INTRODUCTION
Q fever is a worldwide zoonosis caused by Coxiella burnetii, a Gram-negative bacterium that can survive for a prolonged time in the environment in a spore-like stage. Until 2007, Q fever was a rarely reported notifiable disease in The Netherlands, with 5–20 cases presenting annually and without seasonal trend. However, underdiagnosis and consequently underreporting were suspected as very few laboratories consistently tested pneumonia cases for Coxiella infection. Q fever emerged in 2007, followed by subsequently larger epidemics in 2008 and 2009 [Reference Schimmer1, Reference Schimmer2], which were probably related to intensive dairy goat farming [Reference Karagiannis3–Reference van der Hoek5]. There is some evidence for a few retrospectively identified clusters of hospital admissions for respiratory illness in 2005 and 2006 that might have been caused by Q fever [Reference van den Wijngaard6]. A seroprevalence study in the 1980s by Richardus et al. found very high prevalence estimates among blood donors and certain risk groups ranging from 15% to 65% [Reference Richardus7].
A recent community-based study from the USA showed a seroprevalence of 3·1% [Reference Anderson8], while 4% was found in a blood donor study in southern France in the late 1980s [Reference Tissot9] and 3·6% in Japanese blood donors in the late 1990s [Reference Abe10]. Higher seroprevalences were observed in other western countries: in Northern Ireland, 12·8% in sera collected in 1986–1987 [Reference McCaughey11], 7·9% in rural Wales in the mid-1990s [Reference Davies12], similar to the 7·5% found in southwestern Germany in 2008–2009 [13]. In northwestern Russia a marked increase in seroprevalence in a healthy population was observed, from 1·1% in 1993 to 11·1% in 2003 [Reference Tokarevich14]. In the UK the seroprevalence was 11·2% in an occupational control cohort in the late 1980s [Reference Thomas15]. Mediterranean countries report even higher seroprevalence levels: in Spain, 23·1% in blood donors in Albacete [Reference Bartolome16], 48·6% in Eastern Cantabria [Reference Pascual-Velasco17], and 15·3% in the Barcelona region [Reference Cardenosa18], while 13·5% was found in north Turkey [Reference Gozalan19] and 52·7% in Cyprus [Reference Psaroulaki20]. A more detailed overview of human seroprevalence studies done in European countries was recently presented in reports by the European Food Safety Authority and the European Centre for Disease Prevention and Control [13, 21]. To understand the baseline epidemiology of Q fever in The Netherlands prior to the epidemics, available serum samples from the general population, collected between February 2006 and June 2007 for evaluation by the Dutch National Immunization Programme [Reference van der Klis22] were used to obtain a seroprevalence estimate of the general Dutch population just before the recent epidemics. In addition, risk factors for seropositivity were identified.
METHODS AND MATERIALS
Study population and questionnaire data
A large population-based seroprevalence study, called the PIENTER project, was carried out primarily for the evaluation of the Dutch National Immunization Programme. Eight municipalities within each of five geographical Dutch regions (Northeast, Central, Northwest, Southwest and Southeast) were sampled with probabilities proportional to their population sizes (Fig. 1). In addition, there was an oversampling of non-Western migrants in 12 of the above municipalities. Data collection started in February 2006 and was finalized in June 2007. The study design and details of the data collection in the PIENTER project have been published [Reference van der Klis22]. The participants donated blood and completed a questionnaire, one version for children aged ⩽14 years, and another version for persons aged ⩾15 years. The questionnaire covered, among others, data on demographics, health perception and diseases, and activities possibly related to infectious diseases (e.g. travelling, profession, food habits, gardening).

Fig. 1. Selected municipalities in the seroprevalence study. Light grey municipalities are included in the nationwide sample (n=40). Dark grey municipalities are low immunization municipalities that are included in the PIENTER project but not in the present study.
Laboratory analysis
Stored sera of the nationwide sample of the PIENTER project were screened for the presence of C. burnetii IgG phase-2-specific antibodies by a commercial enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Serion ELISA classic, Virion/Serion, Germany). A positive ELISA test was defined according to the manufacturer as a concentration of ⩾30 U/ml, and a borderline-positive ELISA was defined as a concentration between 20 U/ml and 30 U/ml. We considered ELISA-borderline-positive and ELISA-positive samples as a positive test result. Immunofluorescence assay (IFA) is considered the reference method for diagnostic screening of C. burnetii and a lower sensitivity of the ELISA test compared to IFA was anticipated, with a similar specificity [Reference Blaauw23]. ELISA-borderline-positive and ELISA-positive samples were subsequently confirmed by IFA (Focus Diagnostics, USA) for IgG phase-1 and phase-2 specific antibodies using a 1:32 and 1:128 dilution. In addition, a random subset of ELISA-negative samples (n=504) was screened for IgG phase-1 and phase-2 specific antibodies by IFA at an initial dilution of 1:32 to estimate the proportion of false-negative test results. A positive IFA sample was defined as a sample with an IFA IgG phase-2 titre of ⩾32 (either or not combined with positive IgG phase 1 of ⩾1:32). By extrapolation we additionally estimated the national seroprevalence adjusting for the proportion of false-positive and false-negative results using the IFA as the gold standard.
Statistical analysis
The national sample that was screened by ELISA was used to estimate the seroprevalence of C. burnetii IgG antibodies representative of the general population of The Netherlands. Oversampled migrant participants were included to allow studying differences in seroprevalence by country of origin in more detail. Participants from municipalities with low immunization coverage were excluded from the seroprevalence estimation. To produce national estimates, the weighted frequencies were averaged over the 40 participating municipalities in eight different provinces. To avoid missing possible infections, borderline laboratory results were considered positive in the statistical analysis. Weights were calculated proportional to the reference population (Dutch population, 1 January 2007) taking sex, age, ethnicity and degree of urbanization (>2500 vs.<2500 addresses per km2 in the neighbourhood) into account. We adjusted for the two-stage cluster sampling by taking into account the strata (regions) and clusters (municipalities). The weighted overall and age-, sex- and region-specific seroprevalences were estimated for the Dutch population.
Based on the ELISA results of the complete sample we performed the further risk factor analysis. Univariate odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for selected variables possibly relevant for C. burnetii exposure, i.e. geographical region, degree of urbanization, country of birth, religion, educational level, household income, number of persons in the household, consumption of raw meat and unwashed vegetables, being a vegetarian, gardening, playing in a sandbox (only children aged <15 years), keeping a pet (past 5 years), keeping livestock (past 5 years), tick bites (past 5 years), and occupational contact with animals (past 5 years). Information from Statistics Netherlands was collected on goat, sheep and cattle density in the participating municipalities. Variables which reached a significance level of P<0·20 in the univariate analysis were included in a multivariate logistic regression model. Multivariate analysis was done by a General Logistic Mixed Model with municipality added as a block effect. Selection of model terms was done by backwards elimination to determine independent risk factors for seropositivity using P<0·05 as significance level (R, version 2.10; R Foundation, Austria).
RESULTS
Overall seroprevalence
The 5654 stored sera available from the nationwide sample of the PIENTER project were screened for the presence of C. burnetii IgG phase 2 by ELISA. Antibodies were detected in sera of 85 study participants, of which 47 had borderline levels, resulting in a weighted rough seroprevalence of 1·5%. Based on ELISA test results only, the weighted seroprevalence was higher in males than in females (1·65% vs. 1·28%). Seropositivity increased with age from 0·48% (95% CI 0·00–0·96) in the 0–4 years age group to 2·30% (95% CI 1·46–3·15) in the 60–79 years age group (Fig. 2). The seroprevalence for those born abroad was slightly higher than for persons born in The Netherlands. Persons with low educational level (i.e. no education or elementary-school level) and those living in a household with a very low monthly income (⩽€850) had the highest seroprevalence. No regional differences in seroprevalence were observed (Table 1).
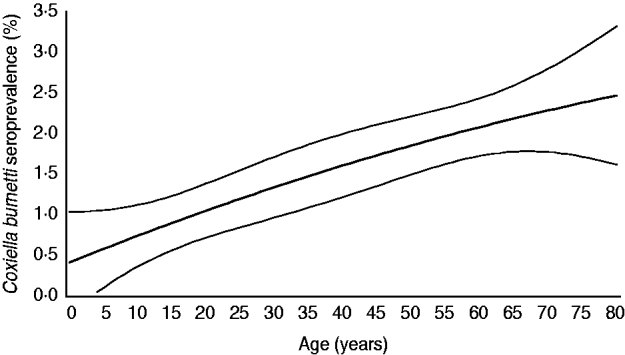
Fig. 2. Age-specific weighted seroprevalence of C. burnetii IgG antibodies in a representative sample of the Dutch population aged 0–79 years (n=5654), PIENTER project, 2006–2007. Prevalence rates per age group were estimated using a linear model with a spline function for age (i.e. second-degree polynomial).
Table 1. WeightedFootnote * seroprevalence of Coxiella burnetii IgG phase-2 antibodies (Serion ELISA, IgG phase 2) in the Dutch population aged 0–79 years (n=5654), PIENTER 2 project, The Netherlands, February 2006–June 2007
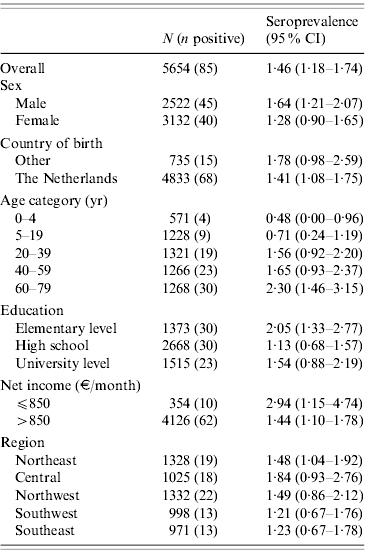
CI, Confidence interval.
* Weighted for sex, age, ethnicity and degree of urbanization.
The 85 ELISA-positive samples were subsequently screened by IFA. Fifteen samples (17·6%) turned out IFA-negative. Of the remaining 70 IFA-positive sera, 13 had a low-level IgG phase-2 titre ranging between 1:32 and <1:128, while 57 sera had a titre of ⩾1:128. In the IFA-tested subset of 504 ELISA-negative samples, six samples (1·2%) had low-level IgG phase-2 titres ranging from 1:32 (n=5) to 1:128 (n=1). Correcting the ELISA results with the IFA results, the adjusted overall seroprevalence estimate was 2·4% (70 true positives and 66 false negatives divided by the total number of 5654 study participants).
Determinants of C. burnetii seropositivity
Based on ELISA test results only, risk factors significant at the P<0·20 level were increasing age, being male, marital status, not being born in The Netherlands, net income, religion, contact with cats (past 12 months), kept livestock (past 5 years), frequency of eating raw meat, and occupational contact with animals (Table 2). No significant association was found between seropositivity and goat, sheep or cattle density in the 40 municipalities. In the multivariate logistic regression model, seropositivity was found to be associated with keeping ruminants (past 5 years) with or without other farm animals [adjusted OR (aOR) 8·2, 95% CI 3·3–20·8 and aOR 3·8, 95% CI, 1·1–13·1, respectively], being born in Turkey (aOR 5·1, CI 2·1–12·5), and increasing age (aOR 5·4, 95% CI 1·4–20·4 for the 15–39 years age group; aOR 6·0, 95% CI 1·6–22·9 for the 40–59 years age group, and aOR 6·6, 95% CI 1·8–24·0 for the oldest age group of 60–79 years compared to the reference age group of 0–14 years), with borderline significance being observed for occupational contact with animals. Other factors contributing to the multivariate model were low monthly net income (⩽€850), and being male, although these did not reach statistical significance (Table 3). In the multivariate analysis, keeping pets seemed to play a protective role (aOR 0·53, 95% CI 0·32–0·87). This variable was included based on the significance of keeping rabbits as a variable in the univariate analysis (P=0·18).
Table 2. Coxiella burnetii IgG antibodies (%) in study population (n=5654) and adjustedFootnote * univariate analysis of factors associated with seropositivity to Coxiella burnetii PIENTER 2 project, The Netherlands, February 2006–June 2007
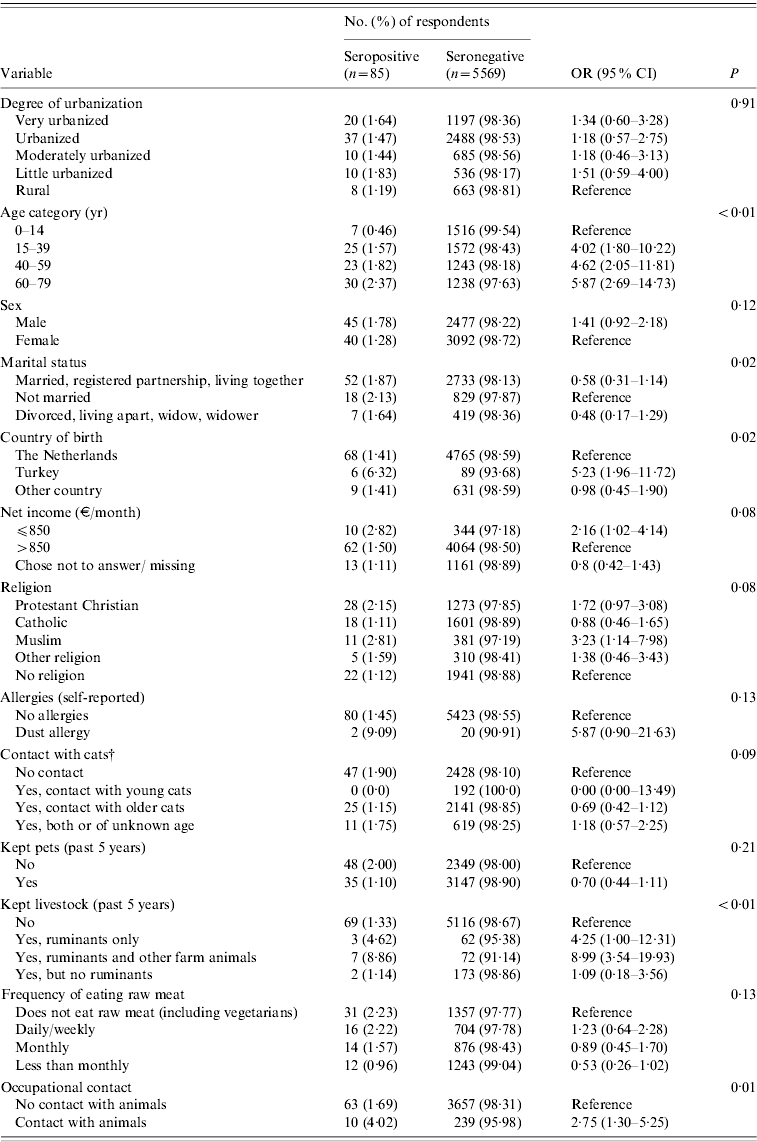
OR, Odds ratio; CI, confidence interval.
* Adjusted for sex, age and ethnicity.
† Adjusted for sex and age.
Table 3. Final multivariable model for risk factors associated with seropositivity to Coxiella burnetii, PIENTER 2 project, The Netherlands, February 2006–June 2007

OR, Odds ratio; CI, confidence interval.
DISCUSSION
The overall Q fever seroprevalence, based on this representative sample of the Dutch population from 2006 to 2007 is relatively low. The overall estimate of 1·5%, and IFA-corrected estimate of 2·4% reflect the seroprevalence in a pre-epidemic period as the tested sera were collected in 2006 and the first half 2007, just before the major Q fever epidemics in 2007–2009. The a-priori expected C. burnetii IgG phase-2 seroprevalence was estimated to be around 4% based on screening of serum samples of pregnant women outside the epidemic area carried out in 2007 by IFA [Reference Meekelenkamp24]. For comparison, since the epidemic rise in 2007, seropositivity rates of the general population have probably increased, as shown by a 24% seropositivity rate in the population of the Q fever epicentre of 2007 [Reference Karagiannis3]. In the 1980s, the seroprevalence for C. burnetii in blood donors was studied in different regions of The Netherlands using an IFA developed in-house. Using a low cut-off for positivity in this IFA (screening dilution of 1:16), a high seroprevalence was found, ranging between 15% and 65%, depending on region, sex and age [Reference Richardus7]. Unfortunately, no information was available on exact titres measured. It is unknown how current IFAs and ELISAs relate to this previously used in-house IFA, as neither the test nor the sera are available at present. The high seroprevalences found in the 1980s led to the suggestion that C. burnetii may have been far more prevalent for a considerable time than the number of notifications suggested, and that before 2007 clinical cases were not detected because of the aspecific presentation of most symptomatic Q fever infections as well as a large proportion of asymptomatic and subclinical infections. Few laboratories had serology for C. burnetii in their standard panel for pneumonia patients before national awareness was raised by the Q fever outbreaks.
Several other population studies observed seroprevalences between 2% and 4%, similar to the estimated seroprevalence found in our study [Reference Anderson8–Reference Abe10]. However, comparisons of seroprevalence estimates should be made with caution as different study populations, different serological assays and criteria for positivity are used. The latter is especially important in population surveys, while comparisons of assays in The Netherlands for diagnoses of acute cases demonstrate a high concordance of test results. Increasing age, being born in Turkey, keeping ruminants and to a lesser extent occupational contact with animals were identified as independent risk factors for Q fever seropositivity in this study. The increase in seroprevalence with age is consistent with findings from other seroprevalence studies [Reference Anderson8, Reference Whitney25]; however, the range of age-specific seroprevalences in our study is smaller. In accord with other studies [Reference Anderson8, Reference McCaughey11], we showed that young children and adolescents have a very low seroprevalence, which is supported by a very small proportion of children in the notified clinical cases in our routine Q fever surveillance. Keeping ruminants was an independent risk factor in our study as shown also in other studies [Reference Anderson8, Reference McCaughey11, Reference Whitney25]. Turkey as country of birth was an independent risk factor. In accord with this observation, recent studies in Turkey indicate that Q fever is highly prevalent: prevalence of C. burnetii anti-phase-2 IgG was 13·5% in healthy subjects in the west Black Sea region [Reference Gozalan19] and 32·3% by ELISA in blood donors from Ankara [Reference Kilic26].
In southwestern Germany, the seroprevalence showed a linear increase with sheep density in different municipalities [13]. In our study we did not find an association between goat, sheep and cattle density, and seropositivity in the 40 municipalities, suggesting that the dominant role of the dairy goats in the epidemiology of Q fever in The Netherlands is a relatively recent occurrence. Occupational animal contact was not a strong independent predictor in the multivariate analysis as there was a large overlap with those keeping ruminants. The kind of occupational animal contact was not further specified in the questionnaire which also included contact with non-ruminant species. Livestock farmers, veterinarians, slaughterhouse workers and animal laboratory staff are known occupational risk groups for Q fever, as exemplified by higher seroprevalence levels than the general population [Reference McCaughey11, Reference Whitney25]. In order to investigate the actual risk for professionals dealing with livestock, separate seroprevalence studies in livestock farmers, veterinarians and persons actively involved in culling activities at infected dairy farms are currently performed in The Netherlands. Although poverty was a risk factor in a seroprevalence study from the USA [Reference Anderson8], we cannot fully support this observation as the category with the lowest monthly net income (⩽€850) was not statistically significant in the multivariate model. This is possibly caused by a large proportion in this income group that chose not to disclose their monthly income. Males generally have higher seroprevalence than females, which is often explained by occupational contact. In our study, being male is a possible confounding factor, at least partially explained by occupational exposure to animals. In laboratory surveillance studies, males are more likely to be diagnosed as they more often develop symptoms, as shown in our routine laboratory surveillance. Others suggested that this is explained by sex hormones that may control the host's immune response to a C. burnetii infection [Reference Leone27], resulting in gender differences in clinical attack rates. In the case-control study performed during the first epidemic season in 2007 in the main cluster area in the south of The Netherlands we observed a higher proportion of males developing symptoms, while seroprevalence due to the airborne exposure was equally distributed among males and females [Reference Karagiannis3]. It is unclear why keeping pets turned out to be a protective factor. Other studies did not find any association with keeping pets, or in contrast, found pets to be a risk factor, such as in a recent study from Germany where pet rats were surprisingly found as a risk factor (S. Brockmann, unpublished observations). Moreover, several Q fever outbreaks in the USA and Canada were associated with parturient cats and dogs [Reference Pinsky28, Reference Buhariwalla, Cann and Marrie29].
Our study has several limitations as the exposure information collected in the questionnaire was mainly focused on vaccine-preventable infections instead of zoonotic infections. Information on exposures does not necessarily relate to the relevant time period as we do not know at what moment the actual infection with C. burnetii occurred in those testing serologically positive. Close contact with animals other than through occupation or ownership and proximity to animal stables or farms could not be studied. Further, the questionnaire did not include information on exact occupation. Possible associations between history of miscarriage or stillbirth in female participants and seropositivity could not be studied, as no information on reproductive history was collected. Our study demonstrated a low prevalence of C. burnetii infection in the period 2006–2007, around 1·5% based on ELISA. The Serion IgG ELISA has shown a suboptimal sensitivity of 58% [Reference Blaauw23], which at least led to an underestimate of the national seroprevalence, which was adjusted in retrospect although based on only a subset of seronegatives. We consider it unlikely that misclassification of serological status was related to the exposure variable and has systematically biased our risk-factor analysis. However, risk factors will be flawed because of dilution of the effect by probable random misclassification. A two-step screening approach, using ELISA as a screening tool and IFA for confirmation, was used in a recent seroprevalence study from the USA [Reference Anderson8]. We confirmed that using the same approach, with only the 70 IFA-confirmed ELISA-positive samples in the statistical analysis instead of 85 ELISA-positive samples, the same risk factors were observed as in the current study (data not shown). A separate risk-factor analysis only based on the subset of IFA-tested samples was not possible due to low seroprevalence.
Further modelling studies are necessary to study the relationship between IFA and ELISA and improve or replace the cut-off for ELISA as a binomial outcome to a probability, which will improve the multivariate analysis of the risk factors, but confidence intervals will remain large. Municipalities in the highest-incidence Q fever areas in 2007–2010 were not part of the study sample. In 2007 a much larger part of Noord-Brabant province was already affected. Several other municipalities in this early-affected province were included in our study sample. However, adjacent municipalities did not show a higher seroprevalence nor was a relationship of seroprevalence with goat density observed.
This study will serve as a baseline for future population-based seroprevalence studies performed after the emergence of Q fever. Prior to the recent Q fever epidemics the overall seroprevalence of C. burnetii infection in The Netherlands was relatively low and it was not associated with goat, sheep or cattle density, but merely with keeping ruminants or occupational contact with animals. This supports the hypothesis that The Netherlands has been confronted with a newly emerging Q fever problem in the general population since the spring of 2007. Before the start of the epidemics, high-risk groups for Q fever were individuals with animal contact, including occupational exposure, and Turkish immigrants, probably infected in their home country. Further modelling studies are needed to study the relationship between IFA and ELISA in order to improve comparison between seroprevalence studies.
ACKNOWLEDGEMENTS
We thank the participating municipal health services and the municipalities involved in this study, the PIENTER project team and all participants for their contribution. We also thank Wim van der Hoek and Tineke Herremans (CIb/RIVM) for their useful comments on the manuscript.
DECLARATION OF INTEREST
None.









