Introduction
Invasive plant species are considered a major threat to biodiversity conservation and ecosystem stability. Their negative impact is usually due to displacement of native species as well as changes in the dynamics and availability of resources such as nutrients, water, and organic matter (Ehrenfeld Reference Ehrenfeld2010; Senator and Rozenberg Reference Senator and Rozenberg2017). For this reason, abundant research has been conducted on the prediction of invasiveness as well as how to detect and control invasive species (Goodwin et al. Reference Goodwin, McAllister and Fahrig1999; Mack et al. Reference Mack, Simberloff, Lonsdale, Evans, Clout and Bazzaz2000; Moracová et al. Reference Moracová, Pyšek, Jarošik and Pergl2015; Pheloung et al. Reference Pheloung, Williams and Halloy1999). Today, although there is still debate about how to manage invasive species, it is widely accepted that the preferred management strategies are prevention and, if introduction occurs, early detection and rapid response to prevent dissemination and eradicate the invasive species when possible (Anderson Reference Anderson2005; Lodge et al. Reference Lodge, Williams, MacIsaac, Hayes, Leung, Reichard, Mack, Moyle, Smith, Andow, Carlton and McMichael2006; Reaser et al. Reference Reaser, Burgiel, Kirkey, Brantley, Veatch and Burgos-Rodriguez2020). These approaches are more affordable and more effective than strategies intended to control widespread established populations (Lodge et al. Reference Lodge, Williams, MacIsaac, Hayes, Leung, Reichard, Mack, Moyle, Smith, Andow, Carlton and McMichael2006; Myers et al. Reference Myers, Simberloff, Kuris and Carey2000).
An additional obstacle for the management of invasive plant species is the type of control actions that can be implemented depending on the ecological, economic, and social contexts in which the invasion occurs (Gardener et al. Reference Gardener, Atkinson and Renteria2009). For example, reductions in populations of invasive species in areas in which aggressive control actions, such as the use of herbicides, cultivation and tillage, or prescribed fire, occur more rapidly compared with reductions in invasive species populations in areas in which there might be restrictions on the type of control tools that can be used (Bennion et al. Reference Bennion, Ferguson, New and Schultz2020; Rinella et al. Reference Rinella, Maxwell, Fay, Weaver and Sheley2009). Thus, in protected areas, where it is important to ensure that native species and ecological dynamics are maintained, managers might prefer to use low-impact control tools that minimize negative effects on non-target species (Bennion et al. Reference Bennion, Ferguson, New and Schultz2020; Blossey Reference Blossey1999; Genovesi and Monaco Reference Genovesi, Monaco, Foxcroft, Pyšek, Richardson and Genovesi2013; Rinella et al. Reference Rinella, Maxwell, Fay, Weaver and Sheley2009). Long-term experiments represent a similar scenario in which aggressive control actions could potentially modify the historical trend of the multiple factors being studied (Genovesi and Monaco Reference Genovesi, Monaco, Foxcroft, Pyšek, Richardson and Genovesi2013). The reality is that managers must face a challenging trade-off between ensuring control or eradication of the invasive plant species and protecting the ecosystem dynamics in the area. For the former, aggressive control might be needed, while for the latter, low-impact actions might be preferable (Rinella et al. Reference Rinella, Maxwell, Fay, Weaver and Sheley2009). Furthermore, finding an adequate solution to this conundrum gets even more complicated, because managers must consider not only the ecosystem dynamics but also the opinions, interests, and involvement of multiple stakeholders (Blossey Reference Blossey1999) such as policy makers, regulators (i.e., local, state, or federal agencies), researchers, and participants in the local education and economic activities that benefit from the protected/experimental area (Gardener et al. Reference Gardener, Atkinson and Renteria2009; Maguire and Lind Reference Maguire and Lind2003).
The use of multistakeholder governance has been proposed as an efficient decision-making approach to find solutions that maximize the benefits of a management strategy for which diverse stakeholders have different interests and preferences (De Lange et al. Reference De Lange, Stafford, Forsyth and Le Maitre2012). When using this approach, managers might have to assign less weight to ecological science when making decisions, but they might be able to gain more support from stakeholders to ensure proper long-lasting funding to address the invasion using tactics such as monitoring at different spatial scales (Epanchin-Niell et al. Reference Epanchin-Niell, Hufford, Aslan, Sexton, Port and Waring2010). Additionally, there is shared responsibility for the outcome of the management strategy, so the manager does not have to bear all of the burden if the program fails, and conversely, a shared success could bring more interest in the operation of the protected area/experiment (Lévesque et al. Reference Lévesque, Dupras and Bissonette2020).
A major challenge for the use of multistakeholder governance in invasive plant species management is the difficulty in determining how ecological data can be incorporated as a core component of the decision-making process to bring balance to the uneven power roles among participants (Warner and Kinslow Reference Warner and Kinslow2011). In other words, because not all stakeholders will have the same level of influence in determining the final management strategy, it is important that the strategy is based on data/evidence that all parties consider credible and applicable to the case (De Lange et al. Reference De Lange, Stafford, Forsyth and Le Maitre2012; Maguire and Lind Reference Maguire and Lind2003). Thus, regulators will likely have more power than managers, and managers more than neighbors, but if the decisions are made in a manner that most participants consider to be backed by scientific evidence or correspond with the parameters defined as acceptable at the beginning of the negotiation, the group will support the final strategy. Conversely, if stakeholders with more power take decisions against the consensus, the process will generate distrust and future resistance (Maguire and Lind Reference Maguire and Lind2003). One might ask why a stakeholder and/or policy maker would act against the consensus of the group and/or ecological evidence? This is a very important question that is usually ignored in the invasive plant species management literature. More research focuses on the technical aspects of detection and control, while the social aspects of how invasive plant management decisions are made remain elusive. Not considering how we, as managers and decision makers, use information other than the ecological data in hand to design management plans can limit the efficacy and success of those plans.
An example of multistakeholder governance is described using tropical spiderwort (Commelina benghalensis L.), which was added to the federal noxious weed species list because of its rapid expansion and invasion of agroecosystems from tropical to subtropical and transition zones in the 1990s and early 2000s. The major contributing factor to this invasive behavior was the introduction and widespread adoption of glyphosate-resistant crops and concomitant increase in glyphosate use (Webster et al. Reference Webster, Burton, Culpepper, Flanders, Grey and York2006). Commelina benghalensis is naturally tolerant to glyphosate, so when many agricultural systems moved to a glyphosate-only weed control system, the survival and reproduction of this species increased dramatically (Culpepper et al. Reference Culpepper, Flanders, York and Webster2004; Webster et al. Reference Webster, Burton, Culpepper, Flanders, Grey and York2006). Commelina benghalensis was first detected in North Carolina in 2001 (Krings et al. Reference Krings, Burton and York2002). This detection prompted a rapid response from North Carolina State University (NCSU) researchers and administrators and local and state authorities to contain and eradicate this invasive species before it could invade nearby agricultural fields. Here, we describe is a real-life case about how a multistakeholder governance approach was used to design, implement, and conclude an eradication program for the noxious weed C. benghalensis in North Carolina, USA.
Site and Local Context
The Farming Systems Research Unit (FSRU) of the Center for Environmental Farming Systems (CEFS), NCSU, is located at the Cherry Research Farm of the North Carolina Department of Agriculture and Consumer Services (NCDACS) in Goldsboro, NC. The FSRU is a large-scale, long-term experiment established in 1999 to compare how different levels of crop diversification and agricultural management, as well as secondary ecological succession, affect ecological processes in Coastal Plains ecosystems. Cherry Research Farm has approximately 910 ha, of which the FSRU comprises 81 ha. The different cropping and management systems include conventional row-crop production with best management practices, organic production with tillage, no-tillage organic production, integrated crop and animal systems, agroforestry systems, and a secondary successional system.
Cherry Research Farm is under the management of the NCDACS, but historically all the research is directed and implemented predominantly by NCSU researchers. Therefore, there is a strong collaboration between the institutions. This collaboration is coordinated between the College of Agriculture and Life Sciences at NCSU and the Research Stations Division of NCDACS. NCSU researchers determine their own research priorities and methodologies, but they must operate within the physical, budgetary, and regulatory constraints determined by NCDACS or those that this latter institution must follow based on state and federal regulations.
Cherry Farm is located in an agricultural region, and it is surrounded by commercial agricultural farms. Also, NCDACS research stations throughout the state usually share agricultural equipment, so the cost-effective operation of the different research stations depends on movement of equipment with the accompanying risk of spread of invasive plant species. This connectivity with other farms, research stations, and other agricultural operations is of critical importance when considering the role of Cherry Farm as a source of solutions for the local stakeholders, but also is a concern if it were the case that the farm introduced invasive species to the area.
Commelina benghalensis Invasion
With the widespread adoption of glyphosate resistant crops in the United States, C. benghalensis populations increased and rapidly spread throughout agroecosystems in the midsouthern, southeastern, and mid-Atlantic United States. In 2001, C. benghalensis plants were detected in the FSRU of Cherry Farm. Verification of the detection of was done by NCSU weed scientists and a plant taxonomy specialist, who were able to distinguish this species from the more commonly occurring climbing dayflower (Commelina diffusa Burm. f.). It is likely that C. benghalensis was present in other parts of the state, but farmers misidentified it as C. diffusa. Because the detection in Cherry Farm was the first official report of this invasive noxious species in the state, and the species had been included in the federal and state noxious weed species lists, NCSU and NCDACS officials initiated a series of meetings to address the issue in a collaborative manner to make sure that any management strategies would minimize any impact on the ongoing research projects at the research farm but would also ensure the containment of the invasion.
Multistakeholder Governance Strategy
The group tasked with managing the C. benghalensis invasion was composed of multiple stakeholders who represented different interests within NCSU, NCDACS, and the community. In the case of NCSU, stakeholders included researchers with studies at the FSRU and Cherry Farm, CEFS leadership, researchers with studies in other research stations that needed to share equipment with Cherry Farm, graduate students, local extension agents, university administrators in charge of day-to-day research operations, and administrators coordinating collaborations with NCDACS. In the case of NCDACS, stakeholders included farm operations personnel at Cherry Farm and other research stations, administrative personnel in charge of coordination of activities with NCSU, and regulators in charge of invasive species and pest management at the state and local levels. Although growers were not directly involved, there was representation of growers at the CEFS advisory board and through local- and state-level noxious weed management programs through NCDACS.
Multistakeholder negotiations were conducted between 2004 and 2005 to design a methodology intended to eradicate C. benghalensis populations at Cherry Farm and in nearby areas. The methodology was based on three core considerations: (1) eradication had to be accomplished in a reasonable time, (2) infestations in organic research plots at the FSRU would not be controlled by herbicides nor fumigants, and (3) a strict and exhaustive quarantine and monitoring system would be implemented to document and ensure progress of the eradication strategy. Also, it was agreed that decisions would be made by consensus, and no party would take actions without previous consent of the other stakeholders.
Eradication Plan
The multistakeholder group met several times to design an eradication plan. The meetings were contentious, and it was difficult to reach agreements. Although all parties wanted to solve the problem, there were very different opinions about how to do it. Regulatory agencies and policy makers were adamant about the need to fumigate all fields with positive detections, while NCSU researchers emphasized the negative effects that this approach would have on long-term data collection at FSRU. Also, NCSU researchers proposed that eradication could be achieved by nonchemical means. Ultimately, the group agreed that the eradication plan had three main goals: (1) C. benghalensis populations would be eradicated before they could spread and invade other areas in North Carolina, (2) C. benghalensis populations in organic and successional fields of the FSRU would not be controlled with synthetic pesticides (i.e., herbicides, fumigants) that could alter ecological processes studied in those fields, and (3) the cost of the eradication plan would be shared by both NCSU and NCDACS.
The specific details of the eradication plan were determined by agronomists, weed scientists, and invasive pest control specialists from NCSU and NCDACS, and those details were later presented to the leadership of both institutions for approval. The plan divided the infested fields into four management areas depending on control tool restrictions. The first area was the FSRU, where C. benghalensis was first detected and where there were restrictions for the use of herbicides in several fields and restrictions for using broad-based fumigant (methyl bromide) in all fields. It is worth noting that regulators considered this a major compromise on their part, because they were taking a risk regarding public opinion for not requiring fumigation from the beginning. The second area was the rest of Cherry Research Farm, where C. benghalensis plants were also found, but fumigation could be used for control. The third area included agricultural and nonagricultural fields within the county. Finally, the fourth area included any other NCDACS research farm that exchanged equipment with Cherry Farm. The zones were created as tiers of control actions and monitoring intensity and frequency.
It was decided that NCSU would provide a budget for a full-time scout and also two part-time scouts, whose responsibilities included scouting the entirety of FRSU fields in continuous 10-d cycles throughout the year. If C. benghalensis plants were found, they had to be removed with a shovel to a 50-cm depth and a 50-cm radius to ensure the removal of all underground reproductive structures. The coordinates of each detection were recorded with an RTK-GPS, so following monitoring events would also focus on previous detections to ensure the elimination of the population. The scouts developed a historical georeferenced database and provided reports to NCSU and NCDACS twice per year. These positions were an important financial commitment from NCSU, representing more than $1 million to date.
As part of the containment actions at the FSRU, it was decided that any equipment entering roads within the FSRU had to be pressure washed before leaving the farm to remove any C. benghalensis seeds or vegetative propagules. Furthermore, any agricultural equipment used in C. benghalensis–infested fields, including tractors, mowers, tillage and cultivation implements, planters, and harvesters, had to be fumigated before they could be used in another field or transported to another location. The cost of fumigation was borne primarily by NCDACS, which was the entity that more frequently had to utilize equipment within and outside the FSRU. However, NCSU researchers conducting studies at the FSRU and in other research farms had to pay for the fumigation of their equipment.
The Cherry Farm eradication plan outside the FSRU was conducted with methyl bromide fumigation of fields where C. benghalensis was detected followed by monitoring the same year and the following 2 yr. If plants were detected after fumigation, a spot fumigation was conducted, and the same monitoring procedure described above was implemented. Beside fumigation, the entirety of the research farm was kept under quarantine until the eradication declaration was made. This meant that any movement of equipment outside Cherry Farm also required fumigation. However, no fumigation was required to move equipment across fields as long as the equipment had not been in the FSRU fields.
For the third management area, scouting was conducted based on reports of local officials of potential presence of C. benghalensis and systematically in fields or farms surrounding areas with confirmed detections. Positive detections were managed with methyl bromide fumigation. A similar approach was followed for the fourth management area; NCDACS personnel were made aware of the eradication program and required to notify the monitoring personnel of any plant in the research farms that could be C. benghalensis.
Outcomes
The eradication plan was initiated in 2005 at the FSRU, a year in which more than 55,000 C. benghalensis plants were detected and physically removed from organic fields or removed from fields managed under conventional agriculture practices using a combination of physical removal and the use of effective herbicides. This intensive approach was very effective in reducing the populations of this invasive species at a logarithmic rate (Figure 1A). Thus, after 3 yr, the number of detections was reduced more than 10-fold. In general, the eradication methodology resulted in a sustained and consistent rate of population decline with a trajectory to achieve eradication in 2024, or 19 yr after its initiation (Figure 1B).
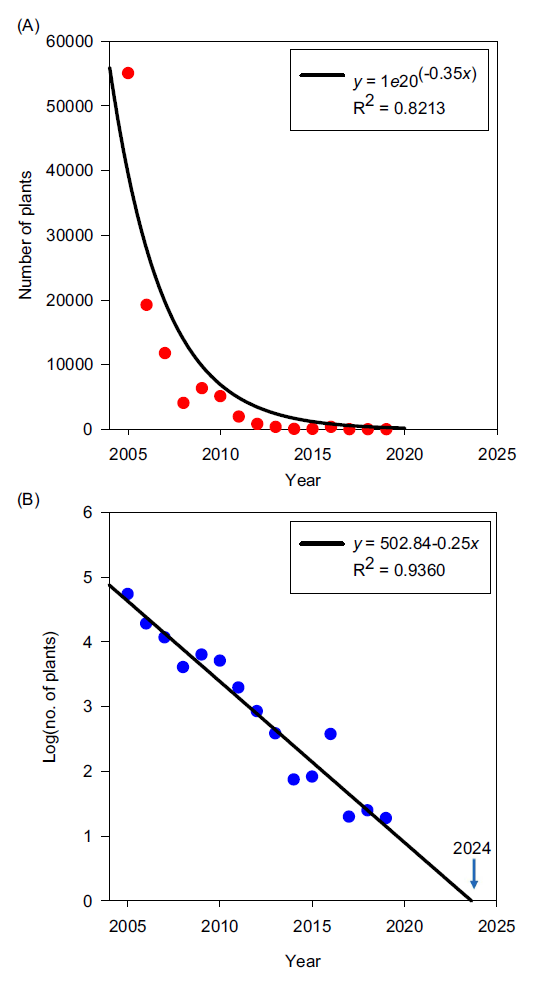
Figure 1. Number of Commelina benghalensis plants detected and eliminated over time at the Farming Systems Research Unit in Goldsboro, NC (A), and the rate of population decline predicting eradication in 2024 (B).
The reductions in C. benghalensis detections were not only for population density, but also for spatial distribution. Although the populations of this species were spread over at least 65 ha at the beginning of the monitoring/eradication actions, the most persistent patches were limited to two fields, and their persistence was associated with their original high densities. By 2019, there was a single patch with 19 C. benghalensis plants in an area covering less than 1 ha (Figure 2).
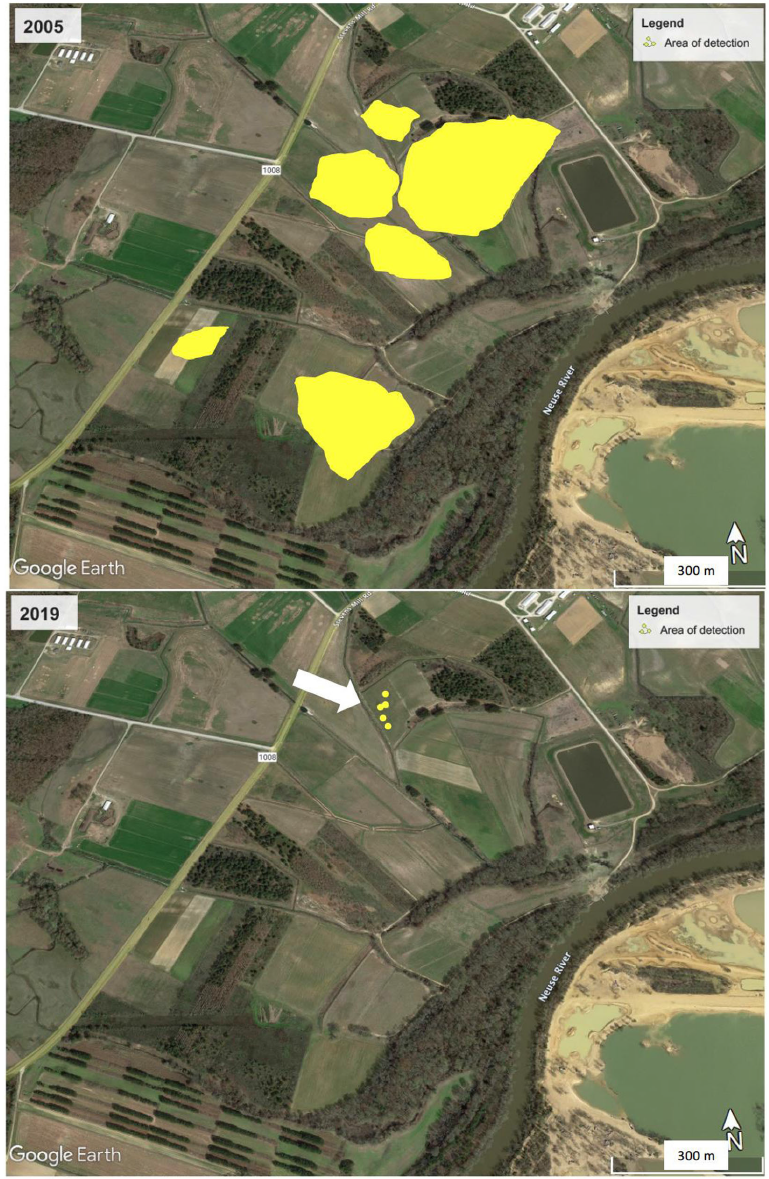
Figure 2. Reduction in Commelina benghalensis distribution from 2005 to 2019 as a result of an eradication program based on physical removal, integrated management, monitoring, and mapping. Yellow indicates the areas in the FSRU where C. benghalensis plants were detected. The white arrow indicates the field where the last 19 plants of this species were found in 2019 (B).
Eradication Uncertainty Impacts on Management and Regulatory Policy
In 2019, the multistakeholder group met to discuss the progress of the eradication program. This was motivated by the financial burden to NCSU and NCDACS of operating Cherry Farm under the quarantine conditions. Furthermore, NCSU researchers and NCDACS-Cherry Farm staff agreed that the terms of the quarantine were limiting the quality of the research projects at the FSRU, so it was necessary to reevaluate the strategy and try to eliminate or reduce the quarantine to address this situation. During the meeting, all members of the group recognized that the eradication program was largely successful, but local and state regulators were concerned that C. benghalensis plants were still being detected, albeit at very low numbers. At this point, it became evident that the original plan lacked a criterion to define eradication for practical purposes. Although some members assumed that a year or two without detections plus continuous monitoring would be a reasonable requirement to lift the quarantine, some regulators decided that the quarantine could not be lifted unless the fields that still presented detections were fumigated or that C. benghalensis individuals were not detected for 10 consecutive years. This period was set by regulators, who had full discretion on determining the terms of the quarantine. Regulators felt obligated to use the most stringent control measures as a way to protect neighbors from this invasive species; any relaxation in the quarantine or the use of control tools other than fumigation would represent an unacceptable risk for the agricultural and natural systems of the area and might create liability issues for the regulators. This decision was not well received by NCSU researchers, who considered that the available data did not support such interpretation of the risk that the C. benghalensis populations represented in 2019 (Figures 1 and 2). NCSU researchers also argued that C. benghalensis was no longer a problem, because glyphosate-only herbicide programs were not used in row-crop systems any more due to the evolution of glyphosate-resistant weeds. The incorporation of other herbicides such as mitosis inhibitors (e.g., acetochlor, alachlor, metolachlor) in herbicide programs provided effective control of C. benghalensis, dramatically reducing its populations (Webster et al. Reference Webster, Burton, Culpepper, Flanders, Grey and York2006). This was in fact one of the tools used to eradicate this weed from the FSRU. Although multiple negotiations followed, the regulators did not change their position.
Due to the lack of funds to maintain the eradication program in its original form, NCDACS officials decided to fumigate the entire fields in which C. benghalensis had been detected during the previous 2 yr, and NCSU researchers reluctantly accepted this, with the condition that the quarantine was lifted in no more than 3 yr. Thus, in the spring of 2020, two fields were fumigated, and in the following 2 yr, they were maintained fallow to eliminate any C. benghalensis left. It is worth noting that this final outcome had irreversible consequences for the long-term soil and ecological processes studied at the FSRU, and several members of the multistakeholder group questioned the value of the entire eradication program, considering that the final outcome was basically what the program was designed to avoid. This was a sore point, because millions of dollars obtained through grants had been invested in the FSRU to establish long-term plots. NCSU scientists considered the final fumigation a major disruptor of the scientific potential of the experimental area, and the unclear consequences limited the identification of mitigating actions.
Successes
The present example illustrates how multistakeholder governance can be used to design eradication strategies that can harmonize different concerns, priorities, and preferences, which was the case before the final decision to fumigate (see “Shortcomings” section). A key component for the success of the negotiations was that all parties agreed that the eradication was necessary. Unlike negotiations in which there is disagreement about the need for taking action, the fact that there was a clear common goal allowed the multistakeholder group form relatively quickly; in the case of invasion containment, such rapid actions are critical for success (Anderson Reference Anderson2005). Another beneficial component of the process was the willingness of both major stakeholders (i.e., NCSU and NCDACS) to commit to fund the implementation of the plan, so the financial burden was distributed equitably (Anderson Reference Anderson2005). Finally, the idea of developing management areas with different restriction levels provided enough flexibility in the implementation of the plan in a manner that progress was documented using the varying criteria of the different stakeholders.
Multistakeholder governance models are applicable not only to long-term research projects but also to natural systems and conservation areas where the use of chemical or aggressive physical options for invasive species control might be restricted and the risk of continuous invasion is high when alternative control practices are adopted. In cases in which an invasive species is detected in a sensitive area or ecosystem, the design of management strategies is commonly left to the managers or the regulators, who have their own priorities and biases and might not have the resources or the context necessary to make a properly informed decision (Maguire Reference Maguire2004). Incorporating points of view from stakeholders who will be directly or indirectly affected by the invasion and its management certainly could make the decision-making process slower and more complicated (Gardener et al. Reference Gardener, Atkinson and Renteria2009). However, this approach can also generate positive opportunities, such as increasing the resources available for the implementation of the plan and by sharing the responsibility for the outcomes of the process (Anderson Reference Anderson2005). The latter is of great importance in case there are uneven power relations within the governance group or the plan fails.
The results demonstrated that intensive monitoring combined with geospatial tracking over time of the positive detections and physical removal of C. benghalensis was an effective eradication strategy that avoided the use of herbicides and fumigants, which would have negatively impacted the biodiversity and ecological processes of the organic fields of the FSRU. This intensive strategy was possible because of the relatively small scale of the area that had to be covered and the resources devoted to the program. Nevertheless, modern remote-sensing techniques have made the detection and tracking of invasive species at larger scales easier (Ahmed et al. Reference Ahmed, Atzberger and Zewdie2020; Vaz et al. Reference Vaz, Alcaraz-Segura, Campus, Vicente and Honrado2018). In the present case, returning to the sites of previous detections and especially prioritizing high-density spots represented an effective approach to ensure C. benghalensis population decline. The fact that this species has relatively large seeds that disperse by barochory and produces seeds from underground flowers contributes to the patch stability observed (Riar et al. Reference Riar, Webster, Brecke, Jordan, Burton, Telenko and Rufty2012). Invasive plant species with mechanisms that favor long-distance dispersal (e.g., anemochory, zoochory) might require more detailed probabilistic approaches for detection and tracking (Heijting et al. Reference Heijting, van der Werf, Stein and Kropff2007).
Shortcomings
The main question in the present case study is that if the eradication plan was working, why was fumigation implemented at the end, undermining the large scientific, managerial, and financial investments of the previous 15 yr? This heavy-handed final action by regulators could be interpreted by other stakeholders as a predetermined agenda that was not part of the original consensus (Maguire and Lind Reference Maguire and Lind2003).
Several shortcomings during the design of the eradication plan provide an answer to this question. For example, no clear milestones for the plan were determined at the beginning of the project. Therefore, there was no clarity about what constituted acceptable progress. Although all parties agreed that a continuous decline in C. benghalensis density and distribution was critical for the success of the plan, the target rate of population decline was never defined. This was in part because, before the plan was implemented, the persistence of the seedbank of this species was unknown (Regan et al. Reference Regan, McCarthy, Baxter, Panetta and Possingham2006; Riar et al. Reference Riar, Webster, Brecke, Jordan, Burton, Telenko and Rufty2012). Thus, it would take a few years of data collection before it was possible to have an idea of the potential rate of population decline based on the control actions used. Also, a lack of predetermined financial limits jeopardized the continuation of the project based on adequate progress (Gardener et al. Reference Gardener, Atkinson and Renteria2009). In the spirit of collaboration and solving the problem, NCSU and NCDACS committed operating funds without setting an expiration date. This was probably because of wishful thinking on the part of the administrators of both organizations, who assumed the eradication could be accomplished faster than it was (Hulme Reference Hulme2020). In cases in which the rate of seed decay or seedbank decline are unknown, techniques such as derivation of cumulative seedling emergence functions can be used to progressively approximate when a seedbank approaches zero (Sousa-Ortega et al. Reference Sousa-Ortega, Royo-Esnal, DiTommaso, Izquierdo, Loureiro, Mari, Cordero, Vargas, Saavedra, Paramio, Fernandez, Torra and Urbano2020).
Perhaps the most critical misstep in the planning and implementation phases was not identifying a priori the conditions that would have triggered the most aggressive control measure, that is, fumigation. Knowing from the beginning of the process that regulators, who had full discretion to determine the acceptable eradication criteria, wanted 10 yr free of detections before lifting the quarantine, would have allowed a better estimate of the duration of the eradication program. For example, using population decline data during the first 5 yr of the plan, it would have been evident that the program required almost 30 yr (i.e., 19 yr to eradication and an additional 10 yr of no detections; Figure 1). This key piece of information would have better informed the decisions regarding the allocation of financial resources and the scientific data collection that the project had to continue generating. When considering a 30-yr eradication program, most stakeholders (personal communications) would have preferred to fumigate from the beginning and avoid the quarantine restrictions and devote more resources to document the impacts of the fumigation and to implement actions to minimize the interruption of the long-term trends of ecological processes in the FSRU fields.
Liability Concerns versus Data-based Decision Making
In the present case study, it is intriguing that regulators required 10 yr free of detections for the nonchemical control program but only 2 to 3 yr for fumigation to lift the quarantine. This suggests that the confidence in the outcomes of the eradication program were based, to a large extent, in the perceived intensity of the control measure and not so much on the data documenting C. benghalensis population decline. During the last negotiations that determined the final conditions to lift the quarantine and that resulted in the fumigation of the fields of the FSRU, it was stated that regulators felt liable for any potential escape that could contribute to the spread of this invasive species throughout the state and region. A major concern for them was that unless they were able to demonstrate that the most rigorous or aggressive control methods available to eradicate C. benghalensis were employed, they could be directly or solely blamed for a failed eradication. We recognize that those concerns are valid and must be addressed as part of the multistakeholder governance model, because regulators have enforcement responsibilities (Burgos-Rodriguez and Burgiel Reference Burgos-Rodriguez and Burgiel2020). It is difficult to ignore the fact that in the case of a failed eradication, regulators might have to state why they did not require the “most aggressive” or “most effective” control actions from the beginning. This raises another important question, what do we consider the “most aggressive” or “most effective” control measure?
Herbicides and fumigants are highly toxic to plants, which increases the possibility of rapid control, but they can also have serious negative effects on native and desirable vegetation and biodiversity. Conversely, a physical removal program combined with intensive georeferenced tracking can also be highly effective, as demonstrated here, and will minimize negative impacts on off-target species, although it is not as fast-acting as chemical options. Managers and regulators must understand that eradication programs represent multidimensional considerations and trade-offs and that success or failure is not determined only by the control tool selected. Acknowledgment of these interlocking factors should reduce the fear of liability on the part of regulators and encourage/force other stakeholders to take ownership of the outcomes of the program, especially considering that proper implementation of the plan and timely corrections when needed might be more important than the original plan in the long run (Nourani et al. Reference Nourani, Krasny and Decker2018).
Eradication and containment programs of invasive species must be result oriented. In the present case, we had two eradication programs that were considered viable by members of the multistakeholder group. One was fumigation and the other was physical removal/integrated management and georeferenced tracking. Once there are 2 yr without detections, would the former program provide more confidence than the latter? The answer should be no. Neither program should give the managers complete confidence that the problem is solved. We contend that the multistakeholder group also failed to prepare a monitoring system after the “eradication declaration” was made. In both programs, there was a risk of plants escaping control, and detection is less likely at very low densities (Regan et al. Reference Regan, McCarthy, Baxter, Panetta and Possingham2006), so those escape plants could potentially increase the seedbank over time. It was incorrect to assume that fumigation would give perfect control in a single or two treatments, which would allow “moving on” and not worrying about reinfestations.
Maintaining some level of monitoring for 5 yr is likely a valuable practice that can help detect new infestations and give confidence to regulators and other stakeholders that the problem is under control (Blossey Reference Blossey1999). This does not mean that one must do this under quarantine conditions, as was required by regulators in the present case. Clear and robust population density data demonstrating that at least the “theoretical zero” has been achieved should be enough to lift the quarantine and initiate normal management of the area.
Conclusions
Plant invasion management does not depend solely on availability of control tools. Financial resources, technical knowledge, access to infested areas, public awareness, managers’ commitment, regulatory constraints, and policies and laws are examples of some of the many factors that influence management design, implementation, and success. This level of complexity makes dealing with many different stakeholders unavoidable for the manager. For this reason, multistakeholder governance is a valuable tool not only to design management strategies (e.g., prevention, containment, eradication) but also to generate the support needed for proper implementation. It is important, however, to recognize that different stakeholders will have different priorities and levels of influence in the decision-making process. In general terms, regulators and those funding the management plan will have the most influence. If they are concerned that the risk to their professional responsibilities or credibility is too high, it is likely that they will choose the approach they perceive as the most “aggressive” or effective, although this can be detrimental to the priorities of the majority of the stakeholders. To avoid this type of situation, the governance model must clearly address concerns about responsibilities and liabilities before the management plan is finished. Also, it is important to define success and failure in a clear time frame agreed a priori. Otherwise, it will be difficult to identify and implement corrective measures within the multistakeholder governance model without more powerful stakeholders exerting their greater influence over the final decision.
Acknowledgments
We are very thankful to Richard Banner for his invaluable, tireless scouting and great contributions to control and eradicate Commelina benghalensis from the FSRU. The authors declare no financial conflict of interest. The authors declare participating as stakeholders in different moments of the negotiations representing researchers involved in activities related to the FSRU.







