Introduction
Lumbar puncture (LP) is a simple technique to sample cerebrospinal fluid (CSF) as a window into central nervous system (CNS) biochemistry. A large body of literature now suggests that a biological diagnosis of Alzheimer’s disease (AD) can be made using measurements of pathogenic proteins in the CSF. Reference Zetterberg, Rohrer and Schott1 We have known for more than 25 years that decreased amyloid β levels in CSF reflect aggregation into plaques of cerebral amyloid β Reference Zetterberg, Rohrer and Schott1-Reference Van Nostrand, Wagner and Shankle5 – a core feature of AD pathological diagnosis Reference McKhann, Knopman and Chertkow6 ; and that high levels of microtubule-associated protein tau in CSF reflect axonal pathology in AD. Reference Blennow, Wallin, Agren, Spenger, Siegfried and Vanmechelen7,Reference Vigo-Pelfrey, Seubert and Barbour8 CSF analysis is now recognized as a reliable diagnostic tool for AD in NIA-AA and IWG-2 diagnostic criteria, Reference McKhann, Knopman and Chertkow6,Reference Dubois, Feldman and Jacova9 and is widely used in European academic memory clinics. Reference Duits, Martinez-Lage and Paquet10-Reference van Waalwijk van Doorn, Kulic and Koel-Simmelink14 In Canada, CSF samples are collected in academic dementia centers as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI), Reference Kang, Korecka and Figurski15 but the clinical use of CSF analysis in neurocognitive diseases remains scarce to this date. Reference Duits, Martinez-Lage and Paquet10-Reference van Waalwijk van Doorn, Kulic and Koel-Simmelink14,Reference Rosa-Neto, Hsiung and Masellis16 In our experience, CSF analysis is a useful tool to reach an earlier and more accurate diagnosis in patients with early onset atypical dementia, for whom the initial dementia workup did not allow to reach a clear diagnosis. In this manuscript, we report our clinical experience with CSF analysis in the diagnostic workup of 262 consecutive patients seen at our tertiary care memory clinic.
Methods
Patient Selection and Diagnostic Workflow
The Clinique Interdisciplinaire de Mémoire du CHU de Québec (CIME) is the oldest memory clinic in Canada. Reference Laforce, Verret, Poulin, Fortin and Houde17 It is visited by an average of 1500 patients per year, generally for further evaluation after an inconclusive initial assessment by a primary care physician. All patients were assessed according to the “Recommendations of the 4th and 5th Canadian Consensus Conference on the Diagnosis and Treatment of Dementia.” Initial consultation typically includes history-taking, physical examination, cognitive screening, targeted cognitive screening, basic blood work, and brain imaging with computed tomography or magnetic resonance imaging (MRI). In our experience, this initial diagnostic workup allows to reach a probable diagnosis in about 70% of patients. Reference Bensaidane, Beauregard and Poulin18,Reference Bergeron, Beauregard and Guimond19 When diagnosis remains unclear, patients can be referred to further neuropsychological testing or 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). When the diagnosis is still unclear, clinicians can repeat FDG-PET Reference Bergeron, Beauregard and Guimond19 or order either an amyloid PET Reference Bensaidane, Beauregard and Poulin18 or an LP with CSF analysis of AD biomarkers (see Figure 1).

Figure 1: Diagnostic workup at Clinique Interdisciplinaire de Mémoire du CHU de Québec, a tertiary care memory clinic in Quebec City, QC, Canada.
Lumbar Puncture and Cerebrospinal Fluid Analysis
All LPs were performed by three experienced neurologists (LV, RWB, RL) according to the Canadian and International Guidelines. Reference Rosa-Neto, Hsiung and Masellis16,Reference Engelborghs, Niemantsverdriet and Struyfs20-Reference Teunissen, Petzold and Bennett22 We collected a total of 5-20 ml of CSF divided into three to four sterile polypropylene tubes of approximately 10ml of CSF each: (1) cell count; (2) glucose, proteins; (3) AD biomarkers; and (4) further infectious and/or autoimmune investigation in select cases. Traumatic taps causing blood contamination of CSF were not used for biomarker study. Patients remained supine for 15–30 min, followed by progressive mobilization. All polypropylene tubes identified for AD biomarker analysis were centrifuged at 2000 × g for 10 min at room temperature within 15 min of LP. They were split into 2 ml aliquots in small polypropylene tubes and kept at –80 °C using dry ice. One 2 ml tube was sent overseas by express mail on dry ice to Amsterdam VUmc Alzheimercentrum for AD biomarker analysis. Other 2 ml was stored locally at –80 °C. Aβ-42, total tau (t-tau), and tau phosphorylated at threonine 181 (p-tau181) concentrations were measured with INNO-BIA AlzBio3 Luminex assay (Fujirebio, formerly Innogenetics, Gent, Belgium). All CSF analyses were performed at the end of the study at the VUmc in Amsterdam in the Netherlands. Reference values were >640 pg/ml for Aβ-42, <375 pg/ml for t-tau, and <52 pg/ml for p-tau181 according to single-center validation studies. Reference Zwan, van Harten and Ossenkoppele23-Reference Mulder, Verwey and van der Flier25 Due to the upward drift of Aβ-42 values measured with Innotest ELISA over the past two decades (caused by changes in ELISA kits over time), reference values for Aβ-42 changed over time, first >550 pg/ml, then >640 pg/ml, >680 pg/ml, then >1000 pg/ml. Reference Schindler, Sutphen and Teunissen26 An AD CSF profile was established when Aβ-42 was lowered and at least one tau measurement was elevated. When absolute values were at the limit of positivity, a ratio of p-tau/Aβ-42 was calculated to facilitate interpretation of the results; a ratio exceeding 0.024 was considered suggestive of Alzheimer’s pathophysiology.
Data Collection and Statistical Analyses
For all patients, we retrospectively retrieved the following variables: age at the time of LP, sex, number of years of education, MMSE score/30 closest to LP (<6 months), date of LP, Aβ-42, t-tau, and p-tau181 level, minor and/or major complications of LP (minor: post-LP headache, minor infection not requiring antibiotics; major: CNS infection requiring antibiotics, significant bleeding, post-LP headache requiring blood patch, etc.), date of amyloid PET if applicable, visual read of amyloid PET (positive/negative), concordance between LP and amyloid PET, primary and alternative diagnoses prior to LP, primary and alternative diagnoses following LP, change in diagnosis and/or management following LP. Analyses were performed using SPSS (version 22; IBM, Chicago, IL, USA) and STATA (version 14; StataCorp; College Station, TX, USA). Differences in demographical characteristics were assessed using ANOVA for continuous variables and χ 2 or Mann-Whitney U tests for dichotomous or categorical data.
Results
Patient Characteristics
Between July 2015 and April 2020, 3755 patients were evaluated at the CIME tertiary care memory clinic; in 262 patients (7%), an LP was performed to help clarify the diagnosis of the neurocognitive disorder. In these patients, the initial memory clinic workup, including history taking, neurological examination, cognitive testing, blood tests, MRI, and FDG-PET did not allow to reach a clear diagnosis. Patient characteristics are shown in Table 1. Patients were on average young (67.5 ± 2 years old), well-educated (12.7 ± 2 years of education), and at an early disease stage (MMSE 26 ± 3). Indication for LP covered a wide range of clinical scenarios (see Figure 2). Over the 262 LP, 121 (46%) were ordered to identify underlying AD pathology in patients with amnestic mild cognitive impairment (aMCI) with a high probability of progression to AD, 45 (17%) were ordered to distinguish the behavioral variant of frontotemporal dementia (bvFTD) from the dysexecutive variant of AD, 27 (10%) to identify the underlying pathology in primary progressive aphasia (PPA) with mixed/unclassifiable features, 29 (11%) to differentiate AD from psychiatric conditions (depression, psychotic disorder, attentional disorders, bipolar disorder, etc.), and 40 (15%) to distinguish AD from other conditions.
Table 1: Demographics of the sample
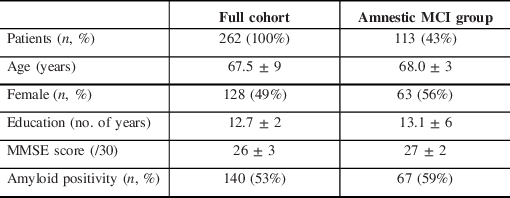
MCI = mild cognitive impairment.

Figure 2: Clinical indications for CSF analysis. A+=amyloid-positive; A−=amyloid-negative; AD=Alzheimer’s disease; aMCI=amnestic mild cognitive impairment; FTD=frontotemporal dementia; PPA=primary progressive aphasia; Psy=Psychiatric disorder.
CSF Results
In the total cohort, 140 out of 262 (53%) patients had lowered Aβ-42 levels compared to 122 patients with normal Aβ-42 levels. Of those, 49 out of 101 (49%) patients 65 years old or less were amyloid-positive compared to 91 out of 151 (56%) older than 65 years old. Seven patients had both CSF analysis and amyloid PET results. CSF and amyloid PET were concordant in 6/7 (86%) of cases for amyloid β status. The discordant case was a patient with chronic alcohol use, depression, and a clinical suspicion of AD whose amyloid PET was negative, CSF Aβ-42 was borderline (550 pg/ml), and t-tau and p-tau181 was normal (163 pg/ml and 25 pg/ml, respectively).
Impact on Diagnosis and Management
The LP was performed to rule out the presence of AD pathology in 121 patients with aMCI (mean MMSE 26.7). The LP showed a CSF profile consistent with AD pathophysiology in 72/121 patients (59%); these patients were diagnosed with aMCI due to AD pathology and treatment with acetylcholinesterase inhibitor was initiated. The 49 patients with normal CSF profiles were reassured that the LP did not reveal changes consistent with AD, and that patients with amyloid-negative MCI generally have a better cognitive prognosis. In patients with an atypical cognitive profile or abnormal FDG-PET, an alternative neurodegenerative pathology was considered. One patient received a diagnosis of bvFTD and a second diagnosis of primary age-related tauopathy (PART).
The LP was performed in 45 patients to distinguish AD versus FTD pathology in patients with atypical behavioral presentation. The LP showed a CSF profile consistent with AD pathophysiology in 25/45 (55%) of patients, leading to a diagnosis of behavioral/dysexecutive AD. 20/45 patients were amyloid-negative. Seventeen patients were diagnosed with bvFTD, one with corticobasal syndrome, and two patients had uncertain diagnoses at the time the charts were analyzed. The LP leads to a change in the primary diagnosis in 23 (51%) of the cases.
The LP was performed in 27 patients with PPA and a clinicoanatomical syndrome, which did not allow confident classification among one of the three variants. The LP showed a CSF profile consistent with AD pathophysiology in 15/27 (55%) of patients, leading to a diagnosis of AD with language presentation (logopenic variant of PPA or mixed PPA due to AD). 12/27 patients were amyloid-negative and was diagnosed with PPA due to FTD pathology. More specifically, amyloid-negative patients were diagnosed with the semantic (four patients) and non-fluent (four patients) variants of PPA, primary progressive apraxia of speech (one patient), and subjective memory complaints (three patients).
In 40 patients, the LP was performed to distinguish AD from other disorders like vascular dementia, normal pressure hydrocephalus, paraneoplastic encephalitis, LGG1 encephalitis, prion disease, etc. The CSF was suggestive of AD pathophysiology in 19/40 (47%) of patients, in whom treatment with acetylcholinesterase inhibitor was initiated. In seven patients, biochemistry, cell count, and culture of the CSF contributed to diagnosing conditions such as limbic encephalitis and multiple sclerosis, or to rule out conditions such as Creutzfeld-Jackob disease. In six patients with disproportionate hydrocephalus on imaging, the LP allowed measurement of the clinical response to CSF drainage (lumbar tap test); four of these patients were diagnosed with normal pressure hydrocephalus and two patients did not improve following the LP and had CSF analysis consistent with AD. In 29 patients, the CSF analysis was allowed to distinguish AD from psychiatric disorders. The CSF profile was consistent with AD pathophysiology in 9/29 (31%) patients, hence a diagnosis of AD with neuropsychiatric symptoms was made. The 20 patients with normal CSF profiles were reassured that the LP did not reveal changes consistent with AD and were diagnosed with primary psychiatric diagnoses (ranging from dysthymic conditions, adult-onset attention disorder to psychosis). One patient was diagnosed with behavioral bvFTD at follow-up.
The ultimate goal of LP is to improve diagnostic accuracy and guide clinical decisions with regard to treatment. In the total cohort of 262 patients, 140 (53%) patients received positive LP results with CSF biomarkers compatible with AD pathology. Of these 140 patients with positive LP, 117 (84%) began treatment with an acetylcholinesterase inhibitor on follow-up. Of the remaining patients, 23 (16%) were already taking an inhibitor prior to their LP (prescribed either by the referring physician or by the memory clinic team based on the etiological hypothesis) and this was continued. In total, 32 (12%) patients who consulted were already taking inhibitors, 27 (84%) were continued following LP, and 5 (16%) were stopped.
Safety of the Procedure
We systematically reviewed patients’ charts for minor or major complications. Six patients had persistent post-LP headaches requiring a blood patch. Nine patients had minor complications such as temporary positional headache not requiring blood patch (six) and back pain (three). No patients had an iatrogenic CNS infection, hematoma, or brain herniation.
Interpretation
In this manuscript, we report our experience with LP and CSF analysis in the diagnosis of 262 patients with neurocognitive disorders in a tertiary care memory clinic. To our knowledge, this is the most significant clinical experience with CSF analysis in this context in Canada. CSF analysis helped reach an early diagnosis of aMCI due to AD in patients with aMCI at high risk for AD, helped distinguish clinical variants of AD (dysexecutive, language) from FTD variants, and helped distinguish AD from other disorders (psychiatric, vascular, inflammatory, etc.). CSF Aβ-42 was concordant with amyloid PET in 83% of the cases, consistent with previous studies. Reference Zwan, van Harten and Ossenkoppele23,Reference Janelidze, Pannee and Mikulskis27,Reference Leuzy, Chiotis and Hasselbalch28 In some patients, the LP allowed to confirm or exclude alternate diagnoses through biochemistry, cell count, and culture of CSF; or through clinical evaluation following lumbar tap test. No major complication occurred following the LP. These results suggest that CSF analysis is a safe and effective diagnostic tool in complex/atypical dementia cases, hence that Canadian memory clinics would benefit to implement infrastructure to handle and analyze CSF biomarkers for AD and other dementias.
Safety of Lumbar Puncture in the Memory Clinic
Our study confirmed the safety of LP in the memory clinic, with only six major complications (2%; persisting positional headache requiring blood patch) and nine minor complications (3%; temporary headache and/or back pain) over 262 patients. In the multicenter LP feasibility study, a consortium of 23 European academic dementia centers recently studied the performance and complications of LP for dementia diagnosis in a cohort of 3868 patients. Reference Duits, Martinez-Lage and Paquet10 After the procedure, 17% of patients reported back pain and 19% of patients reported headaches. Headaches resolved within 4 d in 78% of the cases. Only 11 patients (0.3%) received a blood patch, in which 23 (0.7%) were hospitalized. An atraumatic needle and age >65 years were associated with a lower prevalence of post-LP complaints. Of course, less invasive biomarkers (such as blood biomarkers) would represent preferable diagnostic tools; however, the blood-brain barrier makes it difficult to identify CNS proteins in the blood in sufficient levels to reach acceptable diagnostic accuracy for clinical use. Reference Keshavan, Heslegrave, Zetterberg and Schott29 Some high-fidelity assays have shown reasonable correspondence with CSF amyloid and tau levels, but their accuracy would currently allow for pre-screening at best. Reference Palmqvist, Janelidze and Stomrud30
Diagnostic Properties of CSF Biomarkers and Comparison with Amyloid PET
In this study, the strong diagnostic impact of CSF analysis was derived from the assumption that CSF Aβ-42, t-tau, and p-tau181 reliably reflect AD neuropathology; gold-standard autopsy confirmation was not available in any patient. Converging evidence has highlighted the inverse correlation between CSF Aβ-42 and cerebral Aβ plaques at autopsy or brain biopsy in healthy controls, MCI, AD, and non-AD dementias. Reference Seeburger, Holder and Combrinck31-Reference Strozyk, Blennow, White and Launer34 CSF t-tau and p-tau181 were also shown to correlate with neocortical tangle pathology at autopsy or brain biopsy. Reference Seppala, Nerg and Koivisto32,Reference Tapiola, Alafuzoff and Herukka33,Reference Buerger, Ewers and Pirttila35 Subsequently, the emergence of PET Aβ and Tau ligands have enabled visualization of Aβ plaques in the brain in living patients. Reference Villemagne, Dore and Bourgeat36 Concordance between amyloid positivity shown by CSF Aβ-42 and amyloid PET has consistently remained around 90% regardless of the ligand. Reference Mo, Stromswold and Wilson13,Reference Lewczuk, Riederer and O’Bryant21,Reference Zwan, van Harten and Ossenkoppele23,Reference Janelidze, Pannee and Mikulskis27,Reference Herukka, Simonsen and Andreasen37-Reference Alvarez, Aguilar and Gonzalez43 and CSF t-tau, and p-tau181 showed good correlation with Tau PET tracer binding. Reference Mattsson, Scholl and Strandberg44-Reference Gordon, Friedrichsen and Brier46 In MCI, abnormal CSF Aβ-42, t-tau, and p-tau181 status has been associated with higher prevalence and faster pace of conversion from MCI to AD. Reference Herukka, Simonsen and Andreasen37,Reference van Maurik, Zwan and Tijms47-Reference Handels, Wimo and Dodel49
Limitations and Future Perspectives
Limitations of the use of CSF analysis in tertiary care memory clinics include the invasiveness of the technique, the risk of minor and major complications, the lack of harmonization of methods for the handling of CSF samples, and the upward drift in CSF Aβ-42 values over time due to changes in ELISA kits. Reference Schindler, Sutphen and Teunissen26,Reference Mattsson, Andreasson and Persson50,Reference Verwey, van der Flier and Blennow51 Ongoing multicenter efforts aim to better standardize the handling and analysis of CSF samples in order to facilitate their widespread clinical use. Reference Lewczuk, Riederer and O’Bryant21,Reference Herukka, Simonsen and Andreasen37,Reference Frisoni, Boccardi and Barkhof52-Reference Teunissen, Otto and Engelborghs54 Our study has limitations. Our study was a retrospective evaluation of the use of LP at our memory clinic. This implies a significant selection bias, since the LP was performed only in a minority of patients evaluated at our memory clinic (see Figure 1), when the standard memory clinic workup (clinical and neuropsychological evaluation, lab tests, MRI, FDG-PET) did not allow to reach a clear diagnosis. Nevertheless, our study provides real-world data on the clinical use of CSF analysis in the tertiary care memory clinic. In this study, we only had access to CSF Aβ-42, t-tau, and p-tau181 measurements, consistent with international consensus recommendations. Reference Herukka, Simonsen and Andreasen37,Reference Simonsen, Herukka and Andreasen55 However, the field of CSF-based biomarkers is rapidly evolving, and multiple novel assays are now available to evaluate Aβ metabolism (sAPPα, sAPPβ, Aβ-40, Aβ-38), synucleinopathies (a-synuclein), neurodegeneration (neurofilament, NSE, VLP-1, neurogranin, HFABP), and glial activation (YKL-40, MCP-1, GFAP). Reference Zetterberg, Rohrer and Schott1,Reference Olsson, Lautner and Andreasson56-Reference Teunissen and Parnetti58 Although validated assays for these new biomarkers are not readily available for clinical practice, they hold great potential to improve diagnostic accuracy in AD, but also in FTD and other neurodegenerative disorders. Reference Olsson, Lautner and Andreasson56,Reference Llorens, Schmitz, Ferrer and Zerr57,Reference Vijverberg, Dols and Krudop59-Reference Mattsson, Insel and Palmqvist62 Therefore, while PET currently does not allow the concomitant use of multiple tracers, CSF analysis has the potential to inform us on the homeostasis of a wide range of biological pathways in order to reach a more accurate biological diagnosis of dementia. Finally, some would argue that LP represents an invasive treatment to diagnose disorders for which cannot benefit from disease-modifying treatments. There is evidence, although inconsistent, that initiation of acetylcholinesterase inhibitors early in the disease (even at the MCI stage) can delay the progression to the dementia stage. Reference Kishi, Matsunaga, Oya, Ikuta and Iwata63-Reference Seltzer, Zolnouni and Nunez65 Furthermore, in early onset dementia, a timely diagnosis also greatly reduces the anxiety related to the diagnostic uncertainty and allows to better plan for future care. Reference Bensaidane, Beauregard and Poulin18,Reference Dubois, Padovani, Scheltens, Rossi and Dell’Agnello66
Conclusion
Altogether, our results highlight the safety and clinical utility of CSF biomarkers for the diagnosis of select patients with neurocognitive disorders, and advocate for their increased use in tertiary care memory clinics in Canada.
Acknowledgments
The authors wish to thank the Primary Progressive Aphasia Research Chair – Lemaire Family Fund at Université Laval and the Vanier Graduate Scholarship of the Canadian Institutes of Health Research (CIHR) for funding this research.
Conflict of Interest
We have no competing interests to disclose.
Statement of Authorship
MS and DB contributed equally to this work. DB, MS, and RJrL were involved in study design, data analysis, and drafting the initial version of the manuscript. MS, DB, EP, XR SMD were involved in data collection. KM coordinated the handling of CSF samples. LS, PM YN, MPF, SC, SP, LV RWB, and RJrL are clinicians at the CIME who were involved in implementing CSF analysis as a diagnostic tool for atypical dementia. CT was our main collaborator at VUmc Alzheimercentrum and shared her experience in using CSF analysis for dementia diagnosis. All authors provided input to the manuscript and agreed on the final version of the manuscript.







