Introduction
Microbiological activity can be detected in extreme environments, such as hot springs (e.g. Kashefi and Lovley, Reference Kashefi and Lovley2003; Takai et al., Reference Takai, Nakamura, Toki, Tsunogai, Miyazaki, Miyazaki, Hirayama, Nakagawa, Nunoura and Horikoshi2008); cold environments, such as polar regions (Price, Reference Price2007; Mikucki et al., Reference Mikucki, Pearson, Johnston, Turchyn, Farquhar, Schrag, Anbar, Priscu and Lee2009; Boetius et al., Reference Boetius, Anesio, Deming, Mikucki and Rapp2015); deep subsurfaces (Gold, Reference Gold1992; Edwards et al., Reference Edwards, Becker and Colwell2012; Inagaki et al., Reference Inagaki, Hinrichs, Kubo, Bowles, Heuer, Hong, Hoshino, Ijiri, Imachi, Ito, Kaneko, Lever, Lin, Methé, Morita, Morono, Tanikawa, Bihan, Bowden, Elvert, Glombitza, Gross, Harrington, Hori, Li, Limmer, Liu, Murayama, Ohkouchi, Ono, Park, Phillips, Prieto-Mollar, Purkey, Riedinger, Sanada, Sauvage, Snyder, Susilawati, Takano, Tasumi, Terada, Tomaru, Trembath-Reichert, Wang and Yamada2015; D'Hondt et al., Reference D'Hondt, Pockalny, Fulfer and Spivack2019) and the upper atmosphere (Yang et al., Reference Yang, Itahashi, Yokobori and Yamagishi2008; Smith et al., Reference Smith, Griffin, McPeters, Ward and Schuerger2011; Bryan et al., Reference Bryan, Christner, Guzik, Granger and Stewart2019). Accordingly, researchers have recently discussed the possibility of life on various solar system bodies, such as Mars (Forget and Hauber, Reference Forget and Hauber2015), Europa (Encrenaz, Reference Encrenaz2015a) and Enceladus (Encrenaz, Reference Encrenaz, Gargaud, Amils, Quintanilla, Cleaves, Irvine, Pinti and Viso2015b).
Mars has been studied extensively as a target for the detection of extraterrestrial life, and several Mars missions have been conducted to examine the habitability of Mars, beginning with the Viking mission in 1976. These missions have revealed that in the Noachian period, Mars had large amounts of surface water (oceans/lakes) (Carr and Head, Reference Carr and Head2003), a carbon dioxide-rich dense atmosphere (Kasting, Reference Kasting1987), a humid and warm climate (Pollack et al., Reference Pollack, Kasting, Richardson and Poliakoff1987) and a strong magnetic field (Acuna et al., Reference Acuna, Connerney, Lin, Mitchell, Carlson, McFadden, Anderson, Rème, Mazelle and Vignes1999). Recent missions have also identified methane in the Martian atmosphere (Webster et al., Reference Webster, Mahaffy, Atreya, Flesch, Mischna, Meslin, Farley, Conrad, Christensen and Pavlov2015) and complex organic compounds in mudstones (Eigenbrode et al., Reference Eigenbrode, Summons, Steele, Freissinet, Millan, Navarro-González, Sutter, McAdam, Franz, Glavin, Archer, Mahaffy, Conrad, Hurowitz, Grotzinger, Gupta, Ming, Sumner, Szopa, Malespin, Buch and Coll2018), suggesting that we may eventually detect extant and/or extinct life on Mars (Yamagishi et al., Reference Yamagishi, Yokobori, Yoshimura, Yamashita, Hashimoto, Kubota, Yano, Haruyama, Tabata, Kobayashi, Honda, Utsumi, Saiki, Itoh, Miyakawa, Hamase, Naganuma, Mita, Tonokura, Sasaki and Miyamoto2010).
The Atacama Desert, located in northern Chile, South America, is one of the driest environments on Earth. A number of pioneering and fundamental studies, conducted in the past two decades, have revealed the microbial ecological features of the biosphere that are unique to extremely arid regions (Navarro-González et al., Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003; Warren-Rhodes et al., Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gomez-Silva, Amundson, Friedmann and McKay2006; Bahl et al., Reference Bahl, Lau, Smith, Vijaykrishna, Cary, Lacap, Lee, Papke, Warren-Rhodes and Wong2011; Pointing and Belnap, Reference Pointing and Belnap2012; Bull et al., Reference Bull, Asenjo, Goodfellow and Gómez-Silva2016; Lebre et al., Reference Lebre, De Maayer and Cowan2017; Farías, Reference Farías2020). Life surviving in the pore spaces of sandstone rocks and salt crystals is a particularly interesting and noteworthy feature (Friedmann, Reference Friedmann1980; Walker and Pace, Reference Walker and Pace2007; Wierzchos et al., Reference Wierzchos, Casero, Artieda and Ascaso2018). Due to the aridity of the Atacama Desert, together with its strong ultraviolet (UV) radiation and the presence of perchlorates and evaporites, this region is regarded as one of the best terrestrial analogues for Mars (Navarro-González et al., Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003; Léveillé, Reference Léveillé2015). Moreover, because soil microbial activity in the Atacama Desert appears to be limited, the development of methods for the detection of chemical biosignatures in this extreme environment may be helpful for future missions aimed at detecting life on extraterrestrial bodies, including Mars.
Several biosignatures have been proposed to detect terrestrial and extraterrestrial life (Tsou et al., Reference Tsou, Brownlee, McKay, Anbar, Yano, Altwegg, Beegle, Dissly, Strange and Kanik2012; Stevens et al., Reference Stevens, McDonald, de Koning, Riedo, Preston, Ehrenfreund, Wurz and Cockell2019; Neveu et al., Reference Neveu, Anbar, Davila, Glavin, Mackenzie, Phillips-Lander, Sherwood, Takano, Williams and Yano2020; Yoshimura et al., Reference Yoshimura, Enya, Kobayashi, Sasaki and Yamagishi2021). Additionally, previous reports have discussed methods for detection of life by analysing bioorganic compounds, their homochirality and enzymatic activities (Takano et al., Reference Takano, Sato, Kaneko, Kobayashi and Marumo2003, Reference Takano, Kudo, Kaneko, Kobayashi, Kawasaki and Ishikawa2004a, Reference Takano, Horiuchi, Kobayashi, Marumo, Nakashima and Urabe2004b, Reference Takano, Mori, Kaneko, Ishikawa, Marumo and Kobayashi2006; Horiuchi et al., Reference Horiuchi, Takano, Kobayashi, Marumo, Ishibashi and Urabe2004; Kudo et al., Reference Kudo, Kobayashi, Marumo and Takano2006; Kato et al., Reference Kato, Yanagawa, Sunamura, Takano, Ishibashi, Kakegawa, Utsumi, Yamanaka, Toki, Noguchi, Kobayashi, Moroi, Kimura, Kawarabayasi, Marumo, Urabe and Yamagishi2009; Georgiou, Reference Georgiou2018). Terrestrial organisms express proteins, including enzymes, amino acids (constituents of proteins) and enzymatic activity, and these features may be good indicators for evaluating biological activity in extreme environments. Enzymatic activity is a particularly interesting indicator in the search for life on other worlds because biology anywhere will likely require catalytic activity similar to the catalytic reactions used on Earth even if the details of the constituent molecules are different (Georgiou, Reference Georgiou2018).
Among the wide variety of enzymes expressed by terrestrial organisms, we have focused on phosphatases (phosphoric monoester hydrolases). There are two types of phosphatases: alkaline phosphatases (ALPases) and acid phosphatases (ACPases). Phosphoric monoesters are used as nucleotides in genetic molecules (e.g. DNA/RNA) and as phospholipids in the cell membrane. Moreover, these molecules are essential for all terrestrial organisms, suggesting that phosphatases should be ubiquitously expressed. Accordingly, phosphatase activity may be a useful indicator of biological activity, with wide applications in the global biosphere, including terrestrial, marine, polar and extreme environments (Dick et al., Reference Dick, Cheng and Wang2000; Hoppe, Reference Hoppe2003; Takano et al., Reference Takano, Mori, Kaneko, Ishikawa, Marumo and Kobayashi2006; Nannipieri et al., Reference Nannipieri, Giagnoni, Landi, Renella, Bünemann, Oberson and Frossard2011).
In this study, we analysed two transects in the Atacama Desert: one across the moisture gradient with latitude defined by Navarro-González et al. (Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003) and another with depth below the surface in the core region. In both transects we examined molecular indicators of biological activity and the presence phosphatase (ALPase) activity. The results provide insight into the microbial patterns in the Atacama Desert that is largely consistent with previous work. In addition by comparing the phosphatase activity to the molecular biomarkers we demonstrate its utility as a tool in the search for life in extreme environments.
Experimental
Samples
The Atacama Desert in Chile is the driest natural environment on Earth and is located on the plateau between the Pacific Ocean and the Andes Mountains. This region extends for approximately 1000 km from north to south, and its hyperarid core lies in the northern part. Two sets of soils were collected in the Atacama Desert and used in this study (Fig. 1).
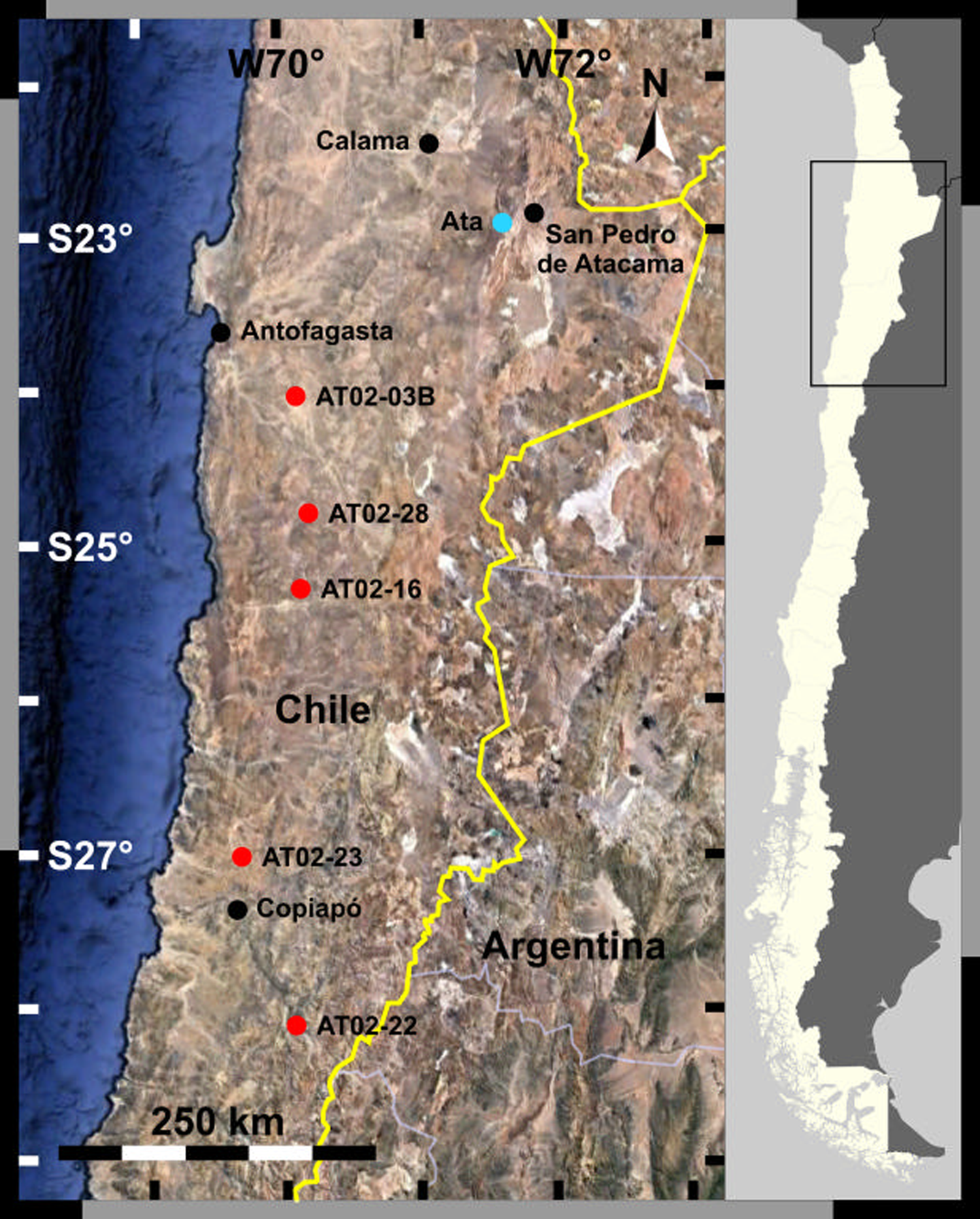
Fig. 1. Sampling sites in the Atacama Desert.
The first set (AT02) was collected in the Atacama Desert along the north–south precipitation gradient in October 2002. These are subsamples of the samples collected by Navarro-González et al. (Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003). The average longitude was approximately 70°W, and the latitude was between 24 and 28°S. The specific locations of the sampling sites are as follows (from north to south): AT02-03B (24°4′11.1″S, 69°51′58.1″W), AT02-28 (24°49′1.2″S, 69°47′2″W), AT02-16 (25°18′17.4″S, 69°50′32.2″W), AT02-23 (27°1′17.4″S, 70°17′40.7″W) and AT02-22 (28°7′4.5″S, 69°55′8″W). Samples were collected from the upper 10 cm soil layer using sterile polyethylene scoops and stored in sterile polyethylene bags at ambient temperature (Navarro-González et al., Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003).
The second set (A, B) was sampled at an alluvial fan, west of the Cordillera Domeyko (Ata: 22°57′42.3″S, 68°24′36.6″W). Soil samples were collected at the surface and every 2 cm from 1 to 21 cm deep using an ethanol-sanitized trowel. The samples were then stored in sterile plastic bags and double-bagged (Nauny, Reference Nauny2019). The samples were labelled as An or Bn, where n represents the sampling depth. Both sample subsets were stored at room temperature; the sample subset An was allowed to retain its humidity, whereas subset Bn was freeze-dried. The samples were sent to Japan at ambient temperature. Sampling was performed by a team from the University of Glasgow, UK on 6 May 2015.
Sea sand (purchased from Fujifilm Wako Pure Chemicals, Japan) heated at 500°C for 4 h was used as a blank.
Water was purified using a Milli-Q system (Merck KGaA, Germany). All glassware was heated at 500°C for 4 h to remove any organic contamination. Teflon tubes were cleaned with 5% Extran (Merck Millipore, USA), 7 M HNO3 and rinsed in purified water successively before use.
Chemicals and reagents
The substrates for ALPase activity used were as follows: (1) disodium p-nitrophenylphosphate hexahydrate (pNPP; Fujifilm Wako Pure Chemicals, Japan) and (2) 4-methylumbelliferyl phosphate (4MUP; Fujifilm Wako Pure Chemicals). The amino acid standard was a mixture of amino acid standard solutions AN-II and B (Fujifilm Wako Pure Chemicals). The hydrolysates p-nitrophenol (pNP) and 4-methylumbelliferone (4MU) were purchased from Fujifilm Wako Pure Chemicals. Tris buffer was prepared with tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl; Sigma Aldrich, St. Louis, MO, USA) and NaOH (Fujifilm Wako Pure Chemicals). MUB buffer was prepared using malic acid (2.32 g l−1), boric acid (1.26 g l−1), citric acid (2.80 g l−1), all purchased from Fujifilm Wako Pure Chemicals, with Tris-HCl (2.42 g l−1). The filters used were hydrophilic PTFE membrane filters (pore size: 0.45 μm; ADVANTEC, Japan).
Instruments
Absorption spectra were recorded using a V-550 UV-VIS Spectrophotometer (JASCO, Japan). Fluorescence spectra were recorded with an FP-6300 spectrofluorometer (JASCO). Amino acids were determined using a Shimadzu Amino Acid Analysis System consisting of two LC-10AT pumps, an RF-20Axs fluorescence detector and a Shimpak ISC-07/S 1504 column (4.0 mm i.d. × 150 mm). For extraction of enzymes from soil samples, an SHK-U3 Universal Shaker (Iwaki, Japan) was used. A CN-25C Cool Incubator (Mitsubishi Electric Engineering, Japan) was used during the incubation to measure enzymatic activity.
Assay of ALPase activity
Phosphatase activity was assayed using absorption and fluorescence spectrometry. For absorption spectrometry (Fig. 2(a)), 0.5 g sample soil, 0.1 ml toluene and 2.5 ml of 1.0 mM pNPP in MUB buffer (pH 8.0) were mixed in a test tube and incubated at 37°C for 1–24 h. Subsequently, 0.5 ml of 0.5 M CaCl2 and 2.0 ml of 0.5 M NaOH were added to terminate the reaction. The mixture was then filtered through a membrane filter, and the absorbance of the filtrate at 410 nm (A 410) was measured. Phosphatase activity (nmol min−1) was calculated using dA 410/dt and the molar absorbance coefficient of ɛ pNP and ɛ pNPP. For fluorescence spectrometry (Fig. 2b), 4MUP and 4MU absorbed the soils very strongly; therefore, we could not directly mix the substrate solution and soil samples. We first extracted enzymes from soils using 0.2 M Tris-HCl buffer (pH 8.0). Tris-HCl buffer (5.0 ml) was added to a 0.5 g soil sample, and the mixture was shaken at 180 rpm for 1 h at ambient temperature and then filtered through a membrane filter. A mixture of 0.4 ml soil extract and 3.0 ml substrate solution (0.5 mM 4MUP in 0.2 M Tris buffer, pH 8.0) was incubated at 37°C for 10 min to 24 h, and the increase in fluorescence (F 451; λx = 362 nm, λem = 451 nm) was monitored. Phosphatase activity was calculated using dF 451/dt and F 451 in a 4MU standard solution.

Fig. 2. Assay methods for phosphatase activity: (a) absorption spectrometry method and (b) fluorescence spectrometry method.
Determination of amino acids
Amino acids were extracted from soil samples using a hot-water extraction method, HCl extraction method, combined method and HF digestion method. For the hot-water extraction method (conventional method), a: a mixture of 0.1 g of soil sample and 3.0 ml water was heated at 110°C for 24 h. The resulting mixture was filtered through a membrane filter, and the filtrate was acid-hydrolysed in 6 M HCl at 110°C for 24 h. For the HCl extraction method, a mixture of 0.1 g soil sample and 2.0 ml of 6 M HCl was heated at 110°C for 24 h. The mixture was then filtered through a membrane filter. For the combined method: the residue from the hot-water extraction method was (1) further extracted with 6 M HCl, as described for the HCl extraction method. For the, as shown in (2), HF digestion method, soil samples (0.1 g) were placed in a Teflon tube, and 3 ml mixture of 5 M HF and 0.1 M HCl was added. The tube was tightly closed with a Teflon lid, placed in a metal holder, and heated at 110°C for 24 h in an oven. After cooling to ambient temperature, the tube was placed on a hot plate to evaporate HF/HCl under an N2 stream. The residue was dissolved in pure water, filtered through a membrane filter and acid-hydrolysed with 6 M HCl at 110°C for 24 h.
After each extraction, the filtered extract was desalted using an AG-50X8 cation exchange resin (Bio-Rad Laboratories, Hercules, CA, USA) and subjected to amino acid analysis by cation-exchange high-performance liquid chromatography (Takano et al., Reference Takano, Marumo, Yabashi, Kaneko and Kobayashi2004c; Kobayashi et al., Reference Kobayashi, Mita, Kebukawa, Nakagawa, Kaneko, Obayashi, Sato, Yokoo, Minematsu, Fukuda, Oguri, Yoda, Yoshida, Kanda, Imai, Yano, Hashimoto, Yokobori and Yamagishi2021a, Reference Kobayashi, Sato, Ito, Nakamoto, Kurizuka, Takano, Obayashi, Kaneko, Kebukawa, Ogawa and Yoshida2021b).
DNA extraction and quantification
DNA was extracted from soil samples using a FastDNA Spin kit for soil (MP Biomedical, USA; approximately 700–800 mg soil for each sample), and DNA was eluted into 50 μl buffer. Despite the risk of low extraction yields because of silt-sized particles in the soil samples (Direito et al., Reference Direito, Marees and Röling2012), this method was chosen to be consistent with that previously used in previous experiments (Thomas, Reference Thomas2018) and to allow for a proper comparison of the results. The concentration of recovered DNA was then measured on a QuBit 2.0 Fluorometer (Life Technologies, USA) using a QuBit dsDNA HS assay kit (Life Technologies).
Determination of loss on ignition at 550°C (LOI550)
For each sample, approximately 5–10 g soil was oven-dried at 105°C overnight and then combusted at 550°C for 16 h. Combusted samples were cooled in a desiccator before being weighed on a precision balance (AG204; Mettler Toledo, Switzerland; deviation: 0.1 mg). The LOI550 was expressed as a percentage of the mass lost during combustion at 550°C using the following formula:
where m 105 is the mass of the sample oven-dried at 105°C, and m 550 is the mass of the sample combusted at 550°C.
Preparation of total lipid extracts (TLEs)
Approximately 10 g soil was freeze-dried for 72 h (6 × 10−4 Pa, −50°C; Alpha 1-2 LD; Martin Christ, Germany) for each sample before homogenization. Freeze-dried samples were weighed before lipid extraction with a CH2Cl2 : MeOH (9 : 1, v : v) mixture on an accelerated solvent extractor (10 MPa, 120°C, 3 × 15 min; Dionex ASE 350; Thermo Scientific, USA). The obtained TLEs were weighed after evaporation of the extraction solvent.
Extraction and analysis of hydrocarbons
The neutral fraction of TLEs was extracted using flash chromatography on ammonium-coated silica gel columns (LC-NH2 SPE silica; Fisher, USA) using a CH2Cl2 : iPrOH (1 : 1, v : v) mixture as the eluent. Aliphatic hydrocarbons were isolated from the neutral fractions using flash chromatography on silica (230–400 mesh, 35–70 μm silica; Fisher), using hexane as an eluent. Molecules were quantified by gas chromatography (GC) with flame ionization detection (FID) and their identities were confirmed by GC-mass spectrometry (MS). An n-alkane standard (NJEPH aliphatic calibration standard; Restek, USA) was measured for every six samples to quantify molecules and to check the reproducibility of the measurements. GC–FID analyses were performed using a Shimadzu 2010 GC (Shimadzu) equipped with a BP1 column (60 m × 250 μm × 0.25 μm; SGE Analytical Science). Hydrogen was used as the carrier gas at a column flow rate of 1.2 ml min−1. For GC, we used splitless injection at 320°C. The oven temperature was programmed as follows: hold at 60°C for 2 min, ramp up to 120°C at 30°C min−1, then to 350°C at 3°C min−1 and then held at 350°C for 20 min. GC-MS analyses were performed using a Shimadzu 2010 GC (Shimadzu) interfaced with an QP2010-Plus MS (Shimadzu). The columns and programmes used were the same as described above. The interphase temperature was set at 300°C, and the ion source temperature was set at 200°C. The carrier gas used was helium at a column flow rate of 1.2 ml min−1.
Fluorescence microscopy
Soil samples (0.025 g each) were placed in multi-well glass-bottom dishes (cat. no. D141400; Matsunami Glass Ind., Ltd.) with 200 μl staining solution containing 5 μM SYTO 24 (Thermo Fisher Scientific) and 50 mM Tris-HCl (pH 8.0). After 15 min, the cells were observed under a fluorescence microscope (Axiovert 135M; Carl Zeiss Inc.) with a B-excitation filter set (excitation: 480–490 nm, emission: >500 nm; Semrock). Fluorescence images were acquired with a digital camera (EOS X6i; Canon Inc.), and microbial cells possessing a cell-like structure and higher green fluorescence intensity than the background were counted.
Results
Figure 3 shows the biomarker data including phosphatase activity for the north–south transect as a function of latitude with the addition of a panel showing the rain data from Warren-Rhodes et al. (Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gomez-Silva, Amundson, Friedmann and McKay2006). Figure 4 shows the biomarker data including phosphatase activity for the depth profile. Figure 5 shows fluorescence microscopic images of four soil samples: two from the north–south transect corresponding to driest and the wettest sites, and two from the depth profile correspond to the surface and a deeper sample.

Fig. 3. North–south profile of rainfall rate and bioindicators: (a) annual rainfall rate (mm year−1), (b) density of culturable heterotrophic bacteria (CFU g−1) taken from Navarro-González et al. (Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003) and (c) phosphatase activity (pmol min−1 g−1), total amino acid concentration (nmol g−1). The comparison indicates a sharp increase in biomass (amino acids), viable microorganisms (CFU) and microbial activity (phosphatase activity) when rain >25 mm year−1 – marking a transition between the Mars-like extreme hyper-arid zone of the Atacama and the hyper-arid zone. Orange squares represent below the detection limit.

Fig. 4. Measurements of several parameters in samples collected along a depth profile (site Ata, Fig. 1). Phosphatase activity (pmol min−1 g−1): (a) A-set (absorption), (b) A-set (fluorescence), (c) B-set (absorption) and (d) B-set (fluorescence). (e) Total amino acid concentration (nmol g−1). (f) Total DNA concentration (ng g−1). (g) LOI550 (% m/m). (h) Total lipids extract (ng g−1). (i) Phytane concentration (ng g−1). n-Alkane concentrations (ng g−1): (j) n-C18, (k) n-C20, (l) n-C29 and (m) n-C31. (n) Water content (% m/m). Measurements (e)–(n) used samples from the A-set, following the preparation procedures described in the Experimental section. Orange squares represent below the detection limit, and red triangle represents not detected.

Fig. 5. FMS images of soil samples from the Atacama Desert: (a) AT02-03B, (b) AT02-22, (c) A0 and (d) A15.
Phosphatase activity in Atacama Desert soils
ALPase activity in Atacama surface soils (A02 set) was measured by both absorption spectrometry (substrate: pNPP) and fluorometry (substrate: 4MUP), whereas that of the AT set was measured only by the fluorometry method. The detection limit (DL) of each method was calculated using the ALPase activity of the heated sea sand sample. The DL using the absorption method was 52 pmol min−1 g−1, whereas that using the fluorometry method was 4.6 pmol min−1 g−1.
The phosphatase activities of Atacama Desert soil samples are shown in Figs 3(c) and 4(a)–(d). The ALPase activity of A19 was 11.2 pmol min−1 g−1 by the absorption method and 62.2 pmol min−1 g−1 by the fluorometry method. These two values may have differed for two reasons. First, the fluorometric method required an extraction procedure, and only a part of ALPase could be extracted. Second, the enzyme (ALPase) showed different substrate specificities for the substrates used in the different methods. Notably, however, the ALPase activities in most A and B samples evaluated using the absorption method were below the DL, whereas the fluorometry method yielded ALPase activity values higher than the detection method for most samples. Thus, the fluorometry method may be useful for analysis of ALPase activity in extreme environmental samples.
Based on the horizontal distribution of ALPase activity (AT02 set), the southern samples (AT02-22) showed much higher activities than the northern samples (e.g. AT02-03B). The vertical distribution of ALPase (A, B sets) indicated that the surface samples showed low ALPase activity, whereas deeper soil samples showed relatively high activity.
Amino acid analysis
Amino acids in geochemical and cosmochemical samples (e.g. meteorites) have often been determined after hot-water extraction followed by acid hydrolysis (Kvenvolden et al., Reference Kvenvolden, Lawless, Pering, Peterson, Flores and Ponnamperuma1970). However, we found that the hot-water extraction method could extract only a portion of the total amino acids from soil samples, whereas the HF digestion method showed greater amino acid yields in soil samples (Kudo et al., Reference Kudo, Takano, Kaneko and Kobayashi2003).
Table 1 shows the procedure blank for amino acids collected using the three extraction methods with heated sea sand samples. The HF digestion method yielded higher blank values than the hot-water and HCl extraction methods. One reason for this result may be that the apparatus used for the HF digestion method was not adequately cleaned by heating at 500°C. Accordingly, we decided to exclude the HF digestion method from further analyses.
Table 1. Procedure blanks for amino acids (nmol g−1)

ND, not detected; ABA, aminobutyric acid.
Table 2 shows the amino acid concentrations of one of the Atacama soil samples (AT02-22) subjected to hot-water extraction and HCl extraction. The residue obtained from the hot-water extraction still contained amino acids, which were subsequently extracted using the HCl method. Therefore, we mainly used the HCl extraction method in our subsequent analyses.
Table 2. Amino acids in Atacama soils (AT02-22; nmol g−1)

ND, not detected; ABA, aminobutyric acid.
a The residue after H2O extraction.
Table 3 shows the amino acid concentrations in Atacama soil samples (AT02 set) subjected to the combined (hot-water + HCl extraction) method. The amino acid concentrations differed greatly among samples. For example AT02-22 showed much higher amino acid concentrations than the other samples and also showed very high ALPase activity (Fig. 3(c)). By contrast, AT02-03, -28 and -16 had low amino acid concentrations and low ALPase activity (Fig. 3(d)). Glycine, alanine and β-alanine, which are the three simplest amino acids, were the most abundant amino acids in most samples.
Table 3. Amino acids in Atacama soils (AT02 set; HCl extraction method; nmol g−1)

ND, not detected.
Figure 4(e) summarizes the total amino acid concentrations in Atacama soil samples (set A) obtained using the HCl extraction method. In this analysis, the surface sample (A0) had a much higher total amino acid concentration than the other samples. Some of the deeper samples (e.g. A19) showed relatively high concentrations of amino acids. However, the standard deviations were quite high, suggesting that the soil samples were quite heterogeneous.
DNA concentrations and other biochemical parameters
DNA was present throughout most of the depth profiles (Fig. 4). Four samples did not contain DNA at concentrations higher than the DL (Fig. 3, orange squares; 25 pg μl−1 in liquid extracts, before normalization to the amount of soil used). When detected, DNA was present at lower concentrations at and near the surface compared with that in samples collected at greater depths.
To assess the quantity of organic matter present in the samples, an analysis of the LOI550 was performed (Heiri et al., Reference Heiri, Lotter and Lemcke2001). Samples contained low amounts (0.30–0.88%) of organic matter (Fig. 4(g)). To better characterize the organic biomarkers present in the samples, TLEs of the samples were also prepared. TLE quantities were globally low along the depth profile (around 30–40 μg g−1), but showed a sudden increase in concentration at a depth of 13 cm and then slowly decreased to their previous level as the depth increased (Fig. 4(h)). Further analysis of the refined fractions of TLEs permitted the detection of aliphatic hydrocarbon biomarkers (Fig. 4(i)–(m)). Odd-numbered long-chain n-alkanes, which are characteristic of the waxes produced by higher plants (Eglington and Hamilton, Reference Eglington and Hamilton1967; Jetter et al., Reference Jetter, Kunst, Samuels, Riederer and Müller2006), were mostly present at a depth of 19 cm (Fig. 4(j) and (k)). Phytane, a diagenesis product of chlorophyll a (Eglington et al., Reference Eglington, Scott, Belsky, Burlingame, Calvin and Cloud1964), was also detected, although the highest concentration was found at a depth of 15–17 cm (Fig. 4(i)). Finally, the concentrations of even-numbered short-chain n-alkanes, which are usually associated with anthropogenic contamination (Brocks et al., Reference Brocks, Logan, Buick and Summons1999), microbial production (Han and Calvin, Reference Han and Calvin1969; Albro, Reference Albro1976) or the diagenesis of longer n-alkanes (Wiesenberg et al., Reference Wiesenberg, Lehndorff and Schwark2009), were highest just under the surface (at a depth of 1 cm) and at a depth of 15–17 cm (Fig. 4(l) and (m)).
Fluorescence microscopy observations
Figure 5 shows the fluorescence microscopic images of soil samples. Small green dots indicate microbial cells. The number of microorganisms in the southern sample (AT-02-22, Fig. 5(b)) was markedly higher than that in the northern sample (AT-02-03B, Fig. 5(a)). In the image of AT-02-22, dozens of microbial cells were observed, whereas in AT-02-03B, no cells were observed. In the depth profile, microbial cells were observed in both the surface sample (A0, Fig. 5(c)) and the deeper sample (A15, Fig. 5(d)). There were no significant differences in the number of microbial cells between the two samples; however, the number of cells was relatively higher in the surface sample.
Discussion
Evaluation and overview of biogeochemical indicators
Table 4 shows the basic statistics and correlation matrix for the total amino acid concentrations (nmol g−1), ALPase activity (pmol min−1 g−1), CFU (g−1), temperature (°C) and precipitation (mm) for the AT02 dataset. Parameters related to amino acids, enzyme activity, CFU, temperature and precipitation showed distinct positive and negative correlations. Therefore, when the principal component loadings (principal component 1 [PC1]) were graphed, we observed strong correlations between indicators other than temperature (Fig. 6, Table 5). Among the principal component loadings, the score of principal component 2 (PC2) was not significant, providing insights into the beneficial index nature of these indicators. Notably, this trend was similar to that described in a previous report (Takano et al., Reference Takano, Mori, Kaneko, Ishikawa, Marumo and Kobayashi2006), indicating the responsiveness of subsurface microbial activity to the contribution of precipitation. In dry regions with large amounts of light, the supply of water and nutrients is a key factor in maintaining the arid biosphere. In this context, atmospheric transport of various chemical species supports primary production in the Atacama Desert, and microbial growth and the community shift due to rainfall response have also been investigated (Ji et al., Reference Ji, Greening, Vanwonterghem, Carere, Bay, Steen, Montgomery, Lines, Beardall and Van Dorst2017; Azua-Bustos et al., Reference Azua-Bustos, González-Silva, Fernández-Martínez, Arenas-Fajardo, Fonseca, Martín-Torres, Fernández-Sampedro, Fairén and Zorzano2019; Uritskiy et al., Reference Uritskiy, Getsin, Munn, Gomez-Silva, Davila, Glass, Taylor and DiRuggiero2019). Thus, the microbiological response of the samples in this study may have been more pronounced because the samples originated from extremely arid regions.

Fig. 6. Representative biogeochemical indicators and their principal component analysis (PCA) with basic statistics, correlation matrix and principal component loadings in Atacama Desert soils: (a) compilation for amino acids, ALP, CFU, temperature and precipitate (n = 5) and (b) compilation for absorbance, fluorescence, amino acids, DNA, LOI, TLE, phytane, n-C18, n-C20, n-C29, n-C31, H2O contents (n = 11). See the raw data of PCA profiles in Table 5.
Table 4. Basic statistics and correlation matrix for biological parameters in Atacama Desert soils

Table 5. Basic statistics and correlation matrix for other biochemical indicators (absorbance, fluorescence, amino acids, DNA, LOI, TLE, phytane, n-C18, n-C20, n-C29, n-C31, H2O contents) in Atacama Desert soils

North–south distribution of phosphatase activity and other parameters
Figure 3 summarizes horizontal (north–south) profile of biological and biochemical indicators and rainfall. Five sampling sites for AT02 set samples were located along the ~70°W meridian: AT02-03B was the northernmost, followed by AT02-28, -16, -23 and -22. In this region, the northern part is more arid than the southern part, and annual precipitation near AT02-03B and -28 was less than 5 mm, whereas that near AT02-22 and -23 was approximately 15 mm (Fig. 3(a); Navarro-González et al., Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003). The density of culturable heterotrophic bacteria (CFU g−1) was taken from Navarro-González et al. (Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003). In desert studies all locations with rain less than 100 or 150 mm year−1 are considered as ‘hyper-arid’ (e.g. Brandt et al., Reference Brandt, Tucker, Kariryaa, Rasmussen, Abel, Small, Chave, Rasmussen, Hiernaux, Diouf and Kergoat2020). The data in Fig. 3 show that there is a transition in biological indicators when the precipitation falls below ~25 mm year−1. The Atacama Desert is one of the only deserts in the world where the precipitation falls below this level. The results shown in Fig. 3 are consistent with previous work in the Atacama that defined these extreme hyper-arid ‘Mars-like’ soils (e.g. Navarro-González et al., Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003; Warren-Rhodes et al., Reference Warren-Rhodes, Rhodes, Pointing, Ewing, Lacap, Gomez-Silva, Amundson, Friedmann and McKay2006; Azua-Bustos et al., Reference Azua-Bustos, Caro-Lara and Vicuña2015).
The phosphatase activities in soil samples from AT02-03B, -28 and -16 were below the DL (4.6 nmol min−1 g−1), whereas that of AT02-22 was ten times larger than the DL (Fig. 3(c)). The total amino acid concentration of AT02-22 was approximately 1400 nmol g−1, which was more than 20 times higher than that of AT02-03B. Navarro-González et al. (Reference Navarro-González, Rainey, Molina, Bagaley, Hollen, de la Rosa, Small, Quinn, Grunthaner, Caceres, Gomez-Silva and McKay2003) reported that the CFU of culturable heterotrophic bacteria at AT02-03B and -28 was approximately 100 CFU g−1, whereas that at AT02-28 was approximately 106 CFU g−1 (Fig. 3(b)). Furthermore, fluorescence microscopy using SYTO 24, which stained both live and dead cells, also showed a higher density of microbial cells in AT02-22 than in AT-02-03B. Thus, these findings suggested that ALPase activity was well correlated with microbial density among the AT02 set samples.
It is important to note that light intensity and flux are also important environmental factors, especially when considering the formation of UV-induced reactive species, which promote oxidative reactions on the soil surface and affect the microbial ecosystem (Georgiou et al., Reference Georgiou, Sun, McKay, Grintzalis, Papapostolou, Zisimopoulos, Panagiotidis, Zhang, Koutsopoulou and Christidis2015).
The vertical distribution of phosphatase activity and other parameters
ALPase activity in the A and B sets of Atacama Desert soils was measured by fluorometry after extraction, and the surface samples (A0, B0) showed activity below the DL, whereas some deeper samples (A15 and A19) showed relatively high activity (>10 nmol min−1 g−1). This activity level was close to that of the AT02-23 sample, but less than that of AT02-22 (the least arid point among the five AT02 sets). There were differences between the ALPase activities of the A and B sets, which may be related to the heterogeneity of the samples.
In the case of the land core of Rikubetsu, the very surface sample showed the highest ALPase activity, total amino acid concentration and cell density. Generally, deeper samples showed lower ALPase activities, total amino acid concentrations and cell densities owing to the fact that the land surface biosphere depends on photosynthesis (Takano et al., Reference Takano, Mori, Kaneko, Ishikawa, Marumo and Kobayashi2006). By contrast, the submarine core of the Suiyo Seamount showed maximum ALPase (and ACPase) activities and amino acid concentrations in the deeper part of the core samples. This suggested that extant microbes may have lived in the subsurface of submarine hydrothermal systems, depending on chemosynthesis (Takano et al., Reference Takano, Edazawa, Kobayashi, Urabe and Marumo2005).
Although no archaeal 16S rDNA was detected after amplification by polymerase chain reaction, bacterial 16S rDNA was found from the surface to a depth of 3 cm and at depths of 11–13 and 19–21 cm (Nauny, Reference Nauny2019). Total DNA amplification by ddMDA (Rhee et al., Reference Rhee, Light, Meagher and Singh2016; Sidore et al., Reference Sidore, Lan, Lim and Abate2016) for the 15 cm deep sample, followed by Illumina sequencing, revealed marker genes primarily associated with bacilli and nitrifying bacteria. However, the low number of reads obtained did not permit definitive conclusions to be reached (Nauny, Reference Nauny2019).
For Atacama Desert A-set samples, the ALPase activity of the surface sample (A0) was below the DL, whereas the samples collected from a greater depth (A15, A19) showed relatively high ALPase activities. The surface sample (A0) contained a high concentration of amino acids and a relatively high density of cells observed by fluorescence microscopy as well as substantial concentrations of DNA and phytane (Nauny, Reference Nauny2019). These results could be related to the observation that prolonged survival of cells (and enzymes) at the surface is difficult owing to the high flux of solar UV and extreme aridity, although photosynthesis did occur at the surface when water was temporarily supplied. Organic compounds could remain at the surface, and some may move to greater depths, thereby feeding subsurface extant organisms that produce enzymes.
Groundwater and the moisture contribution from its sub-surface veins can be a determinant of microbial activity on long timescales (~100 year scale) (Gamboa et al., Reference Gamboa, Godfrey, Herrera, Custodio and Soler2019). There are reports detecting the growth of microbial communities after very rare rainfall events (1–10 year scale) (Schulze-Makuch et al., Reference Schulze-Makuch, Wagner, Kounaves, Mangelsdorf, Devine, de Vera, Schmitt-Kopplin, Grossart, Parro, Kaupenjohann, Galy, Schneider, Airo, Frösler, Davila, Arens, Cáceres, Cornejo, Carrizo, Dartnell, DiRuggiero, Flury, Ganzert, Gessner, Grathwohl, Guan, Heinz, Hess, Keppler, Maus, McKay, Meckenstock, Montgomery, Oberlin, Probst, Sáenz, Sattler, Schirmack, Sephton, Schloter, Uhl, Valenzuela, Vestergaard, Wörmer and Zamorano2018). Therefore, we consider that water-soluble organic matter, which supports microbial growth, is correlated with the total amount of rainfall and the degree of solubility of sedimentary matrix.
Phosphatase activity as a tool for detecting life
We measured ALPase activity in extreme environments such as rock samples in the deep sea hydrothermal subvents of the Suiyo Seamount, Izu-Bonin Arc in the Pacific Ocean (Takano et al., Reference Takano, Edazawa, Kobayashi, Urabe and Marumo2005) and Antarctica soils near the Showa Base (Kobayashi et al., Reference Kobayashi, Mita, Kebukawa, Nakagawa, Kaneko, Obayashi, Sato, Yokoo, Minematsu, Fukuda, Oguri, Yoda, Yoshida, Kanda, Imai, Yano, Hashimoto, Yokobori and Yamagishi2021a, Reference Kobayashi, Sato, Ito, Nakamoto, Kurizuka, Takano, Obayashi, Kaneko, Kebukawa, Ogawa and Yoshida2021b). We also analysed ALPase activity in soil samples from Japan, including core samples from Rikubetsu, Hokkaido (Takano et al., Reference Takano, Mori, Kaneko, Ishikawa, Marumo and Kobayashi2006) and soil samples from the YNU campus. Comparing the current results with previous studies on ALPase activities in geological samples, we identified good positive correlations among ALPase activity, total amino acid concentrations and cell density. Such positive correlations were observed not only for the horizontal distributions in the Atacama AT02 set (this study) and Antarctica samples (Ishikawa et al., Reference Ishikawa, Aoki, Obayashi, Kebukawa, Kaneko, Mita, Ogawa, Navarro-González and Kobayashi2015), but also for the vertical distributions in land core samples at Rikubetsu, Hokkaido, Japan (Takano et al., Reference Takano, Mori, Kaneko, Ishikawa, Marumo and Kobayashi2006) and ocean floor core samples at the Suiyo submarine hydrothermal system (Takano et al., Reference Takano, Edazawa, Kobayashi, Urabe and Marumo2005). In addition, the dynamic range of ALP activity was large. Soil samples in ordinary environments, such as the YNU campus, showed quite high ALPase activity (>10 nmol min−1 g−1) (Kobayashi et al., Reference Kobayashi, Sato, Ito, Nakamoto, Kurizuka, Takano, Obayashi, Kaneko, Kebukawa, Ogawa and Yoshida2021b), whereas samples in extreme environments, such as the most arid part of the Atacama Desert, showed quite low ALPase activity (on the order of 1 pmol min−1 g−1 or less). Thus, ALPase activity may be a good indicator of microbial activity in extreme environments.
Many researchers have discussed the possibility of life on other solar system bodies, including Mars (Forget and Hauber, Reference Forget and Hauber2015), Europa (Encrenaz, Reference Encrenaz2015a) and Enceladus (Encrenaz, Reference Encrenaz, Gargaud, Amils, Quintanilla, Cleaves, Irvine, Pinti and Viso2015b). Mars has been a major target for detection of life, and several missions have been conducted since the Viking Mission in 1976 (Encrenaz, Reference Encrenaz2015c). In the Viking Mission, three ‘Viking Biological Experiments’ were performed, together with organic analysis by thermal volatilization-GC/MS (Biemann et al., Reference Biemann, Oro, Toulmin, Orgel, Nier, Anderson, Simmonds, Flory, Diaz, Rushneck, Biller and Lafleur1977). The former was used to measure the metabolic activity of terrestrial-type organisms using gas exchange, label release and pyrolysis release experiments; however, no conclusive results on the presence of life on Mars were obtained (Klein, Reference Klein1978). The lack of conclusive results could be related to the low sensitivity of the instruments used (Navarro-González et al., Reference Navarro-González, Navarro, de la Rosa, Iniguez, Molina, Miranda, Morales, Cienfuegos, Coll, Raulin, Amils and McKay2006) and problems in the selection of sampling sites because Mars surface soil is irradiated with harsh solar UV light. In addition, we do not know whether the methods used are suitable for possible Martian organisms, even if they are suitable for terrestrial organisms.
DNA analysis is a sensitive technique for the detection of terrestrial microbes. However, for detection of extraterrestrial life (e.g. Martian life), we do not know whether Martian organisms will harbour DNA similar to that of terrestrial organisms. Therefore, if different types of genetic molecules are used by extraterrestrial organisms, DNA analysis may not be effective.
Amino acids are more promising target molecules because amino acids are easily available in various extraterrestrial environments, including carbonaceous chondrites (Glavin et al., Reference Glavin, Burton, Elsila, Aponte and Dworkin2019), and amino acids may be used by extraterrestrial organisms. Although abiotic amino acids can be found in extraterrestrial bodies, it is possible to discriminate these amino acids from amino acids of biological origin. First, life systems could use a limited repertoire of amino acids; the standard genetic code of terrestrial organisms uses only 20 protein amino acids, including amino acids with complex residues (such as histidine and tryptophan). Although more types of abiotically formed amino acids exist, most have relatively simple residues (e.g. aminobutyric acids). Second, amino acids (except for glycine and a few others) have enantiomers, and organisms use only one (d- or l-) enantiomers for their biocatalysts. Abiotic amino acids are racemic. Thus, if we find racemic mixtures of amino acids in Martian soil, they may have an abiotic origin; however, if there are d-rich amino acids present, we can expect to find Martian life with an origin different from ours.
Fluorescence microscopy, which detects organic compounds stained with fluorescent pigments, is another promising technique for detecting extant and extinct microbes. The advantage of this technique is that it is possible to detect a wide range of organic compounds with various types of pigments and visualize their morphological characteristics at high resolution. Yamagishi et al. (Reference Yamagishi, Satoh, Miyakawa, Yoshimura, Sasaki, Kobayashi, Kebukawa, Yabuta, Mita, Imai, Naganuma, Fujita and Usui2018) proposed a life detection microscope that detects organic compounds, such as proteins, membrane structures and catalytic activities. These features are the fundamental framework of life and would be useful as biosignatures for detecting life on Mars as well as on Earth.
Perspectives
In this study, we proposed using phosphatases as targets for the detection of extant life. Phosphatases are enzymes that hydrolyse phosphate esters, including essential biomolecules, such as nucleic acids, adenosine triphosphate (ATP) and phospholipids. These enzymes are expected to be essential for all terrestrial organisms. Moreover, phosphates have been detected in Martian soil (Adcock et al., Reference Adcock, Hausrath and Forster2013). We do not know what types of organisms are present on Mars if they were ever present, but we expect that Martian organisms would use energy-rich phosphate esters in their biological systems. Thus, Martian organisms may have catalysts that hydrolyse phosphate esters. Other hydrolytic enzymes include peptidases and carboxylic ester hydrolases (esterase). The former hydrolyses the enzyme itself, limiting its survivability in the environment. Hydrolysis of the P–O bond is more difficult than that of the C–O bond (Zhang et al., Reference Zhang, Xie, Li, Shang and Wang2008), and the hydrolysis of phosphate esters is difficult without catalysts. Accordingly, we believe that phosphatases may be better targets than peptidases and esterases for detection in extreme environments.
One benefit of using enzymatic activity assays in natural environments is that a single catalytic molecule (enzyme) can facilitate the generation of large amounts of products when appropriate substrates are added. Thus, we can detect enzymes after amplification by catalytic reactions. Detection of active ALPase activity in natural water has been conducted since the 1960s, and identification of ALPase as an active metalloenzyme in seawater was reported in the 1980s (Kobayashi et al., Reference Kobayashi, Matsui, Haraguchi and Fuwa1983). ALPase activity in soil has been studied mainly from the perspective of agriculture (Tabatabai, Reference Tabatabai, Page, Miller and Keeney1982). Even in the recently discovered common ancestor of prokaryotes and eukaryotes, ATP is considered to be an essential compound for energy acquisition and homoeostasis (Spang et al., Reference Spang, Saw, Jørgensen, Zaremba-Niedzwiedzka, Martijn, Lind, van Eijk, Schleper, Guy and Ettema2015; Imachi et al., Reference Imachi, Nobu, Nakahara, Morono, Ogawara, Takaki, Takano, Uematsu, Ikuta, Ito, Matsui, Miyazaki, Murata, Saito, Sakai, Song, Tasumi, Yamanaka, Yamaguchi, Kamagata, Tamaki and Takai2020). We measured ALPase and/or ACPase activity in terrestrial extreme environments, such as core samples and chimney samples in submarine hydrothermal systems and Antarctic soils (Takano et al., Reference Takano, Edazawa, Kobayashi, Urabe and Marumo2005; Kobayashi et al., Reference Kobayashi, Sato, Ito, Nakamoto, Kurizuka, Takano, Obayashi, Kaneko, Kebukawa, Ogawa and Yoshida2021b), and found positive correlations with other parameters, such as amino acid concentration and cell density. These results, together with the current findings, suggest that phosphatase activity could be used to evaluate extant microbial activity in terrestrial environments.
In the current study, we used soil samples from the Atacama Desert; these soil samples had been stored at ambient temperature for years. Thus, the activity values themselves may have changed during storage, although the relative trends in activity seemed to be preserved.
Mars sample return missions are now under consideration by National Aeronautics and Space Administration (NASA) and European Space Agency, and some Mars soil samples have already been cached by the Perseverance Rover during the NASA Mars 2020 Mission in September 2021 (Michalski et al., Reference Michalski, Onstott, Mojzsis, Mustard, Chan, Niles and Johnson2018; Farley et al., Reference Farley, Williford, Stack, Bhartia, Chen, de la Torre, Hand, Goreva, Herd and Hueso2020; Bell et al., Reference Bell, Maki, Mehall, Ravine, Caplinger, Bailey, Brylow, Schaffner, Kinch and Madsen2021). International collaboration between research institutes and the research community will be important for tackling the challenging mission of Martian sample returns (Fujimoto and Tasker, Reference Fujimoto and Tasker2019; Hyodo and Usui, Reference Hyodo and Usui2021). Moreover, it is necessary to demonstrate practical approaches and the establishment of a system for exploration of Martian systems in Japan (Usui et al., Reference Usui, Bajo, Fujiya, Furukawa, Koike, Miura, Sugahara, Tachibana, Takano and Kuramoto2020; Fujiya et al., Reference Fujiya, Furukawa, Sugahara, Koike, Bajo, Chabot, Miura, Moynier, Russell, Tachibana, Takano, Usui and Zolensky2021). After returning the samples to Earth, they would be kept at ambient temperature for years. Our results suggested that such samples could be used to evaluate microbial activity by assay of phosphatase activity. However, it may also be interesting to measure phosphatase activity on site.
Acknowledgements
We greatly appreciate the assistance of the late Prof. Rafael Navarro-González for providing the Atacama Desert soil samples (AT02 set). We also thank Prof. Vernon Phoenix, Dr Nick Thomas and Dr Rory Porteous for their help in collecting the samples at the site near San Pedro de Atacama and Prof. Jaime Toney for providing access to the analytical equipment needed for the analysis of hydrocarbon biomarkers. This work was partly supported by JSPS KAKENHI Grants-in-Aid for Scientific Research (Grant Nos. 19K21895, 19H01955 and 20H02014), the Sir Alwyn Williams Postgraduate Scholarship (University of Glasgow), and the Michael Golden Postgraduate Scholarship (University of Glasgow). We would like to thank Editage (www.editage.com) for English language editing.
Conflicts of interest
The authors have no conflicts of interest directly relevant to the content of this article.
















