Introduction
Stereoelectroencephalography (SEEG) has primarily been used as a diagnostic procedure since its development in the 1950s. Recently, a therapeutic aspect has emerged, specifically, radiofrequency thermocoagulation (RF-TC) delivered via intracerebral electrodes implanted for SEEG.
Stereotactic lesioning itself is not a new concept in epilepsy surgery,Reference Flanigin and Nashold1 but the last two decades have seen a resurging interest in RF-TC delivered via SEEG electrodes implanted for seizure localization. Since the description of the first experience with SEEG-guided RF-TC by Guenot et al. in 2004, a handful of series have been published with experiences from different centers in Europe and Asia.Reference Guénot, Isnard, Ryvlin, Fischer, Mauguière and Sindou2-Reference Fan, Shan and Lu19 These reports have utilized RF-TC techniques to address various pathologies causing refractory epilepsy. Given the safety profile and size of lesion created, as determined by Bourdillon et al in their in vivo and in vitro data, the SEEG-guided RF-TC method may be an ideal technique for these lesions.Reference Bourdillon, Isnard and Catenoix20
SEEG was introduced in North America by Dr. Andre Olivier and has been regularly used in our center since the early 1970s.Reference Olivier, Boling and Tanriverdi21-Reference Hall and Khoo25 It has undergone continuous evolution to take advantage of advances in imaging, computer-based navigation, electrode materials, and robotics. In this paper, we describe our experience of SEEG-guided RF-TC at the Montreal Neurological Institute (MNI). The first case of SEEG-guided RF-TC was performed in May 2016 by the senior author (JH). Patient selection, technique, and outcomes are detailed in this report. To our knowledge, this is the first series from a North American Epilepsy Center utilizing the SEEG-guided RF-TC approach as a potential treatment for refractory focal epilepsy.
Methods
Retrospective chart review, approved by the departmental ethics committee, was conducted. All patients who underwent RF-TC at our institution were included. Patient data were collected in Statistical Package for Social Sciences (SPSS IBM). Variables collected included Demographics: Patient age, gender, age of seizure onset, age at diagnosis, cause if described, time in years until the patient was considered for surgical workup, number of antiepileptic drugs (AEDs) (current and past); Pre-surgical workup: Functional and structural imaging studies (MRI, fMRI, EEG-fMRI, PET, SPECT, MEG, HD-EEG), neuropsychological testing, lesional MRI, location of lesion, type of lesion; Seizure data: Semiology, Epileptogenic zone (EZ) by hypothesis, scalp EEG findings; Surgical data: Prior operation, SEEG, number of electrodes, location of electrodes, cortical stimulation via SEEG, and RF-TC location; Outcomes: Seizure recurrence after RF-TC, complications, repeat operation after RF-TC, and Engel Class.
Operative Technique
Preoperative Planning
At our center, weekly seizure conferences are conducted and candidates for SEEG implantation are discussed in a multidisciplinary fashion. An electroclinical hypothesis is formulated after careful study of the clinical semiology, scalp EEG findings, and ancillary testing (3T MRI with post-processing, PET, SPECT, MEG, EEG-fMRI, and HD-EEG), which generates candidate foci for SEEG implantation. The electrodes have traditionally been placed 8–10 mm apart, with the aim of identifying the seizure onset and propagation zones. With RF-TC, it may be advisable to place the electrodes closer together (ideally within a 5 mm distance), specifically around the region which is being considered as the primary EZ. This provides the team with the option to perform RF-TC across these closely placed electrodes. The preoperative planning is then completed on the ROSA (MedTech. Montpellier, France) robotic guidance system. All patients undergo thin-cut MRI with contrast as well as CT angiogram, which are fused and utilized for electrode planning.
Placement of SEEG Electrodes and Seizure Investigation
Since 2011, robotic SEEG electrode placement has been performed at our institution.Reference Hall and Khoo25 Of note, hair is not shaved for electrode placement and a chlorhexidine solution is used to perform a sterile wash of the scalp and hair. From 2007 onwards, we have used electrodes from DIXI Medical (Besancon, France). Standard electrodes have 10 or 15 contacts. Each contact is 2 mm in length and 0.8 mm in diameter with an intercontact spacing of 1.5 mm. Postoperatively, thin-cut T1 and T2 MRI images are obtained to identify accurate anatomic placement and the exact location of each contact on the electrode. Seizure recording proceeds in the standard fashion in the epilepsy monitoring unit (EMU). Patients may undergo cortical stimulation since stimulation of habitual seizures has been shown to be as reliable as spontaneous seizures in determining EZ.Reference Cuello Oderiz, von Ellenrieder and Dubeau26 This is likely to shorten the duration of implantation and may avoid risks associated with abrupt withdrawal of AEDs.
RF-TC and Removal of SEEG Electrodes
Once the seizure mapping is complete and electrode removal is planned, RF-TC may be considered in consultation with the epileptologist. The technique itself is quite simplistic. The contacts on the MRI are identified across which the RF-TC will be performed. Accurate labeling of each electrode and each contact is recorded in a standardized format. The patient is brought to the operating room and kept awake. The contacts across which RF-TC will be performed are identified on the electrode connectors and the rest are excluded from view (Figure 1). The radiofrequency device (Radionics Medical Products, Model No. RFG-5, Burlington, MA, USA) is set to the maximal RF output. Hand-held applicators, developed by the biomedical staff at the request of the senior author (JH), are then utilized to pass current across the selected contacts (Figure 2). At this point, feedback from the patient is critical. The patient reports a sharp high-pitched sound with interval decrements over the next few seconds. The patient can be instructed to say out aloud the decrement in the sound by percentage (75, 50, 25, stopped). Often, a crackling sound can be heard by the operator. This process is repeated across all planned electrodes. Occasionally, the patient can report some discomfort which dissipates immediately as the passage of current ceases. We have noted occasional vague headache or facial pain in the trigeminal distribution which could be due to proximity of the electrode to the tentorium and/or Meckel’s cave region.

Figure 1: Operating room setup.

Figure 2: Application of RF-TC.
Coagulation Technique
We have utilized two methods of coagulation, Axial coagulation (along an electrode) and Cross-Coagulation (between electrodes). In the axial coagulation technique, coagulum formation occurs around and between two adjacent contacts on a single electrode. In the cross-coagulation technique, when contacts on two electrodes are within a 5 mm distance, coagulation can be performed between those contacts forming a bridging coagulum (Figures 2 and 3).
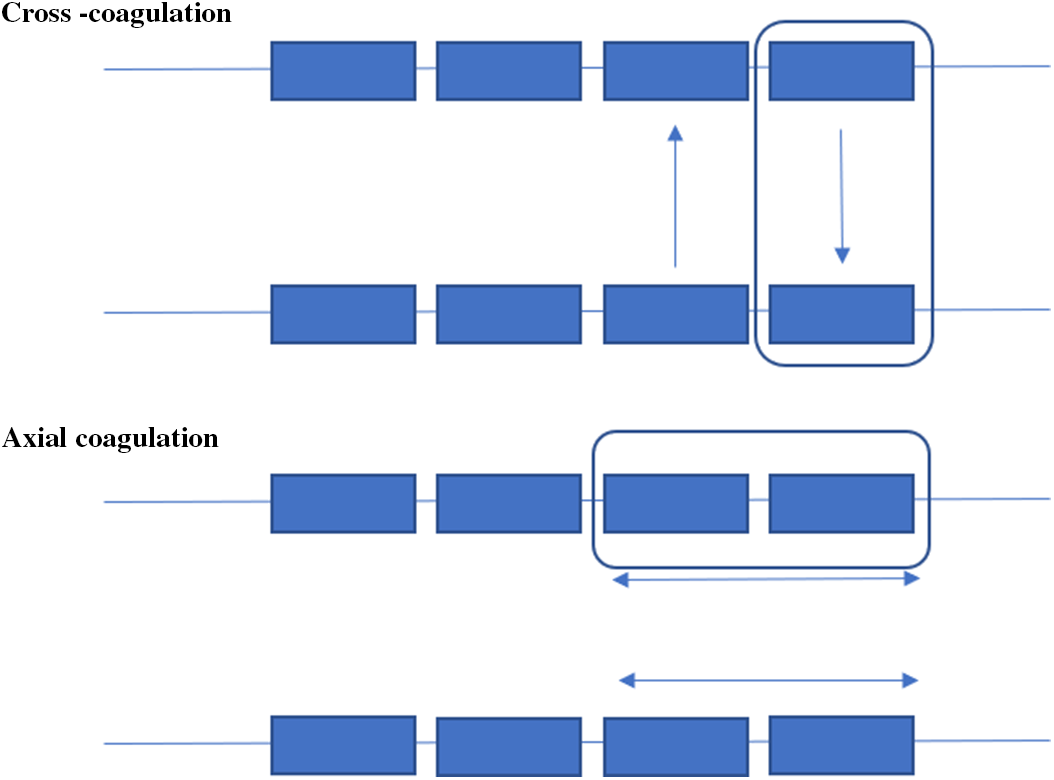
Figure 3: Coagulation techniques.
Once RF-TC is complete, the patient is placed under general anesthesia and electrode removal ensues in the usual fashion. Some patients have undergone additional recording after the thermocoagulation to evaluate the effects of RF-TC prior to SEEG removal.
To remove the electrodes, the head dressing is removed, and the SEEG leads are cut at the caps which secure them to anchoring pegs. The hair and electrode sites are washed with chlorhexidine. A sterile field is established. The electrode caps are unscrewed from the pegs and the electrodes are removed. The pegs are carefully removed using a specially adapted wrench. The peg sites are inspected. A second wash is performed with chlorhexidine and a head wrap is placed. Sutures are not required. The head wrap stays in place for 2–3 days after which time the patient can shampoo their hair normally.
Postoperative Care
Patients are kept one night in the hospital to monitor for any early untoward effects of the ablation. MRI is obtained, specifically thin-cut T1 and T2 images, the same day to ascertain the size of the lesion created (Figures 4–7). Patients are usually discharged on the next day with scheduled follow-up. AEDs are titrated back to baseline dose by the epileptologist prior to discharge.

Figure 4: (Patient #1): Electrode implantation around the previous SMA resection cavity (Row 1). Post-RF-TC inferior and posterior to the resection cavity (Row 2).

Figure 5: (Patient #8): Electrode implantation (Row 1). Post-RF-TC left Heschel’s gyrus – Immediate Post-op (Row 2). MRI FLAIR image 2 years post-RF-TC (Row 3).

Figure 6: (Patient #11): Electrode implantation (Row 1). Immediate post-op RF-TC of right PVNH (Row 2). Immediate post-op RF-TC of left PVNH (Row 3).

Figure 7: (Patient #14): Electrode implantation (Row 1). Immediate post-op RF-TC of right amygdala (Row 2). Immediate post-op RF-TC of right hippocampal head (Row 3). Immediate post-op appearance of lesion on T2-weighted sequences (Row 4).
Results
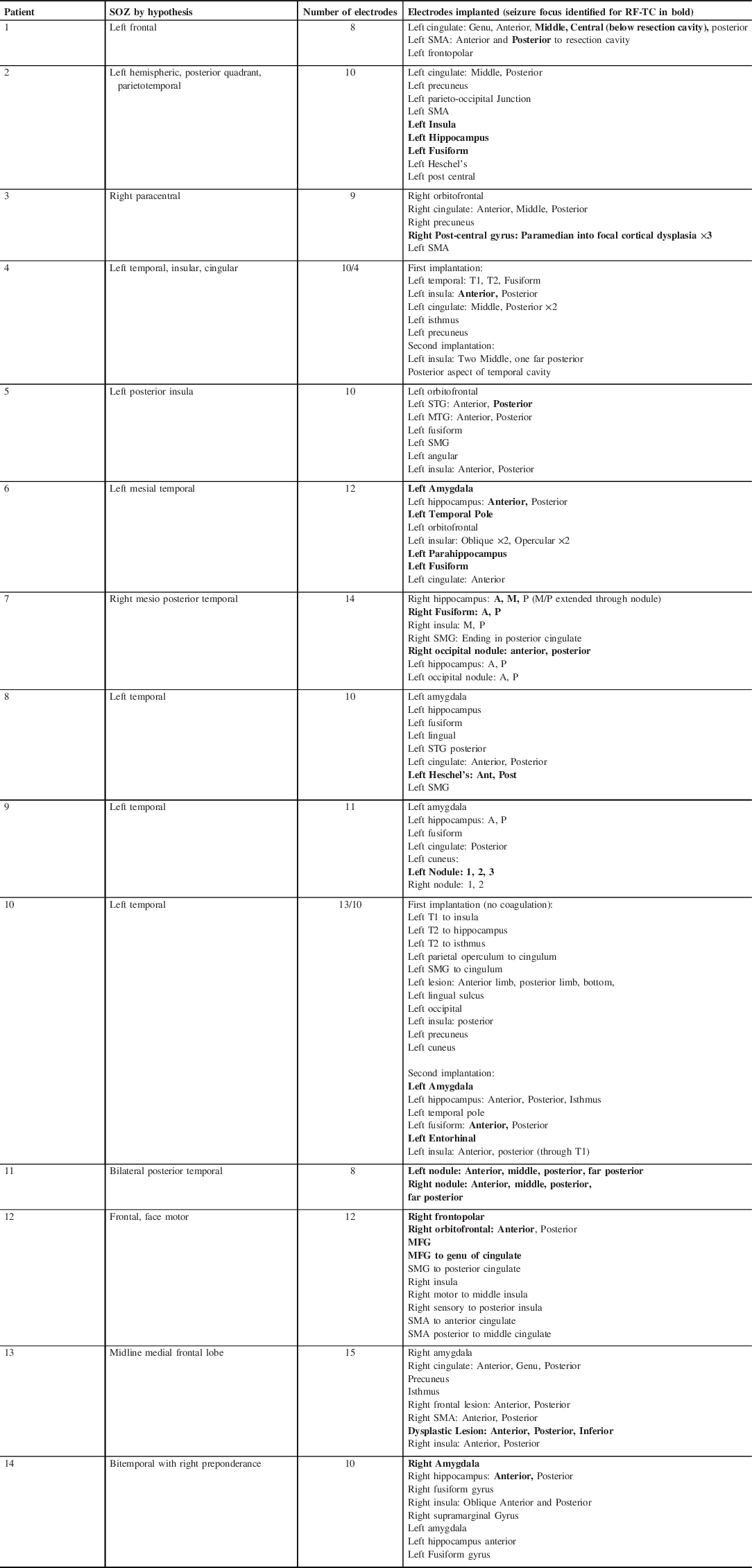
Fourteen patients underwent SEEG-based RF-TC between May 2016 and September 2019 (Table 1). Seven males and seven females. Mean age is 34.43. Mean age at seizure onset was 13.77. Mean time from diagnosis to being assessed for surgery was 14.33 years. All patients underwent a complete pre-surgical workup. Three patients had had prior operations (SEEG, resection, VNS). Eight patients had lesional findings on MRI (four FCD, three PVNH, one Cavernoma). Six had a negative 3T MRI. All patients underwent robotic SEEG implantation to clarify a preimplantation hypothesis regarding the EZ (Table 2). In 13 patients, electrophysiologic data was suggestive of the primary focus which subsequently underwent RF-TC. One patient underwent RF-TC via SEEG electrodes without SEEG recording to bilateral periventricular nodular heterotopia (PVNH) as a primary ablation procedure. No catastrophic perioperative complications were noted with either SEEG implantation, RF-TC, or SEEG removal. One patient had transient verbal memory deficits following hippocampal ablation which improved spontaneously, and one developed symptomatic SMA region edema which resolved with steroid administration.
Table 1: Patient data

Table 2: Hypothesis/electrodes/RF-TC
In patients with lesions identified on MRI, one of the three with PVNH was seizure free after RF-TC (Engel 1a), one had seizure freedom for 18 months (Engel 2b), and one required temporal neocortical/PVNH resection rendering him seizure free (Engel 1a); one of the four patients with FCD were seizure free after RF-TC (Engel 1a); of the remaining three, two attained seizure freedom after resection (Engel 1a and 1b), while one continues to have significant seizures (Engel 4b). One patient with a cavernoma and low central area EZ (suspected FCD based on imaging and SEEG findings) did not derive any benefit from RF-TC and underwent resection. In the MRI-negative group, six patients underwent RF-TC. Two of these achieved seizure freedom for 3 months and 1 year, respectively, and then went on to Engel 1a after resection. Three had seizure recurrence immediately or within weeks of RF-TC (Engel 4b). One patient remains seizure free at 4 weeks after RF-TC of the right amygdala and hippocampus.
With RF-TC alone, only 2/14 (14%) patients achieved Engel 1a, two patients had more than 1 year of seizure freedom (14%), one patient had 3 months of seizure freedom, while the rest had recurrence immediately or within a few weeks. 7/14 patients underwent secondary interventions after RF-TC. Overall, when combining RF-TC and secondary intervention, seven patients (50%) achieved Engel 1a or 1b seizure freedom, one each 2b and 3a, and five Engel 4b. Transient postoperative complications after RF-TC were seen in two patients (14%). One noted a verbal memory deficit with spontaneous resolution, and one developed contralateral weakness due to SMA region edema which responded well to steroid administration.Footnote 1
Discussion
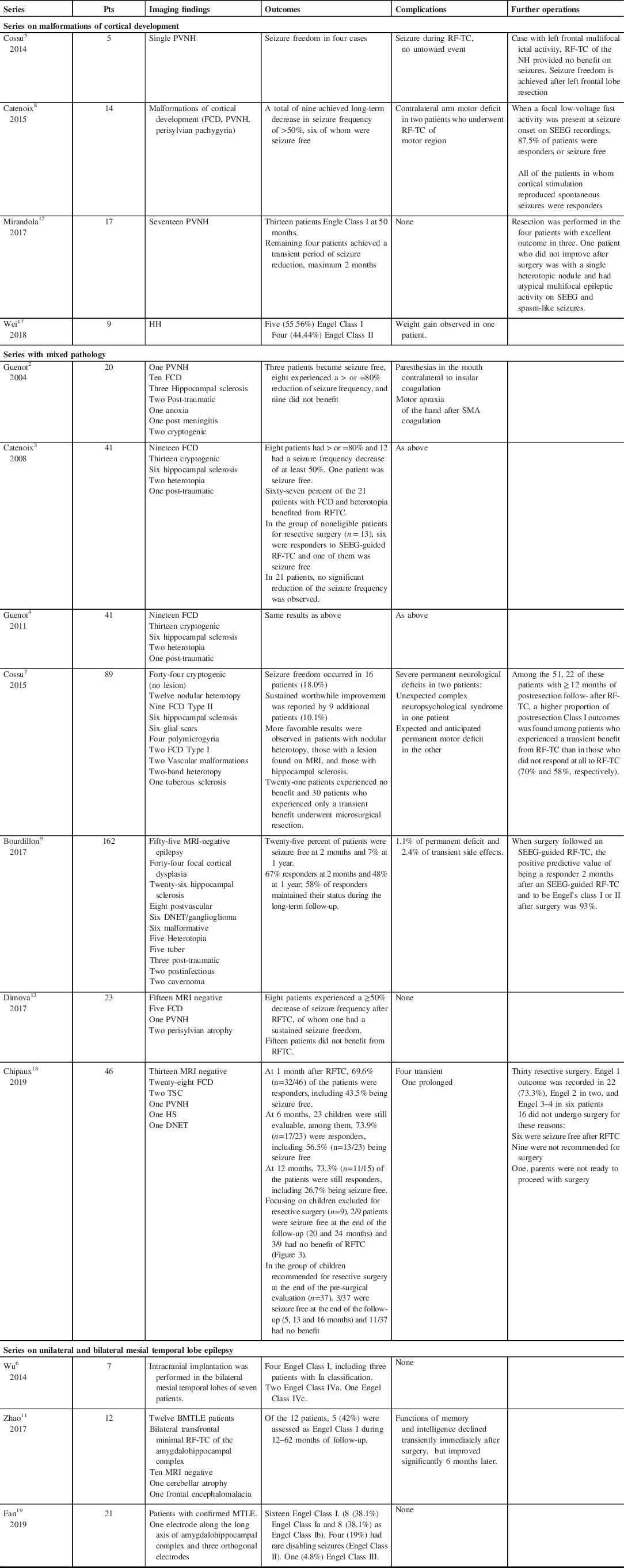
RF-TC is a minimally invasive technique, which can be performed via SEEG electrodes in situ, placed for pre-surgical workup. It does not require a separate incision, passage of probe, or craniotomy. The use of RF-TC has been described in cases of refractory epilepsy from cortical malformations, hypothalamic hamartomas (HH), and PVNH (Table 3). Generally, unilateral lesions have been noted to respond better than bilateral cases. Deep-seated lesions that require disruption of normal white matter tracts to gain surgical access may be better suited to RF-TC as first-line therapy. In non-lesional cases, the utility of this technique appears limited and warrants further investigation.
Table 3: Literature review of series on SEEG RF-TC
Electrodes have traditionally targeted sites to clarify a well-defined preoperative hypothesis with the aim of suggesting an eventual definitive surgical resection. The time has come to consider the potential therapeutic aspect of SEEG-guided RF-TC when planning the investigation, as larger more conformal lesions may be made possible with a more densely placed array.
We have employed two techniques of RF-TC, which we refer to as axial and cross-coagulation. Axial coagulation occurs along adjacent contacts on the same electrode and cross-coagulation occurs between contacts of proximal electrodes. The combination of more dense coverage and tailored RF-TC would be expected to increase the size of the lesion currently reported for SEEG RF-TC. Lesion size is dependent on properties of the electrode (diameter, contact size and spacing, etc.), the amount of energy passed through the tissue, and intrinsic tissue properties. In one study, in vitro analysis was conducted utilizing chicken egg whites. Counterintuitively, larger lesions across electrodes were created at a lower power (3 watts) even if contacts of the two electrodes were 8–12 mm apart. Once a rise in tissue impedance was seen, no further enlargement of the lesion was noted despite the continued application of current for up to 180 s.Reference Staudt, Maturu and Miller27 In the same study, in vivo application revealed similar results. Three patients underwent SEEG-based RF-TC and larger lesions were noted with an application of 3 watts, albeit with more perilesional edema which may not be desirable within or near eloquent regions. As noted in one of our patients, even a small conformal lesion in the premotor region was enough to cause enough peri-lesional edema resulting in delayed onset contralateral weakness which resolved with steroid administration.
In our series, after RF-TC, five patients proceeded to resection (#3, #6, #7, #8, #13) and one patient underwent VNS placement (#2). Of these, Patients #3, #6, #7, and #8 were early responders to RF-TC and fared well overall after subsequent resection. Patient #1 had a marginal response for 1 month but was not a candidate for any further resection. There were four nonresponders, two of whom (#4 and #5) had had prior SEEG as well as multiple resections and generally were not felt to be good candidates for further resection. Both underwent RF-TC as a palliative procedure, but did not derive any significant benefit. Patient #12 also had a minimal response and is planned to undergo resection. Patient #13 had an immediate recurrence of seizures but underwent resection of FCD with excellent seizure control. Good seizure control was achieved in one patient for more than 1 year who had non-lesional epilepsy localized to the left Heschel’s gyrus. Upon recurrence of seizures after an excellent seizure-free period, he underwent dominant Heschel’s gyrus resection and remains seizure free thus far (Patient #8). Two other patients (#10 and #11) achieved seizure freedom, and one (#9) had a major reduction in her seizures after RF-TC alone but they have short-term follow-up. Among the four MRI-negative patients with immediate or early recurrence of seizures, patient #14 is of considerable interest. She underwent bitemporal implantation with a predominantly right-sided temporal hypothesis, and subsequently underwent RF-TC to the right amygdala and hippocampus. Post-RF-TC, she remains seizure free at the most recent follow-up (Figure 7).
In our series, FCD and unilateral PVNH had the best response to RF-TC alone. In MRI-negative or “non lesional” cases, we noted the immediate recurrence of seizures after RF-TC in two patients, and in one within 3 weeks. All three nonresponders had undergone RF-TC as a palliative option, therefore the lack of impact on seizure control was not surprising. Conversely, in lesional cases such as patient #13 with FCD, complete lack of response with RF-TC was not a negative predictor of success of the subsequent surgical operation. This suggests that the lesion created was too small to impact the EZ. The two “non-lesional” cases who were responders for 3 months and 1 year, respectively, went on to have successful resections and are now seizure free. This suggests the time duration of seizure freedom post-RF-TC may be an important distinction within the MRI-negative group of patients, as it may point to more localized EZ which would be amenable to a larger lesion by SEEG-guided RF-TC.
RF-TC does not preclude future resective operations if seizures recur. In the handful of series published on this technique, several have described subsequent resections after RF-TC with successful outcomes.Reference Cossu, Fuschillo and Cardinale5,Reference Cossu, Fuschillo and Casaceli7,Reference Bourdillon, Isnard and Catenoix9,Reference Mirandola, Mai and Francione12,Reference Chipaux, Taussig and Dorfmuller18 Early response, albeit transient, has also been suggested to hold a positive predictive value for Engel Class 1 outcome after surgical resection.Reference Cossu, Fuschillo and Casaceli7,Reference Bourdillon, Isnard and Catenoix9 At this time, we do not feel that RF-TC is a replacement for resective surgery especially in those cases which are easily amenable to resection. In patients with EZs in speech or motor areas who are deemed to be poor candidates for resection, RF-TC may be used as a palliative option.Reference Chipaux, Taussig and Dorfmuller18 There is little downside to performing an ablation, even in cases that are deemed appropriate for future resection, as it may impart some seizure freedom until the patient returns for the definitive operation. Even a short period of improvement in seizures may allow the patient a glimpse into a seizure-free life and provide motivation for the definitive operation.
Strengths and Limitations
This is a single-center, single-surgeon series of complex refractory focal epilepsy patients. The technique we are describing can be easily performed at any neurosurgical center, which is performing SEEG electrode placement and has access to a radiofrequency generator. There is no added cost to this technique, no added length of stay, and no significant discomfort to the patient.
At our quaternary care center, we see complex epilepsy cases referred from within and outside of North America. Many of these have had prior intracranial investigations or surgical interventions either with us or at other centers. Therefore, our patient cohort may not be reflective of the patient population being investigated at other centers. The main limitation is the rather short follow-up time on patients who responded well to their RF-TC and have thus far not required any second intervention.
Conclusion and Future Implications
With the growing number of SEEG implantations in North America, there will likely be an increasing use of this type of RF-TC in treating focal epilepsy. Large-scale multicenter studies will help in determining the true utility of this technique, especially in “non-lesional” cases. In addition to better imaging, algorithmic computer-generated trajectories for SEEG electrodes with a priori hypothesis for EZs based on semiology and scalp studies will improve our ability to target lesional and so-called “non-lesional” MRI-negative epilepsy cases.Reference Zelmann, Beriault and Marinho28 Dense target coverage with a combination of axial and cross-coagulation, and perhaps different parameters of lesion generation can lead to a larger tailored lesion. With this approach, SEEG may be employed not only as a diagnostic method but may also hold therapeutic value in select cases when combined with RF-TC.
Disclosures
No conflict of interest is reported by the authors.
Statement of Authorship
FAM, JAH: Conceptualization of study, data collection, and manuscript development, and approval of the final manuscript.
STROBE/PROCESS Guidelines Followed for Manuscript Development
Significance of the Work
Stereoencephalography (SEEG)-based radiofrequency thermocoagulation (RF-TC) is a safe ablative technique, which is performed via in situ SEEG electrodes. It can be particularly effective for lesional epilepsy due to malformations of cortical development, such as focal cortical dysplasia (FCD) and periventricular nodular heterotopia (PVNH). It may have a role in non-lesional mesial temporal lobe epilepsy as well. It has been utilized in France and Italy for the last several decades, but has not been used extensively in North America. In this paper, we have described our methodology and the two techniques we utilize, axial coagulation and the cross-coagulation. We have attempted to highlight the fact that until now SEEG has been only used as a diagnostic modality. Combining it with RF-TC provides an effective therapeutic option as well. It has a high safety profile and can be performed without any added expense to the institution or patient. We have described our methodology in applying this technique, which will be helpful to epilepsy surgery teams who are considering adopting it for their patients. We believe SEEG-based RF-TC will be an important addition to any epilepsy surgeon’s armamentarium.














