Exposure to chronic early trauma carries negative effects on multiple domains of adaptation (Feldman & Vengrober, Reference Feldman and Vengrober2011; Halevi, Djalovski, Vengrober, & Feldman, Reference Halevi, Djalovski, Vengrober and Feldman2016; Scheeringa, Zeanah, Myers, & Putnam, Reference Scheeringa, Zeanah, Myers and Putnam2005) and these impact overall functioning across childhood and up until adulthood (Carr, Martins, Stingel, Lemgruber, & Juruena, Reference Carr, Martins, Stingel, Lemgruber and Juruena2013; Enoch, Reference Enoch2011; Pechtel & Pizzagalli, Reference Pechtel and Pizzagalli2011). To date, most studies on the long-term impact of early trauma have focused on psychopathology and findings consistently demonstrate marked increases in internalizing and externalizing symptoms, prevalence of psychiatric disorders, and posttraumatic symptoms (Feldman & Vengrober, Reference Feldman and Vengrober2011; Heleniak, Jenness, Vander Stoep, McCauley, & McLaughlin, Reference Heleniak, Jenness, Vander Stoep, McCauley and McLaughlin2016; Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013; Joshi & O'Donnell, Reference Joshi and O'Donnell2003; Pérez-Fuentes et al., Reference Pérez-Fuentes, Olfson, Villegas, Morcillo, Wang and Blanco2013; Yehuda et al., Reference Yehuda, Hoge, McFarlane, Vermetten, Lanius, Nievergelt and Hyman2015). Yet, in the context of chronic early trauma, which leads to excessive activation of the stress response across extended spans of brain maturation, the consolidation of a distinct psychiatric disorder likely reflects an endpoint of impairments in cognitive, social, and emotional regulatory support systems (Shonkoff, Reference Shonkoff2016). Consistent with this view, it has been suggested that the study of psychopathology following early trauma should be complemented by disseminating the global posttraumatic symptomatology into its distinct clusters (Sullivan, Fehon, Andres-Hyman, Lipschitz, & Grilo, Reference Sullivan, Fehon, Andres-Hyman, Lipschitz and Grilo2006; Tursich et al., Reference Tursich, Ros, Frewen, Kluetsch, Calhoun and Lanius2015) and assessing the maturation of regulatory functions across domains (Cicchetti, Reference Cicchetti2013; Feldman, Reference Feldman2015a).
Conceptual models on risk and resilience underscore the need to specify modular systems and functional processes that underpin well-being (Luthar, Cicchetti, & Becker, Reference Luthar, Cicchetti and Becker2000; Masten, Reference Masten2007; Rutter, Reference Rutter2013); for instance, attachment and caregiving, self-regulation, and allostasis and stress management are considered core components of resilience following adversity (Masten, Reference Masten2007; Masten & Narayan, Reference Masten and Narayan2012; Southwick, Bonanno, Masten, Panter-Brick, & Yehuda, Reference Southwick, Bonanno, Masten, Panter-Brick and Yehuda2014). However, the pathways by which risk and protective factors operate across childhood to shape the maturation of specific regulatory functions are unclear and require a longitudinal approach (Rutter, Reference Rutter2013). Longitudinal studies have shown that the quality of parenting is a key predictor of psychopathology following early trauma (Betancourt, McBain, Newnham, & Brennan, Reference Betancourt, McBain, Newnham and Brennan2015; Halevi et al., Reference Halevi, Djalovski, Vengrober and Feldman2016; Koenen, Moffitt, Poulton, Martin, & Caspi, Reference Koenen, Moffitt, Poulton, Martin and Caspi2007; Scheeringa & Zeanah, Reference Scheeringa and Zeanah2001; Yirmiya, Djalovski, Motsan, Zagoory-Sharon, & Feldman, Reference Yirmiya, Djalovski, Motsan, Zagoory-Sharon and Feldman2018). Still, while authors have suggested that resilience is not defined by a mere lack of psychopathology but also indexes systems that enable plasticity, flexibility, and adaptation (Feldman, Reference Feldman2020; Southwick et al., Reference Southwick, Bonanno, Masten, Panter-Brick and Yehuda2014), very few studies focused on specific regulatory systems that support adaptive functioning.
In the current study, we targeted two key regulatory functions that sustain adaptive development; emotion recognition (ER) and executive functions (EF) in youth exposed to chronic early trauma since birth. We utilized a unique cohort of war-exposed children who were followed from early childhood to early adolescence. To specify pathways of risk and resilience, we focused on the avoidance cluster of posttraumatic stress disorder (PTSD) and on mother–child reciprocity in early childhood as precursors of regulatory outcomes. In addition, we tested the role of two stress biomarkers, cortisol (CT) and respiratory sinus arrhythmia (RSA), in shaping pathways to regulatory outcomes. Overall, our goal was to illuminate potential longitudinal pathways that enhance or buffer the effects of trauma on the child's regulatory capacities.
Emotion recognition and trauma exposure: The mediating role of posttraumatic avoidance
The ability to perceive and accurately label emotions in others is a key aspect of humans’ adaptation to their surroundings, which Darwin (Reference Darwin1872) identified as innate and critical for adaptation. Studies have shown that ER – the ability to correctly detect emotions in others – is essential for typical development and pinpointed childhood as a critical period for ER development. By the end of the first year, infants can discriminate several emotions from facial and vocal cues (Caron, Caron, & Maclean, Reference Caron, Caron and Maclean1988) and by late childhood they recognize most emotions as precisely as adults (Durand, Gallay, Seigneuric, Robichon, & Baudouin, Reference Durand, Gallay, Seigneuric, Robichon and Baudouin2007). Recognition of some emotions, such as disgust and anger, gradually mature across childhood and adolescence (Rodger, Vizioli, Ouyang, & Caldara, Reference Rodger, Vizioli, Ouyang and Caldara2015), when sensitivity to subtle changes in emotions is acquired (Thomas, De Bellis, Graham, & LaBar, Reference Thomas, De Bellis, Graham and LaBar2007).
ER provides the foundation for more complex emotional abilities and the ability to recognize emotions predicts positive social interactions, whereas deficits in ER are associated with behavioral and learning problems (Izard et al., Reference Izard, Fine, Schultz, Mostow, Ackerman and Youngstrom2001). ER plays a key role in emotion regulation; it enables children to identify internal states, attach feelings to events, and practice the regulation of distinct emotions (Eisenberg, Sadovsky, & Spinrad, Reference Eisenberg, Sadovsky and Spinrad2005). Deficits in ER, manifested by difficulty to identify and understand emotions, impair social skills, increase negative affect, and lead to psychopathology (Trentacosta & Fine, Reference Trentacosta and Fine2010), and ER deficits predict externalizing and internalizing psychopathologies across childhood and adolescence (Easter et al., Reference Easter, McClure, Monk, Dhanani, Hodgdon, Leibenluft and Ernst2005; Trentacosta & Fine, Reference Trentacosta and Fine2010). Studies have shown that recognition of emotions in others is a precursor of emotion regulation (Di Maggio, Zappulla, & Pace, Reference Di Maggio, Zappulla and Pace2016; Izard, Stark, Trentacosta, & Schultz, Reference Izard, Stark, Trentacosta and Schultz2008b) and, following emotion-based treatment, improvements in emotion regulation were mediated by change in ER (Izard, King, et al., Reference Izard, King, Trentacosta, Morgan, Laurenceau, Krauthamer-Ewing and Finlon2008).
Early exposure to trauma is associated with either augmented or suppressed ER abilities. Studies indicated decreased ability to recognize emotions following early adversity (Dvir, Ford, Hill, & Frazier, Reference Dvir, Ford, Hill and Frazier2014; Shipman, Edwards, Brown, Swisher, & Jennings, Reference Shipman, Edwards, Brown, Swisher and Jennings2005); others assessing ER from an affect-specific perspective suggested that type of adversity shapes recognition of specific emotions. While preschoolers with a history of neglect exhibited suppressed ER abilities, those with a history of physical abuse showed response bias for angry faces (Pollak, Cicchetti, Hornung, & Reed, Reference Pollak, Cicchetti, Hornung and Reed2000). Furthermore, children with a history of abuse required fewer physiological cues to recognize facial expression of anger and this enhanced recognition correlated with parental anger (Pollak, Messner, Kistler, & Cohn, Reference Pollak, Messner, Kistler and Cohn2009), and it is suggested that physically abused children learned to over-tune their perception of anger cues (Pollak, Reference Pollak2008). However, adversity of nonfamilial nature may follow a different course. To date, studies have mainly focused on maltreated/abused children and none, to our knowledge, examined ER in nonfamilial trauma where the family must face the trauma together. In adults, some studies showed reduced ER in war veterans (Poljac, Montagne, & de Haan, Reference Poljac, Montagne and de Haan2011), whereas others found increase in ER in earthquake survivors, both with and without PTSD, and suggested that the traumatic exposure, not the presence of PTSD, is predictive of ER difficulties (Bell et al., Reference Bell, Colhoun, Frampton, Douglas, McIntosh, Carter and Porter2017). Research in adults that tested the differential effects of various PTSD clusters on ER found that avoidance symptoms, but not other posttraumatic symptom clusters, predicted ER impairment (Mazza et al., Reference Mazza, Giusti, Albanese, Mariano, Pino and Roncone2012; Schmidt & Zachariae, Reference Schmidt and Zachariae2009). Such inconsistencies underscore the need for longitudinal studies that may illuminate the developmental course of ER following trauma, taking into consideration the different posttraumatic symptom clusters.
Avoidance symptoms define one of four core symptoms in early childhood PTSD (Zero To Three, 2005) and one of three symptom clusters in adolescents and adults (American Psychiatric Association, 2013). Longitudinal studies pinpointed avoidance symptoms in early childhood as the most notable risk pathway in PTSD chronicity (Feldman & Vengrober, Reference Feldman and Vengrober2011; Feldman, Vengrober, & Ebstein, Reference Feldman, Vengrober and Ebstein2014; Perkonigg et al., Reference Perkonigg, Pfister, Stein, Höfler, Lieb, Maercker and Wittchen2005) and avoidant behavior is related to ER deficits (Schultz, Izard, Ackerman, & Youngstrom, Reference Schultz, Izard, Ackerman and Youngstrom2001; Trentacosta & Fine, Reference Trentacosta and Fine2010). A study from the current cohort showed that early avoidance symptoms predicted disruptions to default mode network (DMN) connectivity in adolescence (Zeev-Wolf, Levy, Ebstein, & Feldman, Reference Zeev-Wolf, Levy, Ebstein and Feldman2020) and these findings are important as the DMN plays a crucial role in ER (Li, Mai, & Liu, Reference Li, Mai and Liu2014). These studies raise the possibility that early childhood avoidance may mediate the relations between early-onset trauma and ER deficits in adolescence.
Executive functions and trauma exposure: The mediating role of parent–child reciprocity
EF are a set of core cognitive processes that facilitate the ability to restrain habitual response, detect errors, and learn by adapting behavior. Three related processes are considered core components of EF: inhibition, working memory, and cognitive flexibility; among these, inhibition is considered the key process that underlies the global EF (Gillie & Thayer, Reference Gillie and Thayer2014; Miyake & Friedman, Reference Miyake and Friedman2012; Valian, Reference Valian2015) and studies have suggested that of the three core aspects, inhibition is the most central (Hall & Fong, Reference Hall and Fong2015). Specifically, the stop-signal task, which is often used to estimate inhibitory capacities, has been suggested to reflect a common EF that underpin EF development (Chevalier, Reference Chevalier2015; Friedman & Miyake, Reference Friedman and Miyake2017). Furthermore, inhibition has shown to be critical for regulating emotional and cognitive arousal (Diamond, Reference Diamond2013). Overall, EF plays a key role in cognitive, social, and psychological development and impacts multiple dimensions of well-being, from physical and mental health to academic achievement and social competence (Diamond, Reference Diamond2013).
The development of EF is most rapid during early childhood and rates of growth decelerate in late childhood and adolescence (Best, Miller, & Naglieri, Reference Best, Miller and Naglieri2011). Global EF, and response inhibition in particular, involve activation of select prefrontal cortex (PFC) regions (Ridderinkhof, Van Den Wildenberg, Segalowitz, & Carter, Reference Ridderinkhof, Van Den Wildenberg, Segalowitz and Carter2004) and due to its prolonged maturation and increased plasticity, the PFC is highly sensitive to both favorable and adverse rearing conditions (Kolb et al., Reference Kolb, Mychasiuk, Muhammad, Li, Frost and Gibb2012). Animal studies indicate that early-life adversities lead to PFC abnormalities, which, in turn, cause impaired EF (Hedges & Woon, Reference Hedges and Woon2011). In humans, EF deficits are linked with psychopathologies and impaired functioning (Fairchild et al., Reference Fairchild, van Goozen, Stollery, Aitken, Savage, Moore and Goodyer2009; Penadés et al., Reference Penadés, Catalán, Rubia, Andrés, Salamero and Gastó2007; Taylor Tavares et al., Reference Taylor Tavares, Clark, Cannon, Erickson, Drevets and Sahakian2007) and early life stress predicts decreased EF skills across childhood (De Bellis, Hooper, Spratt, & Woolley, Reference De Bellis, Hooper, Spratt and Woolley2009; Hostinar, Stellern, Schaefer, Carlson, & Gunnar, Reference Hostinar, Stellern, Schaefer, Carlson and Gunnar2012; McDermott et al., Reference McDermott, Troller-Renfree, Vanderwert, Nelson, Zeanah and Fox2013) and adolescence (Mueller et al., Reference Mueller, Maheu, Dozier, Peloso, Mandell, Leibenluft and Ernst2010). Adolescents exposed to chronic trauma exhibited EF deficiency, particularly inhibition, which correlated with PTSD symptoms and functional impairment (op den Kelder, Ensink, Overbeek, Maric, & Lindauer, Reference op den Kelder, Ensink, Overbeek, Maric and Lindauer2017). Given its key role in adaption, it is important to assess EF in youth exposed to early trauma (Blair & Diamond, Reference Blair and Diamond2008).
Early parent–child relationship quality is a well-known predictor of later EF (Bernier, Carlson, Deschênes, & Matte-Gagné, Reference Bernier, Carlson, Deschênes and Matte-Gagné2012; Conway & Stifter, Reference Conway and Stifter2012; Cuevas et al., Reference Cuevas, Deater-Deckard, Kim-Spoon, Watson, Morasch and Bell2014; Deater-Deckard, Reference Deater-Deckard2014; Lucassen et al., Reference Lucassen, Kok, Bakermans-Kranenburg, Van Ijzendoorn, Jaddoe, Hofman and Tiemeier2015), apart from its role in overall well-being and adaptation. Exposure to early adversity impairs the parent–child relationship (Halevi et al., Reference Halevi, Djalovski, Vengrober and Feldman2016; Scheeringa & Zeanah, Reference Scheeringa and Zeanah2001), particularly the dimensions of synchrony, reciprocity, and attunement (Feldman & Vengrober, Reference Feldman and Vengrober2011; Halevi et al., Reference Halevi, Djalovski, Kanat-Maymon, Yirmiya, Zagoory-Sharon, Koren and Feldman2017; Levy, Yirmiya, Goldstein, & Feldman, Reference Levy, Yirmiya, Goldstein and Feldman2019). Dyadic reciprocity indexes the degree of coordination, synchrony, and mutual responsiveness in the Mother×Child interaction, is individually stable from infancy to adolescence (Feldman, Reference Feldman2010), and mediates the links between early adverse conditions, such as maternal depression, on EF abilities in late childhood (Priel, Zeev-Wolf, Djalovski, & Feldman, Reference Priel, Zeev-Wolf, Djalovski and Feldman2020), suggesting that mother–child reciprocity may mediate the effects of trauma on EF.
Risk and resilience biomarkers: Respiratory sinus arrhythmia and cortisol
Models on resilience emphasize the need to employ longitudinal designs, include theoretically based biomarkers, and test condition-specific factors that tilt children toward a resilient trajectory (Feldman, Reference Feldman2020; Rutter, Reference Rutter2013; Southwick et al., Reference Southwick, Bonanno, Masten, Panter-Brick and Yehuda2014). Furthermore, cognitive, emotional, and parent–child factors should be integrated with biological markers of allostasis and stress regulation (Masten, Reference Masten2007). We measured two biomarkers consistently linked with risk and resilience; hypothalamic–pituitary–adrenal (HPA) axis function indexed by CT and parasympathetic control marked by RSA.
The parasympathetic nervous system, a branch of the autonomic nervous system (ANS), plays an important role in regulatory functions, supporting sustained attention and social engagement (Porges, Reference Porges2001, Reference Porges2007), and RSA is a reliable marker of parasympathetic activity (Porges, Reference Porges1995). High resting RSA indexes flexible adaptation to changing environmental condition, a key feature of resilience (Beauchaine, Reference Beauchaine2001; Feldman, Reference Feldman2020). In contrast, low resting RSA is associated with high-risk rearing, troubled mother–child relationship, and functional impairments (Beauchaine & Thayer, Reference Beauchaine and Thayer2015; Feldman, Reference Feldman2006; Shahrestani, Stewart, Quintana, Hickie, & Guastella, Reference Shahrestani, Stewart, Quintana, Hickie and Guastella2014).
Parasympathetic activity plays a role in allostasis via its connection with PFC activity and its inhibitory function, and RSA has been linked with response inhibition (Thayer, Reference Thayer2006; Thayer & Sternberg, Reference Thayer and Sternberg2006). Response inhibition, a core component of EF, gauges context-dependent responses that enable anticipatory, resource-sensitive (i.e. allostatic) regulation based on ongoing parasympathetic inputs (Gillie & Thayer, Reference Gillie and Thayer2014; Thayer, Reference Thayer2006). Due to the negative effects of adversity on parenting quality and the associations between parenting and EF, it is possible that child RSA may serve a protective role in the pathway leading from early trauma to EF through its impact on the link between dyadic reciprocity and EF. This is supported by a study showing that baseline RSA modulated the link between sensitive parenting in the toddler years and EF at 5 years (Gueron-Sela et al., Reference Gueron-Sela, Wagner, Propper, Mills-Koonce, Moore and Cox2017).
CT is the end product of the HPA axis, the body central stress-management system (Hellhammer, Wüst, & Kudielka, Reference Hellhammer, Wüst and Kudielka2009; Tsigos & Chrousos, Reference Tsigos and Chrousos2002). Early trauma leads to significant alterations in HPA axis activity and CT levels, including cases of abuse and neglect (Cicchetti & Rogosch, Reference Cicchetti and Rogosch2001; Cicchetti, Rogosch, Gunnar, & Toth, Reference Cicchetti, Rogosch, Gunnar and Toth2010; De Bellis & Zisk, Reference De Bellis and Zisk2014) or war-related trauma (Feldman, Vengrober, Eidelman-Rothman, & Zagoory-Sharon, Reference Feldman, Vengrober, Eidelman-Rothman and Zagoory-Sharon2013; Yirmiya et al., Reference Yirmiya, Djalovski, Motsan, Zagoory-Sharon and Feldman2018). However, findings on CT and PTSD are mixed; some studies found that children with PTSD show attenuated basal CT (Feldman, Vengrober, et al., Reference Feldman, Vengrober, Eidelman-Rothman and Zagoory-Sharon2013), others showed that elevated basal CT predicted the development of PTSD following trauma (Pervanidou et al., Reference Pervanidou, Kolaitis, Charitaki, Margeli, Ferentinos, Bakoula and Chrousos2007), while still others found no difference in basal CT between trauma-exposed children with and without PTSD (Carrion et al., Reference Carrion, Weems, Ray, Glaser, Hessl and Reiss2002). Assessment of the different PTSD clusters showed specific associations of CT and the avoidance cluster (Goenjian et al., Reference Goenjian, Pynoos, Steinberg, Endres, Abraham, Geffner and Fairbanks2003; Yehuda et al., Reference Yehuda, Kahana, Binder-Brynes, Southwick, Mason and Giller1995) and this is supported by a study showing higher basal CT in healthy children with avoidant behavior (Blair, Peters, & Granger, Reference Blair, Peters and Granger2004).
To further understand the inconsistencies in the associations of CT and trauma, one solution is to view the role of HPA system activity within a complex net of mutual influences leading from trauma exposure to regulatory outcomes. CT has shown to function as risk indicator in the longitudinal course leading from trauma and PTSD symptoms to various aspect of development (Dunlop & Wong, Reference Dunlop and Wong2019; Kamin & Kertes, Reference Kamin and Kertes2017; Olff & van Zuiden, Reference Olff and van Zuiden2017; van Ast et al., Reference van Ast, Cornelisse, Marin, Ackermann, Garfinkel and Abercrombie2013). These studies suggest that CT may have a modulating role in the complex pathways leading from early trauma to the maturation of ER and EF through its effect on avoidance symptoms. For instance, under conditions of chronic stress both avoidance and CT were related to ER processing (Roelofs, Elzinga, & Rotteveel, Reference Roelofs, Elzinga and Rotteveel2005). Similarly, CT moderated the links between early adversity and children's emotion regulation (Von Klitzing et al., Reference Von Klitzing, Perren, Klein, Stadelmann, White, Groeben and Hatzinger2012), which is closely related to ER (Eisenberg et al., Reference Eisenberg, Sadovsky and Spinrad2005), and CT interacted with social withdrawal in predicting ER processing (van Peer, Spinhoven, Dijk, & Roelofs, Reference van Peer, Spinhoven, Dijk and Roelofs2009). Finally, early-life stress interacted with genes implicated in HPA axis functioning in predicting ER in healthy adults (Hartling et al., Reference Hartling, Fan, Weigand, Trilla, Gärtner, Bajbouj and Grimm2019). These studies support the hypothesis that CT may impact the pathway leading from early trauma to later ER abilities through its interaction with avoidance symptomatology in early childhood.
The current study
We addressed the effects of early and chronic exposure to war-related trauma on children's EF and ER at the transition to adolescence, focusing on two regulatory outcomes critical for well-being and adaptive functioning. While studies have indicated that early adversity impairs EF and ER, the mechanisms and pathways underlying these associations are mainly unknown. Utilizing a unique cohort of war-exposed preadolescents exposed to the same external trauma, we were able to utilize a unique “natural experiment” to test these longitudinal pathways. Our goal was to assess the interplay of biological, relational, and clinical components as they shape each other over time toward a more comprehensive model, consistent with models that emphasize the need to study resilience from a longitudinal, biobehavioral perspective (Feldman, Reference Feldman2020; Masten, Reference Masten2007; Rutter, Reference Rutter2013; Southwick et al., Reference Southwick, Bonanno, Masten, Panter-Brick and Yehuda2014).
Three hypotheses were formulated. First, we expected that preadolescents exposed to chronic war-related trauma would show impaired EF and ER compared to non-exposed controls. Second, we expected that the association between trauma exposure and EF would be mediated by the degree of reciprocity experienced in early childhood and that this indirect pathway may be modulated by the child's RSA. Finally, we hypothesized that avoidance symptomatology in early childhood would mediate the association between early exposure and preadolescence ER and this cascade may be moderated by child's CT levels. Overall, our goal was to test pathways from chronic early trauma to ER and EF in preadolescence as mediated by the parent–child relationship, avoidance symptoms, CT, and RSA to specify mechanisms of risk and resilience.
Method
Participants
Participants were recruited in early childhood and followed four times from early childhood to early adolescence. Here, we used data collected in early childhood (T1) and early adolescence (T4).
Early childhood (T1)
A total of 232 war-exposed and control children–mother dyads were initially recruited (M = 2.76 years, SD = 0.91). The war-exposed group included 148 children and mothers living in Sderot, a small Israeli town located near the Gaza border whose residents experienced frequent and unpredictable rockets and missile attacks for nearly 20 years, with periods of exacerbation occurring every few months. During these years, dozens of civilians were killed, and thousands were injured. Thus, putting children and families in daily tangible threat of death or severe injury. Children in this group all live in the same neighborhoods, thereby exposed to similar daily threat characteristics since birth. The control group comprised 84 dyads from comparable towns in the central part of Israel, matched for age, gender, birth order, parental age and education, maternal employment, and marital status. Other forms of trauma, abuse, or neglect were excluded (Feldman & Vengrober, Reference Feldman and Vengrober2011). Children were followed in middle childhood (T2) (M = 7.68 years, SD 0.7) and late childhood (T3) (M = 9.3 years, SD = 1.41) and data from the T2 and T3 visits appear elsewhere (Feldman, Vengrober, et al., Reference Feldman, Vengrober and Ebstein2014; Halevi et al., Reference Halevi, Djalovski, Kanat-Maymon, Yirmiya, Zagoory-Sharon, Koren and Feldman2017, Reference Halevi, Djalovski, Vengrober and Feldman2016).
Early adolescence (T4)
Children were revisited at preadolescence (11–13 years). The current study utilized data from 111 children who participated in both T1 and T4. Of these, 58 were from the war-exposed group and 53 were controls. Ten children did not have valid RSA data due to excessive movement artifacts or device failure. Ten children did not complete the response inhibition task, six did not complete the ER task, and eight participants did not have valid T1 CT data. Attrition was mainly related to inability to locate families and no demographic differences were found between families who did or did not participate in the current stage in any demographic variable. The study was approved by the local Institutional Review Board. Mothers signed an informed consent and children received a gift certificate for their participation.
Procedure
Early childhood (T1)
Mother–child dyads were visited at home in the afternoon hours to control for diurnal variability in CT. Following acquaintance, baseline saliva samples were collected by the mother by placing a Salivette (Sarstedt, Rommelsdorft, Germany) in the child's mouth for one minute. Mother and child then engaged in a 10-min free interaction with preselected toys. Next, child PTSD symptoms in the different clusters were diagnosed through a detailed maternal interview, consistent with the guidelines of the Diagnostic classification of mental health and developmental disorders of infancy and early childhood: (Revised edition) (Zero To Three, 2005)
Early adolescence (T4)
Following arrival at the lab, children were connected by a trained research assistant to a Mindware device (Mindware Technologies Ltd, Gahanna, OH) which collected electrocardiogram (ECG) and respiration (RSP) data for a three-minute baseline. Following, children completed the ER and EF tasks.
Measures
Early childhood (T1)
Child PTSD avoidance symptoms
Trained clinicians with a background in early childhood development and psychopathology visited families at home and diagnosed child's PTSD using the Diagnostic Classification: Zero-to-Three (Zero To Three, 2005). Mothers were interviewed extensively, and clinicians elicited detailed information concerning PTSD symptoms by focusing on the nature of the trauma, proximity to the traumatic event, and child's emotional reaction to specific traumatic events. Clinicians were supervised by a senior clinical child psychologist and a child psychiatrist, and cases were conferred every few weeks. A similar interview was conducted with the control group mothers. Control mothers were asked to describe events in the last few months experienced by the child as “traumatic”, stressful, or emotionally challenging. Posttraumatic symptoms were assessed in the four categories of early childhood PTSD: re-experiencing, avoidance, hyper-arousal, and fears and aggressions, and the avoidance symptom cluster was used here.
Dyadic reciprocity
To assess dyadic reciprocity, mother–child free play interactions were videotaped during the home visits, and interactions were coded offline using the Coding Interactive Behavior (CIB) manual (Feldman, Reference Feldman1998). The CIB is a well-validated global system comprising 45 codes aggregated into several constructs that have been widely used in the research of typical and high-risk contexts across multiple cultures and developmental stages (for review, Feldman, Reference Feldman2012a). Coding was conducted by trained coders, blind to any other information, and reliability on 20% of the interactions exceeded 93% on all codes (k = .84, range .78–95). Each scale included in the constructs is coded from 1 – target behavior has minimal expression during the interaction to 5 – target behavior has maximal expression during the interaction. Scales are averaged to create the final construct consistent with wide-ranging earlier research (Feldman, Reference Feldman2012b). Here we used the dyadic reciprocity construct that indexes the degree in which the dyad participates in a joint activity, interact in a “give-and-take” manner and the interaction is fluent, rhythmic, and mutually adaptive.
Cortisol
Salivettes were kept cooled until thawed before being centrifuged at 48C at 1000g for 15 min. The samples were then stored at –208C until assayed. CT levels were assayed using a commercial ELISA kit (Assay Design, MI). Measurements were performed in duplicate according to the kit's instructions. Levels were calculated by using MATLAB version 7 (MathWorks, Natick, MA) according to the relevant standard curves.
Early adolescence (T4)
Respiratory sinus arrhythmia (RSA)
Child's ECG and RSP signals were collected using MindWare Bionex device (Gahanna, OH). ECG was recorded using a modified lead II configuration and RSP was assessed with a respiratory belt placed around the chest. ECG and RSP data were sampled at 1000 Hz. Data were transmitted to a computer in an adjacent room monitored by a research assistant and captured using BioLab Software version 3.0 (MindWare Technologies Ltd, Gahanna, OH). Data were processed offline using HRV 3.0.21 software (Mindware Technologies Ltd, Gahanna, OH). ECG data were visually inspected for artifacts – missing or errored heartbeats were manually deleted or inserted as appropriate. Data of participants with more than 10% problematic R-waves were excluded. Following, the data were tapered with a Hamming window and submitted to a fast Fourier transform to derive the spectral distribution. RSA was quantified as the integral power within the respiratory frequency band of 0.2–0.42 Hz appropriate for child age (Fleming et al., Reference Fleming, Thompson, Stevens, Heneghan, Plüddemann, MacOnochie and Mant2011).
Executive function (EF)
Child's EF was indexed by the stop signal task from the computerized Cambridge Neuropsychological Testing Automated Battery system (CANTAB, Cambridge Cognition, Cambridge, UK). The stop signal task measures the ability to inhibit a prepotent response. The task consists of an arrow stimulus presented on a screen and randomly pointing to either right or left direction. The child was instructed to press the corresponding button on a two-button press pad depending on the arrow's direction as quickly and accurately as possible. Following participant's introduction to the test and completion of one block of 16 practice trials, the participant was told to withhold response and not select the button if he/she hears an auditory signal that is paired with the presented arrow. The CANTAB system utilizes a staircase design for the delay between the go stimulus and the inhibitory stimuli. This allows the task to adapt to the performance of the participant, narrowing in on the 50% rate of success rate for inhibition. Stop signal reaction time (SSRT) is an estimate of the length of time between the go stimulus and the inhibitory stimulus at which the subject was able to successfully inhibit their response on 50% of the trials. SSRT is considered a reliable parameter of response inhibition capacity and reflects the individual's capability to internally suppress a response. SSRT has been widely used in both human and animal studies, with SSRT scores representing EF capacity in a reversed manner (Aron, Reference Aron2007, Reference Aron2008; Aron, Fletcher, Bullmore, Sahakian, & Robbins, Reference Aron, Fletcher, Bullmore, Sahakian and Robbins2003; Boehler, Appelbaum, Krebs, Hopf, & Woldorff, Reference Boehler, Appelbaum, Krebs, Hopf and Woldorff2010). Thus, higher SSRT was used here to index lower EF and lower SSRT was used to index higher EF.
Emotion recognition (ER)
We used the Emotion Recognition Toolbox, a computerized ER paradigm developed in our lab and validated in prior research (Priel et al., Reference Priel, Zeev-Wolf, Djalovski and Feldman2020). The toolbox tests the recognition and knowledge of single and complex emotions that are presented at different levels of intensity. The software includes 15 short 30-s videos that demonstrate happy, sad, angry, and neutral emotions of low or high intensity. After each movie, the child is asked by the program whether each emotion appeared in the movie and once the child pressed yes or no, another set appears and asks if these emotions also appeared. The proportion scores for each emotion were calculated and the final score was the overall percentage of correct recognition of emotions from the child's overall response, consistent with our prior work in children of a similar age (Priel et al., Reference Priel, Zeev-Wolf, Djalovski and Feldman2020).
Data analytic plan
Data were analyzed by SPSS 23 (IBM Corp., Armonk, NY). Differences between exposed and controls were examined using the t test for the continuous variables and chi-square test for the categorical variables. For testing of our first hypothesis regarding group differences in EF and ER, we utilized two t tests and applied a Bonferroni correction to reduce errors due to multiple testing (α = 0.05/2 = 0.025). Pearson correlations measured the relationships between study variables. Next, we used conditional process analysis to assess a comprehensive model which includes the direct and moderated mediated paths leading from early trauma exposure to youth regulatory outcomes. Exposure was entered as the independent variable and was dummy coded (control = “0”, war-exposed = “1”). Early childhood avoidance symptoms and dyadic reciprocity were entered as parallel mediators. CT and RSA were entered as moderators and EF and ER as the dependent variables.
We used PROCESS for SPSS (v. 3.3). PROCESS employs bootstrapping calculations, a nonparametric resampling procedure, which provides the most powerful and reasonable method of obtaining confidence limits for conditional indirect effects at different levels of the moderators (Hayes, Reference Hayes2018a). This approach accounts for violation of normality assumptions that can occur in more traditional approaches to mediation and moderation (Hayes, Reference Hayes2018a). For this analysis, bias-corrected standard errors and confidence intervals (CIs) were generated using 10,000 bootstrapped resamples drawn to derive the 95% CI. Conditional mediation is considered present when the CI for the estimation of the indirect effect does not contain zero. Specifically, we used PROCESS Model 16, as it provides indices of partial moderated mediation that quantify the linear relationship between a moderator and an indirect effect when a second moderator is held constant (Hayes, Reference Hayes2018b). This model allowed us to test moderated mediation with the two independent moderators, CT and RSA, rather than performing separate moderated mediation analysis for each moderator. Consistent with Hayes’ guidelines for analyzing a model with multiple dependent variables, we performed two similar analyses with the dependent variable, ER and EF, entered alternatively, while generating a random bootstrapping seed used in both analyses (Hayes, Reference Hayes2018a, pp. 145–146).
Results
Preliminary analysis
Sociodemographic information of the exposed and control groups appears in Table 1. No differences in demographic variables were found between the groups. For a fuller model, we tested group differences of study variables that were not hypothesized to have group differences (Figure 1a–d). As can be seen, in early childhood, exposed children exhibited more avoidance symptoms and lower dyadic reciprocity with their mothers compared with controls.
Table 1. Comparison on child and mother sociodemographic variables between the groups
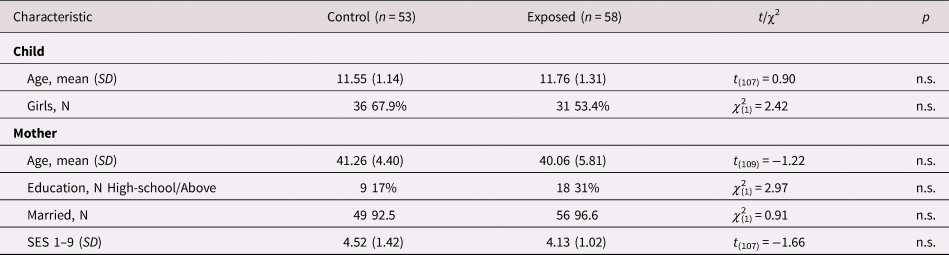
SES = socioeconomic status
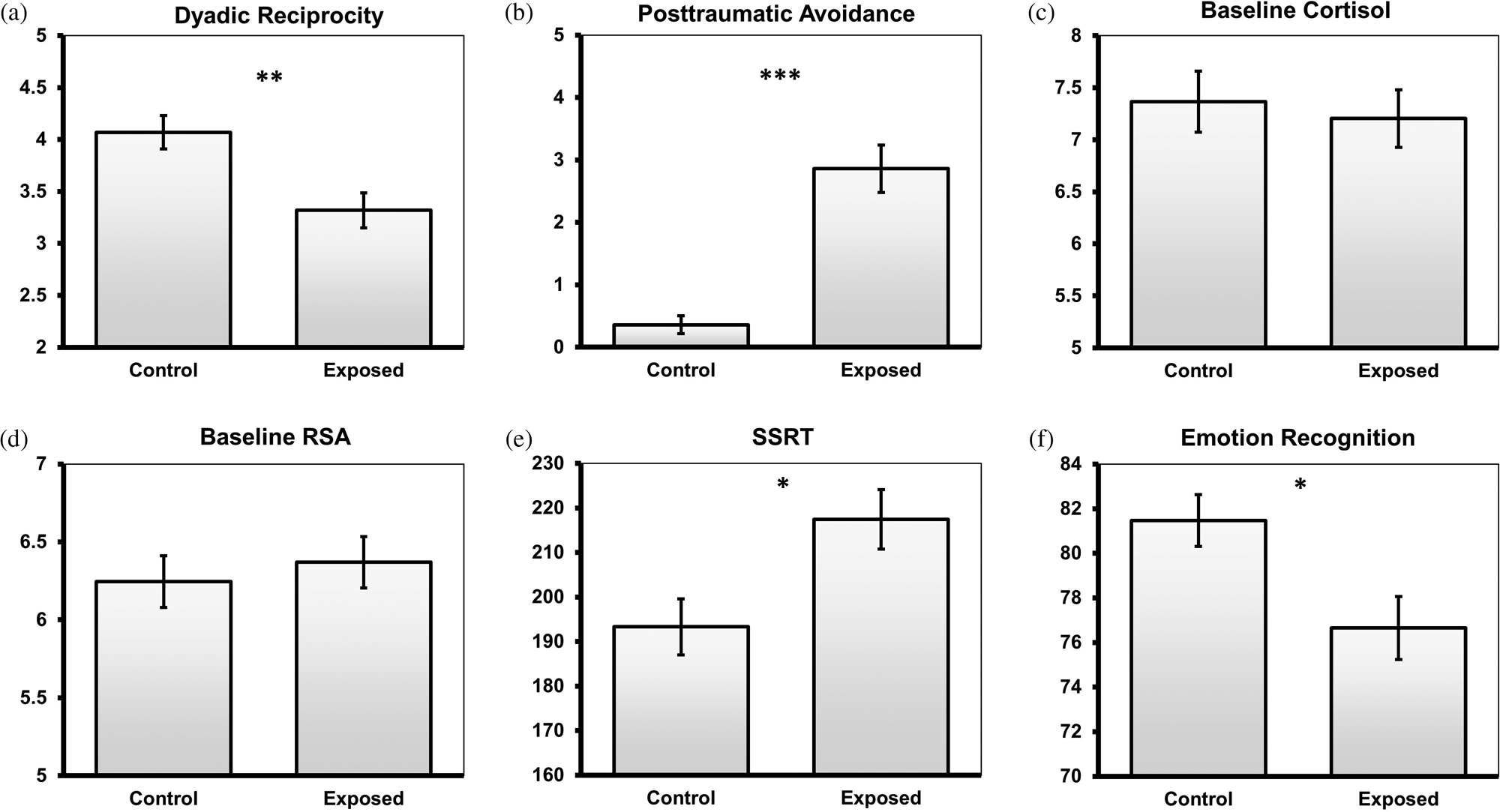
Figure 1. Group differences in study variables. SSRT, stop signal reaction time. RSA, respiratory sinus arrhythmia. Error bars represent the standard error of the mean. *p < .05; **p < .01; ***p < .001
Group comparisons and correlations
Consistent with our first hypothesis, exposed preadolescents (M = 217.44, SD = 47.97) showed significantly higher SSRT (and thereby lower EF) than controls (M = 193.30, SD = 43.98), t (99) = 2.63, p = .01. In addition, exposed preadolescents (M = 76.65, SD = 10.53) showed significantly lower ER than controls (M = 81.45, SD = 10.53), t (103) = 2.59, p = .01 (Figure 1e–f).
Pearson's correlations among study variables appear in Table 2. As seen, early childhood avoidance symptoms were significantly correlated with diminished ER in preadolescence as well as with lower dyadic reciprocity in early childhood. Dyadic reciprocity longitudinally correlated with better ER and EF skills; dyadic reciprocity also correlated with lower basal CT levels. The two regulatory outcomes, ER and EF were interrelated.
Table 2. Pearson correlations among study variables
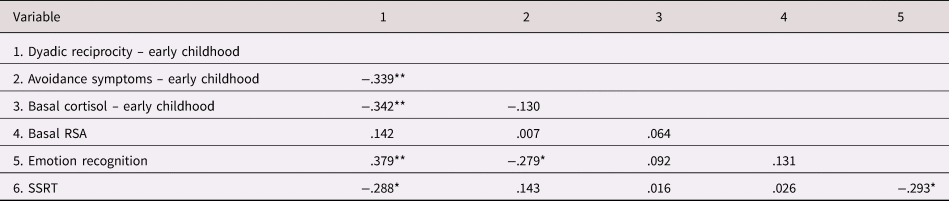
* Bonferroni adjusted two-sided significance level of α < 0.05/15 = p < .0033.
** Bonferroni adjusted two-sided significance level of α < 0.01/15 = p < .00066.
RSA = respiratory sinus arrhythmia; SSRT = stop signal reaction time
Conditional process analysis
Pathways to executive function
Results of the moderated mediation model with EF (indexed by SSRT in a reversed manner) as the outcome is presented in Figure 2 and unstandardized path coefficients are presented.
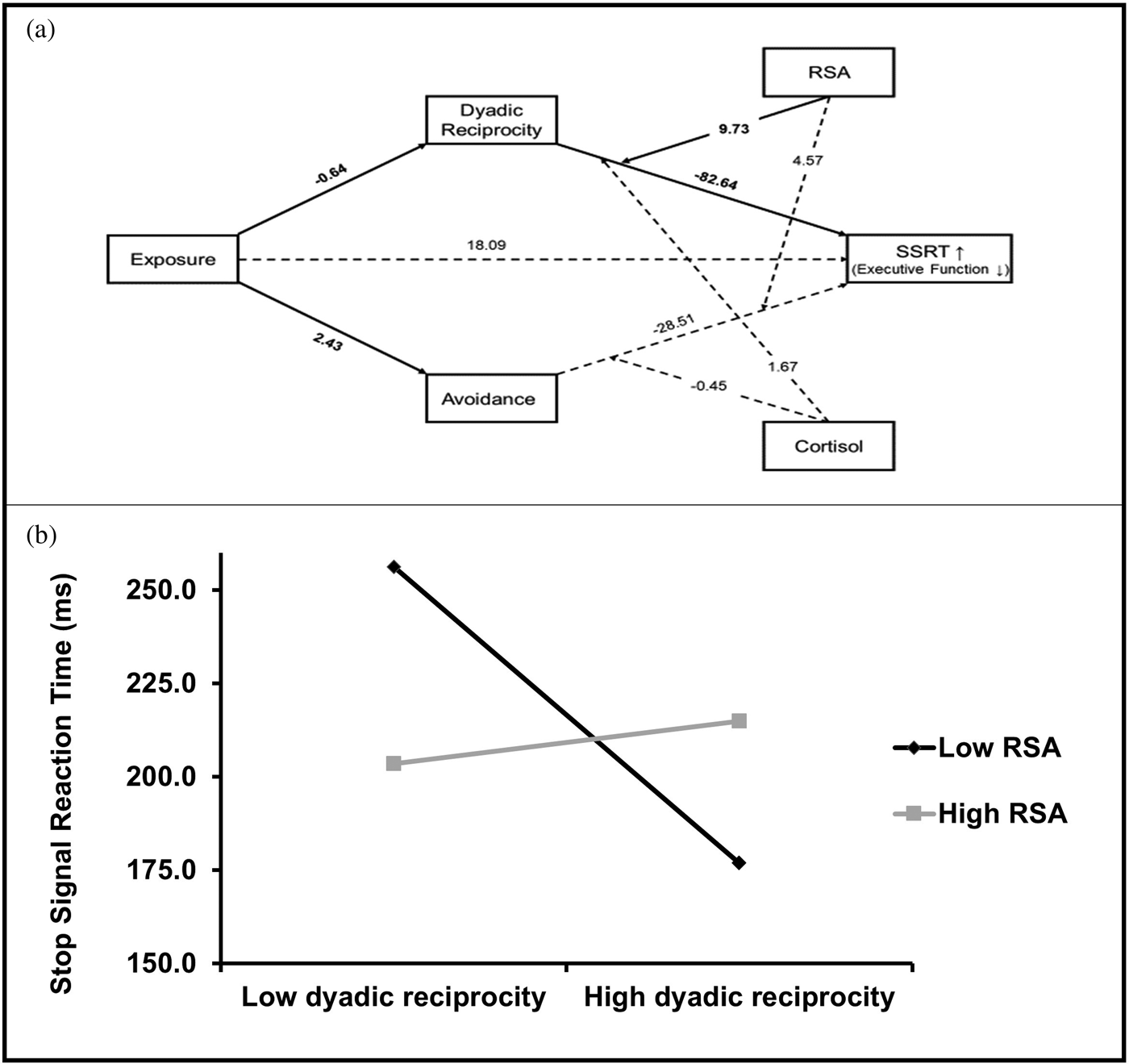
Figure 2. (a) Moderated mediation model of the pathway from early trauma exposure to preadolescence SSRT. Reported are unstandardized coefficients. Emotion recognition score was included as covariate (not depicted). Solid lines represent significant effects, and dashed lines represent nonsignificant effects. (b) Simple slopes of early-childhood dyadic reciprocity predicting preadolescence SSRT for 1 SD below and above the mean of RSA.
Since EF and ER were interrelated, ER scores were entered as covariates in this analysis (of note, analysis without covariates showed similar pattern of results; see Supplementary Materials Table S.1). As seen, trauma exposures linked with lower dyadic reciprocity and higher avoidance symptoms in early childhood, but only dyadic reciprocity was then significantly related to EF in preadolescence. In addition, the direct association of exposure and later EF was not significant in the presence of the other intervals, indicating an indirect path. The partial moderated mediation index allows to quantify the linear relationship between a moderator and an indirect effect when a second moderator is held constant (Hayes, Reference Hayes2018b). RSA had a significant moderation effect on the indirect pathway leading from trauma exposure to SSRT via dyadic reciprocity (index = −6.29, SE = 3.57, 95% CI [−14.25, −0.51]).
To further understand this indirect effect, we evaluated this path at high (1 SD above mean) and low (1 SD below mean) levels of RSA while conditioning the other moderator (CT) at mean level, consistent with Hayes’ recommendations (Reference Hayes2018b, p. 16). A significant simple slope of dyadic reciprocity was found when RSA was low (B = −19.85, SE = 8.01, 95% CI [−35.83, −3.86]). In contrast, when RSA was high the simple slope of dyadic reciprocity was nonsignificant (B = 2.85, SE = 7.85, 95% CI [−12.82, 18.52]). Hence, at low levels of RSA, there was a negative link between early dyadic reciprocity and preadolescence SSRT, whereas at high levels of RSA such association was not evident. Furthermore, the overall path leading from early exposure to SSRT via dyadic reciprocity was significant only when RSA levels where low (B = 12.84, SE = 6.94, 95% CI [1.57, 28.34]). Overall, these results suggest that while chronic trauma exposure impacts preadolescents’ EF via reducing parenting quality in early life, better parasympathetic functioning can buffer this effect.
Pathways to emotion recognition
Results of the moderated mediation model with ER as the outcome is presented in Figure 3, showing unstandardized coefficients. Here, EF (SSRT score) was used as covariate (analysis without covariates showed similar pattern of results; see Supplementary Materials Table S.2). As seen in Figure 3, trauma exposure linked with lower dyadic reciprocity and higher avoidance symptoms, but, unlike the previous model, none was significantly associated with ER. However, an interaction of avoidance and CT was significant [B = −0.67, SE = 0.2, 95% CL [−1.06, −0.27]. Furthermore, significant unconditional mediation is not a prerequisite for a significant moderated mediation (Hayes, Reference Hayes2018a, p. 426). The conditional process analysis revealed that the partial moderated mediation index was significant, indicating CT having a significant moderation effect on the indirect pathway leading from early exposure to ER via avoidance symptoms (index = −1.77, SE = 0.7, 95% CI [−3.39, −0.67]) and this conditional pathway being the only significant path leading from exposure to ER.

Figure 3. (a) Moderated mediation model of the pathway from early trauma exposure to preadolescence emotion recognition. Reported are unstandardized coefficients. SSRT was included as covariate (not depicted). Solid lines represent significant effects, and dashed lines represent nonsignificant effects. (b) Simple slopes of early-childhood posttraumatic avoidance predicting preadolescence emotion recognition for 1 SD below and above the mean of early-childhood basal cortisol. SSRT, stop signal reaction time. RSA, respiratory sinus arrhythmia.
Evaluation of this path at high (1 SD above mean) and low levels (1 SD below mean) of CT revealed a significant negative simple slope of avoidance when CT was high (B = −1.61, SE = 0.55, 95% CI [−2.71, −0.5]), but a nonsignificant simple slope of avoidance when CT was low (B = 0.81, SE = 0.59, 95% CI [−0.37, 1.99]). Hence, at high levels of CT, there was an association between avoidance and preadolescence ER, whereas at low levels of CT such association was not significant. In accordance, the overall path leading from early exposure to ER through dyadic reciprocity was significant only when CT levels were high (B = −1.61, SE = 0.55, 95% CI [−2.71, −0.5]). In combination, these results suggest that the effect of early exposure on later ER deficits is mediated by avoidance symptoms in early childhood, but this indirect pathway depends on CT levels. Specifically, this pathway was evident in children with high levels of CT in early childhood, but not in those with low CT.
Discussion
The current study examined the effects of chronic early trauma on the maturation of two regulatory functions: ER and EF, abilities that play a key role in the individual's well-being and social adaptation. To test the interplay of risk and resilience factors on these regulatory functions, we capitalized on a “natural experiment” and followed a unique cohort of preadolescents who were exposed to the same war-related trauma from early childhood to early adolescence. This design allowed us to trace longitudinal biobehavioral pathways by which early trauma exposure impairs the development of core psychological functions at the transition to adolescence. Specifically, we examined mother–child reciprocity and avoidance symptoms as differential mediators linking trauma exposure with EF and ER. Furthermore, we tested two biomarkers consistently shown to index stress reactivity; RSA and CT production as factors that may condition these pathways. The components of our model and the dynamic interplay among them received substantial empirical attention in the context of early adversity; yet, this is the first study, to our knowledge, that examines a comprehensive model that specifies the longitudinal interplay of these factors in shaping ER and EF as modulated by stress-related biomarkers.
Research on the effects of chronic early trauma on later development has mainly addressed its impact on the prevalence of psychopathology, externalizing and internalizing behaviors, and posttraumatic symptoms. Here we wished to expand the developmental psychopathology perspective by assessing cognitive and emotional regulatory support systems. Both ER and EF consolidate during childhood and by the transition to adolescence enable children to function adaptively in their environment. Still, these two abilities have not, to our knowledge, been tested in combination as dual outcomes of early adversity. Overall, our results demonstrate that chronic early trauma compromises children's EF and ER capacities at this important developmental transition. The two potential pathways measured in early childhood as leading to such disruptions involved factors impacted by trauma; the mother–child reciprocal exchange and the increase in avoidance symptoms in early childhood. Our findings demonstrate two differential indirect and conditional pathways leading from trauma exposure to compromised EF and ER, which, in their presence, the direct associations between early trauma and later regulatory outcomes are altered. These pathways included psychological and social factors that interact with the child's biological components via two key biomarkers: RSA and CT. As such, our findings are consistent with both the biobehavioral synchrony model (Feldman, Reference Feldman2015b, Reference Feldman2017) and models on resilience that emphasize the need for condition-specific perspective that considers the interchange of psychological, cognitive, social, and biological factors (Feldman, Reference Feldman2020; Masten, Reference Masten2007; Rutter, Reference Rutter2013; Southwick et al., Reference Southwick, Bonanno, Masten, Panter-Brick and Yehuda2014).
Deficiencies in EF following trauma exposure were mediated by the trauma's negative effect on the mother–child relationship. We have previously shown that disruptions to the reciprocal component of the mother–child relationship across the first decade of life mediated the effects of chronic maternal depression on child EF at ten years (Priel et al., Reference Priel, Zeev-Wolf, Djalovski and Feldman2020), suggesting that executive abilities may be particularly sensitive to the experience of reciprocal caregiving beginning in early childhood. Further cascade of this path related to the adolescent's parasympathetic activity. Further analyses showed that the decrease in EF following trauma, which was mediated by reduced early reciprocity, is only evident under a specific condition: when the child's parasympathetic functioning is less adaptive and baseline RSA is low, highlighting the resilience-promoting impact of autonomic-system functionality. Results also confirmed our third hypothesis, that the links between trauma and compromised ER are mediated by the increase in avoidance symptoms as moderated by early baseline CT levels. Further analysis revealed that CT interacted with avoidance symptoms and thus, the path leading from trauma exposure to ER via elevated avoidance symptoms was evident in children with higher basal CT levels in early childhood but was absent in children who exhibited lower stress response.
Careful examination of our results outlines a complex pattern of interactions between automatic nervous system activity and dyadic factors in the context of early trauma. Higher dyadic reciprocity was linked with better child EF a decade later but only among children with lower RSA. These results are consistent with a study indicating that RSA moderated the association between sensitive parenting and later EF such that a positive association was found only among children with low basal RSA (Gueron-Sela et al., Reference Gueron-Sela, Wagner, Propper, Mills-Koonce, Moore and Cox2017). Our study expands previous knowledge to the context of trauma and the important topic of resilience within a longitudinal context. Because RSA was only measured in our study in adolescence, it was not possible to see its longitudinal effects. However, in a longitudinal study that followed premature infants from birth to adolescence, we found that RSA at age 10 impacted concurrent EF (Feldman, Rosenthal, & Eidelman, Reference Feldman, Rosenthal and Eidelman2014), but ten-year EF were unrelated to RSA measured in infancy, suggesting that the maturation of parasympathetic control across childhood may be an important buffer for the development of cognitive control, and further longitudinal studies are needed to specify the earliest time point when RSA becomes a resilient biomarker of later cognitive and regulatory abilities.
Studies have shown that parents exposed to trauma tend to form impaired relationships with their children (Feldman & Vengrober, Reference Feldman and Vengrober2011; Halevi et al., Reference Halevi, Djalovski, Vengrober and Feldman2016) and these altered patterns of Parent×Child interaction carry negative effects on the child's future mental health and symptomatology (Scheeringa & Zeanah, Reference Scheeringa and Zeanah2001). Since low RSA is considered a marker of deficient self-regulatory capacities (Porges, Reference Porges2007), our findings raise the possibility that children exhibiting lower RSA rely more on their mother's reciprocal and attuned parenting for self-regulation. The parent–child relationship plays a vital role in the emergence of self-regulation, which sustains EF, and the reliance on the parents is especially crucial during early childhood and under conditions of adversity (Cuevas et al., Reference Cuevas, Deater-Deckard, Kim-Spoon, Watson, Morasch and Bell2014; Feldman, Reference Feldman2015a). In the context of mass trauma, mothers are exposed to the same external danger and have diminished resources to develop carefully attuned reciprocity with their young children, leading to a cascade of regulatory difficulties that may impede cognitive flexibility and EF.
Our results on the modulating role of RSA in the context of trauma may echo the differential susceptibility model (Belsky, Bakermans-Kranenburg, & Van Ijzendoorn, Reference Belsky, Bakermans-Kranenburg and Van Ijzendoorn2007), which suggests that environmental hazards impact outcome as a function of the child's innate regulatory abilities. Extant research has indicated that high baseline RSA is considered to be adaptive (Beauchaine, Reference Beauchaine2001; Porges, Reference Porges2007), and buffers in the association between unfavorable environmental contexts and child outcomes (Mezulis, Crystal, Ahles, & Crowell, Reference Mezulis, Crystal, Ahles and Crowell2015), while low RSA is typically framed as a risk factor in this association (Benjamin Hinnant, Erath, & El-Sheikh, Reference Benjamin Hinnant, Erath and El-Sheikh2015; Dyer, Blocker, Day, & Bean, Reference Dyer, Blocker, Day and Bean2016). However, other studies showed that vulnerable children, particularly those with by low basal RSA who are more reactive (Beauchaine, Reference Beauchaine2001; Rottenberg, Reference Rottenberg2007), are more susceptible to variations in environmental provisions and are able to gain more from positive parenting (Belsky & Pluess, Reference Belsky and Pluess2009), consistent with the differential susceptibility model which proposes that negatively reactive infants require especially responsive parenting for positive outcome (Belsky & Pluess, Reference Belsky and Pluess2013).
Results of our model can be addressed from two different perspectives that incorporate the differential susceptibility frame. Implementing a narrow perspective on the association between dyadic reciprocity and EF, our results can indicate that higher dyadic reciprocity is beneficial for later EF only for children with low basal RSA. Viewing the results from this narrow perspective may also suggest that low RSA children who developed in the context of high dyadic reciprocity showed the highest EF capabilities. However, when observing the overall longitudinal path, it becomes apparent that trauma exposure significantly decreased dyadic reciprocity, hence placing exposed children with low RSA in a vulnerable position, while not impacting the high RSA children who seemed less dependent on the mother's attuned caregiving for the development of EF skills. Dyadic reciprocity is individually stable from infancy to adolescence (Feldman, Reference Feldman2010; Feldman, Bamberger, & Kanat-Maymon, Reference Feldman, Bamberger and Kanat-Maymon2013), and thus, the early decrease in reciprocity probably continues across childhood and thus, the impairments in EF in preadolescence among children with low RSA emerge in the context of chronic disruptions to caregiving. These seemingly conflicting results underscore the importance of a longitudinal perspective and illustrate how the same biomarker can serve as both a risk factor on one level, but a resilience factor if viewed from a wider perspective. In addition, these results demonstrate the complex manner by which autonomic activity shapes the relationship between early trauma exposure and later EF and add a longitudinal angle to the differential susceptibility model.
Findings for the emotional arm of our study showed that the maturation of ER is sensitive to different biological and behavioral features, particularly avoidant symptoms and HPA axis functionality. The avoidance cluster of PTSD has been noted as the most risky pathway in PTSD chronicity following early trauma exposure (Feldman et al., Reference Feldman, Vengrober and Ebstein2014; Feldman & Vengrober, Reference Feldman and Vengrober2011; Perkonigg et al., Reference Perkonigg, Pfister, Stein, Höfler, Lieb, Maercker and Wittchen2005) and is a salient precursor of social dysfunction and professional maladjustment in adults exposed to childhood-onset trauma (Van Voorhees et al., Reference Van Voorhees, Dedert, Calhoun, Brancu, Runnals and Beckham2012). Our results expand knowledge on the role of early avoidance symptoms, particularly their sensitivity to HPA axis functioning in early childhood, in shaping future disruptions to emotional development. Studies have demonstrated that the avoidance symptoms cluster is the one most strongly linked with dysregulation of CT secretion and various aspects of HPA axis functioning (Yehuda et al., Reference Yehuda, Bierer, Sarapas, Makotkine, Andrew and Seckl2009, Reference Yehuda, Kahana, Binder-Brynes, Southwick, Mason and Giller1995) and dysregulation of CT metabolism has been suggested as a causal factor in the expression of avoidance symptoms and the degree of recovery following trauma (Whitaker, Farooq, Edwards, & Gilpin, Reference Whitaker, Farooq, Edwards and Gilpin2016; Yehuda et al., Reference Yehuda, Bierer, Sarapas, Makotkine, Andrew and Seckl2009). As suggested by Rutter (Reference Rutter2013), resilience involves a complex interplay of biological and behavioral factors as they impact each other over time, and our findings may pinpoint condition-specific, age-specific, and biomarker-specific pathways to the maturation of distinct regulatory outcomes.
The early-childhood PTSD avoidance cluster mediated the path from early trauma to later ER while also interacting with early childhood basal CT levels, such that the overall path was only evident in children who had high CT levels and absent in children exhibiting low CT. ER matures through the practice afforded by social interactions, which allow the child to read, understand, and respond to others’ emotions and place emotions within specific social context; hence, avoidance seriously limits the child's opportunities for practice (Schultz et al., Reference Schultz, Izard, Ackerman and Youngstrom2001). Yet social interactions are insufficient for the proper development of ER, as this skill also depends on cognitive factors, such as memory consolidation (Schultz et al., Reference Schultz, Izard, Ackerman and Youngstrom2001). Basal CT levels are associated with impaired memory consolidation of emotional faces (Van Honk et al., Reference Van Honk, Kessels, Putman, Jager, Koppeschaar and Postma2003) and thus, it is possible that while avoidance limits the child's ability to practice ER, a significant and long-lasting deficit in ER is evident only when HPA axis dysfunction may interrupt the consolidation of these experiences into meaningful emotional knowledge.
Several study limitations should be mentioned in the interpretation of our findings. , Although this study utilized a prospective longitudinal design, it is important to emphasize that no causal inferences can be derived from the results. Moreover, important biological, neural, or psychological measures that have not been tested here possibly interact with the pathways presented, and further research is needed to assess such multidimensional links. Furthermore, we did not collect EF and ER across childhood and such data could have shed valuable light on the developmental points in which these functions are most sensitive to adversity in order to specify intervention effort. EF was evaluated by one task of response inhibition, which, although central to EF, should be enhanced by attention to other aspects of EF. Moreover, there are finding that suggest a low reliability of individual differences in chief cognitive paradigms, among them the stop-signal task (Hedge, Powell, & Sumner, Reference Hedge, Powell and Sumner2018). These findings underline the need to evaluate inhibition using other tasks as well as other aspects of EF. Our study did not include fathers, and the father–child relationship could have added valuable insights. Finally, this study is based on a unique cohort of dyads exposed to repeated wartime attacks and further research replicating our findings in other situations of political violence or family-related trauma is essential to generalize our results.
The prevalence of armed conflicts has increased substantially during the past decade and approximately one in five children worldwide is growing up in the context of ethnic, national, or tribal war and violence (Fox, Reference Fox2019; Østby, Rusted, & Tollefsen, Reference Østby, Rusted and Tollefsen2018). Much further research is needed to fully understand how such chronic conditions impact children and families. Our study has important clinical implications by demonstrating the lasting effects of war exposure on key functioning of regulatory support systems at a key developmental transition, underscoring the importance of early PTSD diagnosis and highlighting the centrality of early avoidance symptoms. Our results on the interaction between RSA and early dyadic reciprocity emphasize the importance of building dyadic interventions to young children and their family that incorporate biofeedback elements and suggest that children with reduced physiological regulation may benefit from such dyadic interventions. The findings, therefore, may help the construction of more targeted interventions that focus on social engagement and reciprocity, begin early in life, include both mother and child, and consider biological systems that provide the support for the development of long-term adaptation.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579421000067
Acknowledgment
We are grateful to the mothers and children who participated in this study.
Financial Statement
The study was supported by the Brain and Behavior (NARSAD) independent investigator award to RF, the Simms-Mann Foundation and the Irving B Harris Foundation. KY is supported by Azrielli grant.
Conflicts of Interest
None.









