the intestinal microbiota metabolise choline/phosphatidylcholine and l-carnitine to produce trimethylamine, which is oxidised to proatherogenic trimethylamine N-oxide (TMAO)( Reference Koeth, Wang and Levison 113 , Reference Tang, Wang and Levison 114 ). TMAO levels are elevated in HF compared with controls( Reference Tang, Wang and Fan 115 ). Further, TMAO levels have been correlated with brain-type natriuretic peptide( Reference Trøseid, Ueland and Hov 116 ) and associated with HF severity( Reference Tang, Wang and Fan 115 – Reference Suzuki, Heaney and Bhandari 118 ) and HF mortality( Reference Tang, Wang and Fan 115 , Reference Trøseid, Ueland and Hov 116 , Reference Suzuki, Heaney and Bhandari 118 ). Foods rich in l-carnitine (for example, red meat) and choline/phosphatidylcholine (for example, eggs) have been linked with HF incidence/severity (Tables 2–6). TMAO production may also explain the inconsistent effects observed with fish, dairy products and poultry (all rich sources of choline). Interestingly, those eating primarily plant-based diets, with limited choline/phosphatidylcholine and l-carnitine ingestion, do not seem to produce significant quantities of TMAO, even after ingestion of l-carnitine/choline( Reference Koeth, Wang and Levison 113 ).
Limitations
The focus here was on associations between dietary pattern, dietary components as well as nutrients and HF risk/severity. Therefore, evidence focused on salt/Na or fluid restriction, micronutrient supplementation, alcohol, over-/undernutrition as well as animal/cell model data was omitted.
The literature searches and the data presented here focus on HF. However, HF is related to multiple cardiometabolic risk factors and disorders. It is possible that factors influencing these processes may also influence HF incidence/severity. However, a comprehensive review of nutritional factors contributing to HF and non-HF cardiometabolic perturbations is outside the scope of this review. Further, there are many HF manifestations, for example, preserved v. reduced ejection fraction. It is possible that different HF manifestations may require different dietary approaches. However, data specific to specific HF manifestations are limited.
Many studies reviewed herein included only one measurement of dietary intake or blood nutrient level. These studies cannot determine whether participants changed their diet during the follow-up. Additionally, many of these studies assessed dietary intake via self-report (for example, FFQ). On the other hand, some studies directly measured blood nutrient level or microbiome metabolites but did not assess dietary intake. Therefore, the possibility of reverse correlation cannot be ruled out.
It is important to recognise that grouping categories of similar foodstuffs together can influence observations. For example, two prospective studies reported no association between consumption of nuts and HF incidence( Reference Nettleton, Steffen and Loehr 60 , Reference Djoussé, Rudich and Gaziano 119 ). However, neither of these studies collected information on nut type. However, roasted and salted groundnuts may have a less favourable effect than raw, unsalted walnuts. Similarly, another prospective study reported that sweetened beverages were associated with HF risk( Reference Rahman, Wolk and Larsson 120 ). However, this study did not differentiate between sugar-sweetened beverages and artificially-sweetened beverages. Finally, type and preparation of fish appear important( Reference Mozaffarian, Gottdiener and Siscovick 64 ).
Most available data are observational. Although retrospective, cross-sectional and prospective studies each have their strengths, flaws remain. Although the reported observations could be real, residual confounding by measured and unmeasured factors is a major concern, despite adjusted analysis. Caution with over-adjusting for confounding factors in dietary studies is recommended. For example: if red meat is associated with HF and diabetes and the results adjust for diabetes, then the association between HF and red meat diminishes. However, it is possible that red meat contributes to HF through increased diabetes risk.
Many reports included here were re-analysis involving the same cohorts. Although many of these studies were large and prospective, they often enrolled limited cohorts, for example, the Physicians’ Health Study enrolled male physicians who were mostly Caucasian.
In the context of other CVD and HF precursors (for example, hypertension, obesity, diabetes) there is a notable lack of interventional trials regarding HF. Further, many existing intervention studies were pilot studies with small samples and short follow-up. Nevertheless, existing interventional trials of plant-based diets in HF have reported improvements in cardiac function, functional capacity and quality of life (Table 6), inferring a remarkable response. Based on consistent evidence of CVD benefit, the DASH diet was formally adopted into American College of Cardiology/American Heart Association CVD guidelines( Reference Eckel and Jakicic Ard 28 ).
Future recommendations
The potential role of nutrition in HF prevention/treatment was first suggested in 1996( Reference de Lorgeril, Salen and Martin 30 ). The field has grown since then but remains limited, with many unanswered questions. It is recommended that future studies take account of type of HF and severity as well as pharmacological treatments and co-morbidities. Further, it is recommended that adequately powered sample sizes and relevant follow-up periods are utilised in investigator-blinded, randomised and controlled trials.
Conclusion
There is growing evidence that nutrition is a critical factor in the incidence and progression of HF. The existing but limited observational and interventional evidence from human studies suggests that a plant-based diet rich in fruit, vegetables, legumes and whole grains is likely to be beneficial, acting through multiple pathways.
Considering the relative safety and cost of dietary intervention combined with the limited knowledge on HF and diet, clinical trials are urgently needed to help elucidate the effect of dietary patterns/components on HF incidence/severity. This has been highlighted in the strategic plan of a joint National Institutes of Health and National Heart, Lung, and Blood Institute working group.
A seminal 1999 editorial( Reference Leaf 121 ) regarding the famous Lyon Diet Heart Study stated ‘relatively simple dietary changes achieved greater reductions in risk of all-cause and coronary heart disease mortality in a secondary prevention trial than any of the cholesterol-lowering studies to date’. This editorial details the cost-effectiveness and high benefit:risk ratio of dietary manipulation compared with ‘drugs and invasive procedures’ and concludes that ‘dietary factors must be very important’. Diet does seem important and I quote a more recent, expert editorial: ‘in our search for the silver bullet, we have overlooked the silver plate. It is regrettable that we remain so imprecise and ill-informed about a cornerstone in patient care. Diet is important. We can and should know more’( Reference Van Horn and Yancy 7 ). Physicians, dietitians, scientists, funding agencies and others are urged to help conduct further research in this crucial area.
Acknowledgements
C. P. K. made substantial contributions to review design and manuscript collection and interpretation of data; drafted the submitted article; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
There are no conflicts of interest.











