Taxonomic descriptions inevitably reflect the shapes of organisms in reduced form since the dimensions of all possible diagnostic characteristics are routinely considered at the planar level. By contrast the three-dimensional conformations of structures are difficult to define and remain unspecified. At present, it is difficult to assess the importance of volumetric characteristics for diagnostic taxonomy; however, some biological detail might be obtained from spatial data. When the pycnidia of lichenicolous fungi are examined under a microscope, as in the case of Lichenoconium pyxidatae (Oudem.) Petr. & Syd. (Fig. 1A), it is evident that the production of conidia by a single pycnidium is prolific, although hard to quantify. The question as to how many conidia are contained inside one pycnidium may arise. This study reveals previously unknown information about the structure and function of lichenicolous fungi. In particular, it gives an insight into the scale of reproductive effort represented by a single pycnidium, the quantity of conidia produced by conidiogenous cells and the general volumetric proportions of the wall with conidiogenous cells, internally generated conidia and empty space between them.

Fig. 1 A, pycnidium of Lichenoconium pyxidatae in the process of mass conidium ejection (conidia spilled on the surface of lichen are also visible); B, partly immersed pycnidia of L. pyxidatae on an apothecium of Cladonia cariosa. Scales: A=30 µm; B=0·5 mm. In colour online.
Lichenoconium Petr. & Syd. 1927 (Petrak & Sydow Reference Petrak and Sydow1927) is one of the most common genera of lichenicolous fungi, comprising 14 species that live exclusively on lichens. These fungi are generally characterized by an immersed or sessile dark brown pycnidial opening, usually with an irregular ostiole, simple conidia that are globose to ellipsoid and often truncate at the base, and elongate conidiogenous cells that are hardly or only slightly pigmented (Hawksworth Reference Hawksworth1977, Reference Hawksworth1981; Cole & Hawksworth Reference Cole and Hawksworth2004). The reproductive mechanism of Lichenoconium is simple and consists of the uncontrolled production and passive release of conidia. Dispersal of propagules depends on both abiotic and biological vectors. This simple reproductive strategy seems to be perfectly sufficient, at least for survival, considering the large number of common species with worldwide distributions (see also Lawrey & Diederich Reference Lawrey and Diederich2003). Host specificity is rather weak in most species of Lichenoconium; some of them colonize weakened or dead lichens. The taxonomy of the genus is artificial, relying only on the characteristics of conidiogenous cells and conidia (Cole & Hawksworth Reference Cole and Hawksworth2004). Nevertheless, a recent phylogenetic study supports the monophyly of the genus (Lawrey et al. Reference Lawrey, Diederich, Nelsen, Sikaroodi, Gillevet, Brand and van den Boom2011). Lichenoconium pyxidatae is confined to various species of Cladonia, infecting their podetia in particular (Zhurbenko & Pino-Bodas Reference Zhurbenko and Pino-Bodas2017). Its occurrence in Poland is rather well documented with several localities being reported and with various Cladonia species as hosts (e.g. Czyżewska et al. Reference Czyżewska, Hachułka, Łubek and Zaniewski2008; Kukwa & Flakus Reference Kukwa and Flakus2009).
This investigation was designed to define the spatial characteristics of the average pycnidium of lichenicolous fungi and its capacity to produce conidia. To explore these issues it was necessary to create a physical three-dimensional model of a standard pycnidium. Lichenoconium pyxidatae served as a biological reference for the model.
The study material was collected from a site representing a homogenous patch of the cryptogamic association Cladonietum rei (see Paus Reference Paus1997; Rola et al. Reference Rola, Osyczka and Nobis2014) located in the vicinity of the town of Piekary Śląskie (southern Poland). Despite unrestricted host availability, fungal infections within the patch were not numerous, involving 12 individuals of Cladonia pyxidata (L.) Hoffm., eight of C. cariosa (Ach.) Spreng. (chemotype I, atranorin and rangiformic acid as major substances), seven of C. rei Schaer. (chemotype I, fumarprotocetraic and homosekikaic acids as major substances) and one of C. fimbriata (L.) Fr. Podetia gathered from a 0·02 m2 area of the patch were examined. Infections related to apothecia only for the second lichen species (Fig. 1B) and to podetia in the case of the others. The selected samples of Cladonia infected by L. pyxidatae were sectioned with a rotary microtome (Microm, Adamas Instrumenten). Every effort was made to obtain undamaged, undeformed cross-sections from the base to the top of the pycnidia. Clipped or shapeless snippets were not used for further measurements. Moreover, only pycnidia filled to the brim were used, to ensure that the structure was preserved in its entirety. Pycnidia were divided into five roughly equal segments. The following variables were considered: total height (H) and width (W; measured at the top of 5 segments, S1-S5), thickness of darkly pigmented outer and hyaline inner layers of the wall with conidiogenous cells (WT; six measurements on the circumference between the segments and at the bottom), and width of the opening (O). The variables H, W and WT were determined for 30 mature pycnidia and photographed using light microscopy. Since the top parts of the pycnidia were usually densely covered with masses of conidia and the openings were not easily visible, the width of the opening was measured for eight pycnidia captured from the top by means of SEM (Fig. 2B) after being sputter-coated with a thin layer of gold (Hitachi S-4700 and Noran Vantage). The schema of segmentation and method of measurement are outlined in Fig. 2A. Finally, 10 conidia from each of 30 pycnidia were measured. The measurements were performed using Motic Images Plus 2.0 ML software after appropriate scaling. The results were as follows ((min) mean±SD, (max) (µm)): H, (81·0–)87·5±5·9(–105·5); W (thickest part), (55·3–)61·4±4·8(–72·9); WT (based on all measurements), (7·8–)12·1±2·5(–15·6); O, (11·1–)16·9±3·3(–21·1); conidium diameter (shape reduced to a sphere), (2·1–)2·6±0·4(–3·4).
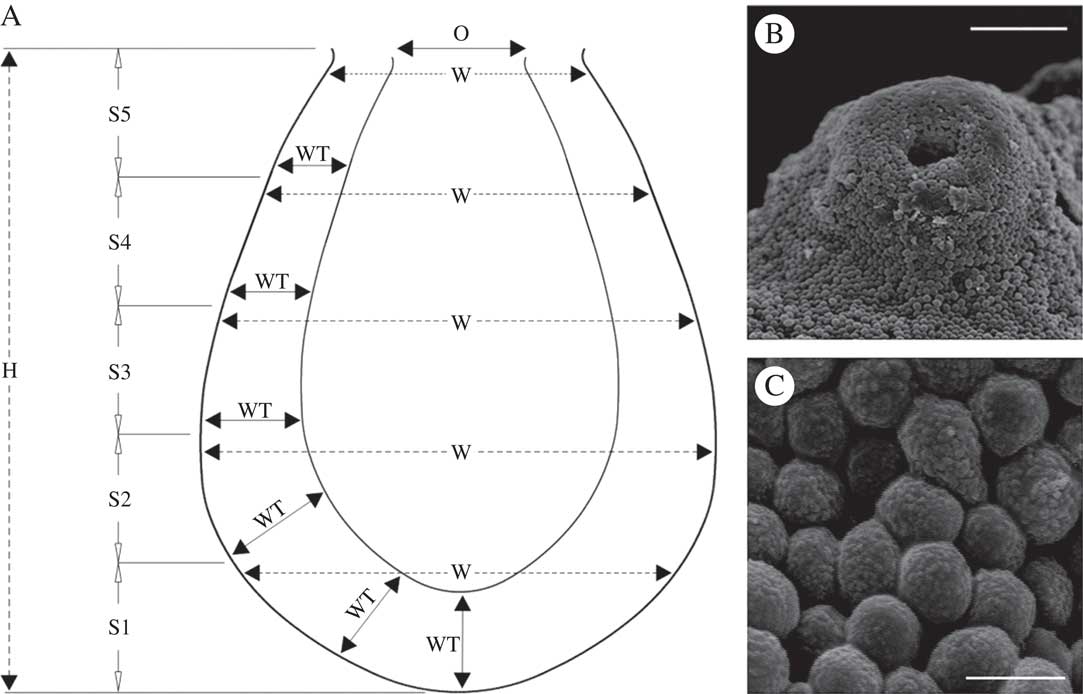
Fig. 2 A, schematic showing the measurement method for pycnidium parameters used in the design of the three-dimensional model; B, masses of conidia entirely covering the ostiole of the pycnidium of Lichenoconium pyxidatae (SEM); C, densely packed conidia, deformed into polygons, inside the pycnidium of L. pyxidatae (SEM). H=height, W=width, WT=wall thickness, O=ostiole width, S1-S5=delimited segments. Scales: B=30 µm; C=3 µm.
Subsequently, one shaped pycnidium was selected as the template for drawing tentative contours. For this purpose, its microscopic image was transformed into a 1-bit black and white form which was used to sketch the outline of the model. The mean size of conidium and uniform glass pellets, 3 mm in diameter, were the basis for defining the scale which was finally calculated at 1154:1. The scaled mean values of H, W, WT and O were applied as appropriate dimensions for the flat model. For example, the H mean value of real pycnidia was 87·5 µm; therefore a height of 100·1 mm for the model was assigned (0·0875×1154=100·975). All parameters used for scaling and modelling coincided with the values reported for L. pyxidatae in the literature (e.g. Hawksworth Reference Hawksworth1977, Reference Hawksworth1981; Cole & Hawksworth Reference Cole and Hawksworth2004; Randlane & Saag Reference Randlane and Saag2004). However, it should be stated that the final 2D drawing of the model is simplified to reflect the regular form based on mean values resulting from the measurements rather than a schematic drawing of a pycnidium cross-section as usually viewed under a microscope. Once the dimensions were resolved, the technical drawing was projected into the 3D model space using 3D CAD tools provided by Geomagic Design software (Fig. 3A). The physical model was made using rapid prototyping by means of fused deposition modelling technology (FDM) with ABSplus material, using a Stratasys 3D printer (Fig. 3B). In addition to the whole model, a half-model was also printed for illustrative purposes (Fig. 3C & D). The volumetric parameters of the model were computed using Geomagic Design X software; the values were subsequently transposed into the true size of the pycnidium using appropriate mathematical (third power) transformations. Conidium content was determined by filling the entire printed model with glass pellets (Fig. 3B) and counting them after removal. Since these pellets could be arranged in the interior in slightly different ways, the procedure was repeated 10 times. The number of pellets was determined by measuring the mass of the total filled load and then using the mass of 200 pellets to estimate the quantity of pellets within the model pycnidium.

Fig. 3 3D model and cross-section of pycnidium of Lichenoconium pyxidatae. A, image of pycnidium model generated by Geomagic Design software; B, solid printed model (scale=1154:1) filled with glass pellets (3 mm diameter); C, lower part of the pycnidium (the wall with conidiogenous cells and the cavity entirely filled with conidia); D, corresponding part (to C) of the solid model filled with glass pellets. Scales: A & B=25 mm; C=20 µm; D=20 mm. In colour online.
The procedure described above enabled the volumetric parameters of a model pycnidium to be defined and the pellets/conidia to be counted and related to an actual pycnidium. For clarity, most of the values are rounded to the nearest full integer: the total volume of the model computed by software is 263098 mm3, of which 188 664 mm3 consists of printed material and 74 434 mm3 represents the hollow interior. Based on these values, the following specifications for the pycnidium were calculated: the total volume is 171944 µm3, of which 123299 µm3 accounts for the wall with conidiogenous cells, while the internal space destined for conidia deposition measures 48645 µm3 (=0·000048645 mm3, or 153015795 times less than the hollow interior of the model). The volume of one glass pellet is 14·14 mm3; the calculations yielded a rounded result of 5264 portions of this volume to fit inside the model. The actual number of pellets that can be poured into the model is much lower; over ten attempts, the amount ranged from 3194 to 3252, with a mean (±SD) of 3225 (±23). The results indicate that 39% of the interior of the model filled with pellets represents the empty space between them. The volume of one average conidium is 9·2 µm3; thus the conidia themselves occupy 29674 µm3 inside the cavity. Calculations show that the conidia constitute up to one-fifth (19%) of the total fungal volume of the pycnidium. This estimate is accurate if it is assumed that the maximum number of conidia within one pycnidium is the same as the number of pellets within the printed 3D model. Certain simplifications related to the use of the model should be taken into account when considering the above specifications. Conidial form was reduced to a spherical pellet instead of the elliptical (and truncated) solid of an actual conidium. The number of internally packed conidia might be slightly higher than the number of pellets as the pressure inside the pycnidium deforms the conidia which consequently adhere to each other to a great extent (Fig. 2C). Finally, variable thickness of walls and accompanying conidiogenous tissue could cause conidium production to vary enormously even in species with similar-sized pycnidia.
Generating a perfect model that perfectly reflects the natural structure is an almost impossible task. However, due to its structural simplicity, the pycnidium of L. pyxidatae was the most suitable natural design on which to base an attempt to create a universal model. Since we routinely work with flat cross-sectional images, the spatial aspect of the pycnidium structure might escape our attention or our perception of it might be misleading. Looking at cross-sections of a mature Lichenoconium pycnidium (e.g. Cole & Hawksworth Reference Cole and Hawksworth2004; Lawrey et al. Reference Lawrey, Diederich, Nelsen, Sikaroodi, Gillevet, Brand and van den Boom2011; Diederich et al. Reference Diederich, Ertz, Stapper, Sérusiaux, Van den Broeck, van den Boom and Ries2017; Fig. 1A), it would seem that a substantial part of it consists of an internal cavity filled with conidia. In fact, the interior volume constitutes less than one-third of the entire volume and c. 3200 conidia are needed to completely fill the pycnidium. To conclude, the function, in this case conidium production, cannot easily be inferred from the simple structural characteristics that usually accompany taxonomic descriptions.
I would like to thank my colleagues Professor Martin Kukwa (Gdańsk) for taxonomic verification of lichenicolous material and Dr Adam Flakus (Kraków) for valuable comments and general discussion on this group of fungi, and for examining some Cladonia specimens for other fungal infections.







