Polyneuropathy is a common neurological disorder, with an overall prevalence of 2.4%, increasing to 8% in people older than 55 years.Reference Martyn and Hughes 1 The diagnosis of polyneuropathy is typically based on a combination of clinical symptoms, signs, and electrophysiological findings.Reference England, Gronseth and Franklin 2 Laboratory tests are used to screen for specific etiologies of polyneuropathy. Although the highest yield of abnormalities is found when testing blood glucose, serum B12 with metabolites and serum immunoelectrophoresis, broader laboratory testing is commonly performed. Additional testing frequently includes complete blood count (CBC), comprehensive biochemical and endocrine panel (including renal function, liver function, thyroid function, folate), erythrocyte sedimentation rate (ESR), rheumatological and other tests. Many of these laboratory tests have low specificity, and an uncertain relationship to the polyneuropathy, so the results must be interpreted in the context of other clinical information.Reference England, Gronseth and Franklin 3
In this study, we aimed to determine the frequency of laboratory abnormalities found when screening polyneuropathy patients with a broad battery of tests, and to explore their association with electrophysiological findings.
We performed a retrospective chart review of patients diagnosed with polyneuropathy, attending the Prosserman Family Neuromuscular clinic at the University Health Network, from January 2013 to September 2015. The Research Ethics Board of the University Health Network approved the current study protocol, and waived informed consent.
Inclusion criteria included a diagnosis of polyneuropathy in symptomatic patients who had confirmation by electrophysiological findings, and performed laboratory tests in order to screen for specific etiologies.
Nerve conduction studies were performed using the Sierra Wave instrument (Cadwell Laboratories Inc., Kennewick, Washington), according to the standards of the Canadian Society of Clinical Neurophysiology and the American Association of Neuromuscular and Electrodiagnostic Medicine. For the purposes of this study, at least one abnormal electrophysiological parameter in the lower limb was required for inclusion. Vibration perception thresholds (VPT) were determined using a Neurothesiometer (Horwell Scientific, London, UK) by the method of limits.
Routine laboratory tests consisted of an extended chemistry panel, including fasting glucose, 2-hour glucose tolerance test (75 g), hemoglobin A1c (HbA1c), renal and liver function, creatine kinase (CK), angiotensin-converting enzyme (ACE), uric acid (UA), CBC, ESR, antinuclear antibodies (ANA), rheumatoid factor, C3, C4, antineutrophil cytoplasmic antibodies, vitamin B12, folate, thyroid-stimulating hormone (TSH), serum protein immunoelectrophoresis, and anti-GM1 ganglioside antibodies. These tests were performed as part of routine testing, even in patients with an apparent diagnosis, in order to rule out alternative diagnoses or contributing factors.
Statistical analysis was performed using SAS version 9.2 (SAS Institute, Cary, North Carolina), and Prism 6 (GraphPad Software, La Jolla, California). Correlations of laboratory test finding and electrophysiological results were explored using point-biserial correlation coefficients, considering electrophysiological data as continuous variables, and the presence or absence of abnormal laboratory tests, as dichotomous variables.
The total cohort included 226 patients with a mean age was 61±14 years, comprising 29% females. The most common etiology for polyneuropathy was diabetes mellitus in 33%, followed by idiopathic in 19%, prediabetes in 14%, and chronic inflammatory demyelinating polyneuropathy in 13%. Their electrophysiological parameters are presented in Table 1.
Table 1 Electrophysiological findings and vibration perception thresholds (VPT) in 226 patients with polyneuropathy
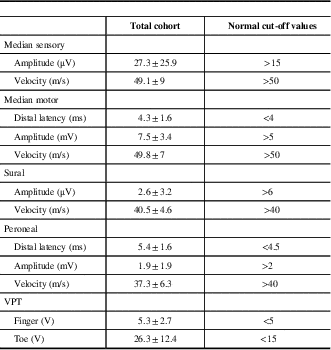
Data are shown as means±SD.
µV=microvolts; m/s=meters per second; ms=milliseconds; mV=millivolts; V=volts.
Abnormal laboratory test results were found in 91%, with an average of 2.3 abnormal test results per patient (Table 2). Abnormal glucose handling tests were the most common (54%), followed by paraproteinemia (21%) and anemia (21%). The most common paraprotein was IgG κ (Table S1), and the frequency of paraproteinemia increased with age. There were no differences in the frequency of laboratory test abnormalities between patients with and without DSP, except higher frequency of glucose handling tests in those with DSP, and high UA levels of 29% in those with diabetic sensorimotor polyneuropathy (DSP), compared with 13% in those without DSP (p=0.03) (data not shown).
Table 2 Abnormal laboratory tests results in 226 patients with polyneuropathy
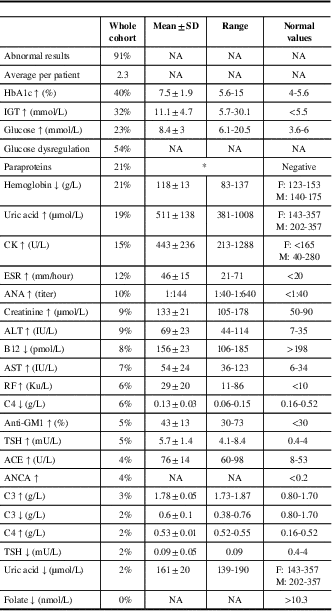
Data are shown as frequencies, means±SD, or ranges.
HbA1c=hemoglobin A1c; IGT=impaired glucose tolerance; glucose dysregulation=abnormal glucose or HbA1c; CK=creatine kinase; ESR=erythrocyte sedimentation rate; ANA=antinuclear antibodies; ALT=alanine transaminase; AST=aspartate transaminase; RF=rheumatoid factor; TSH=thyroid-stimulating hormone; ACE=angiotensin-converting enzyme; ANCA=antineutrophil cytoplasmic antibodies.
* Additional details can be found in Table 1S.
Impaired glucose tolerance, paraproteinemia, low hemoglobin, elevated UA levels and ESR, associated weakly with electrophysiological indices of median neuropathy at the wrist, and elevated VPT at the fingers. None of the laboratory abnormalities were associated with sural nerve amplitudes or conduction velocities. Low hemoglobin and elevated UA levels correlated weakly with higher VPT at the toes (Table S2).
Our study found that laboratory test abnormalities were present in the majority of patients, with at least one abnormality found in 91% of patients after a broad screening test panel. Glucose dysregulation was the most frequent abnormality as expected, since diabetes is the most common cause of polyneuropathy.Reference Visser, Notermans, Linssen, van den Berg and Vrancken 4 We found a higher than expected frequency of paraproteinemia when compared with previous studies in patients with polyneuropathy, and compared with the normal population of a similar age.Reference Saleun, Vicariot, Deroff and Morin 5 , Reference Kyle, Therneau and Rajkumar 6 However, IgG κ was the most common, similar to its distribution in the general population, and the frequency increased with age, as in the general population.Reference Kyle, Therneau and Rajkumar 6 The high prevalence of gammopathy suggests that inflammatory changes might be present in many patients with polyneuropathy. The reduced hemoglobin levels in 21% of patients were generally mild, and mostly normocytic. Although anemia is known to be a common accompaniment to diabetes,Reference Thomas, MacIsaac, Tsalamandris, Power and Jerums 7 and was found in 26% of patients with diabetes in our cohort, a high frequency of 17% was found also in those without diabetes, compared with an expected frequency of 10% in the general population.Reference Patel 8 This may represent anemia of chronic disease, although polyneuropathy is not thought to have systemic impact leading to anemia. Another interesting finding was the presence of high UA levels in 19% of all patients, most commonly in association with the presence of diabetes, consistent with previous literature.Reference Papanas, Katsiki and Papatheodorou 9
Elevated CK levels were common, demonstrated in 15% of patients. Although elevated CK is an indicator of skeletal muscle disease, this finding is by no means specific, and can be demonstrated in neurogenic disorders as well, including polyneuropathies, most likely as a result of axonal loss and denervation leading to impaired muscle membrane integrity.
The frequencies of other laboratory abnormalities, such as elevated creatinine, AST, ALT, TSH, ESR, ANA and ACE were similar to their frequencies in the general population. The yield of routine folate testing was found to be extremely low, similar to previous reports. It should be noted that when ordering multiple independent laboratory tests in a healthy person, there is a chance that one in 20 of the results will be abnormal by random chance alone, as the reference range of a laboratory value is set arbitrarily based on the 95% confidence interval, meaning that by definition, 5% of normal individuals may have abnormal values. It was therefore predicted, that most patients in our cohort would have at least one abnormal finding, as 25 different laboratory tests were explored in each patient, but the frequency of abnormalities remains higher than expected in the normal population, even allowing for this statistical consideration.
Several laboratory abnormalities, including impaired glucose tolerance, paraproteinemia, low hemoglobin, elevated UA levels and high ESR, correlated with median neuropathy at the wrist, but not with sural nerve amplitude or conduction velocity. Although median neuropathy at the wrist might reflect a more severe neuropathy, the current study results suggest a preferential susceptibility of the median nerve at this location, to various hematological, biochemical, metabolic and immunological influences. This association of median neuropathy at the wrist has been described previously with paraproteinemia, diabetes, hypothyroidism and acromegaly. Interestingly, within the battery of glucose handling tests, only impaired glucose tolerance was found to correlate with median neuropathy at the wrist, which might underscore its importance in the evolution of polyneuropathy. Changes in hemoglobin, UA or ESR, have not been described in association with median neuropathy at the wrist previously.
Our study has several limitations. Although we explored a relatively extensive panel of laboratory tests, others were not done routinely. In addition, we did not evaluate associations of electrophysiological parameters with less frequent laboratory abnormalities (<10%), due to statistical limitations of low numbers. We did not evaluate patients with and without clinical carpal tunnel syndrome, and therefore could not correlate laboratory tests abnormalities with clinical findings, although the presence of clinical carpal tunnel syndrome is not associated with electrophysiological findings in diabetic polyneuropathy.Reference Perkins, Olaleye and Bril 10 In addition, a referral bias exists, as our clinic is a tertiary center, and this may lead to misrepresentation of the frequency of different polyneuropathies. However, the most common etiologies in our cohort were DSP and idiopathic neuropathy, similar to their distribution in a community setting. Another limitation is the exclusion of patients with isolated small fiber neuropathies or mononeuropathy, and therefore we are unable to draw conclusions about these peripheral neuropathies. Finally, we did not compare laboratory test findings to a control group comprised of normal subjects, or patients with various other neuromuscular disorders, and therefore cannot address their frequencies in these groups.
In conclusion, most patients with polyneuropathy have some abnormality on laboratory testing, mostly glucose dysregulation, and the frequencies of paraproteinemia and anemia in our cohort were considerably higher than previously reported. Median neuropathy at the wrist was found to be associated with metabolic and hematologic derangements not previously described, and these findings should be confirmed and further explored in prospective studies.
Disclosures
AA, HDK, MN, LEL and VB have nothing to disclose. AB reports grants from Grifols Inc., other from Pfizer, outside the submitted work. CB reports grants from Octapharma, personal fees from CSL, outside the submitted work. BAP reports grants from NIH and JDRF, and speaker honoraria from Medtronic, Johnson & Johnson, Roche, GlaxoSmithKline Canada, Novo Nordisk, and Sanofi; he has received research grant support from Medtronic and Boehringer Ingelheim; and serves as a consultant for NeuroMetrix, during the conduct of the study.
Statement of Authorships
AA, LEL: study concept and design, analysis and interpretation of data; AB, MN, CB: acquisition of data, analysis and interpretation of data; HDK: study concept and design, acquisition of data; BAP: study concept and design, study supervision; VB: study concept and design, analysis and interpretation of data, study supervision, critical revision of manuscript for intellectual content.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2017.298






