INTRODUCTION
Sepsis is defined by a systemic host response to infection by pathogenic microorganisms. The acute phase reaction includes immune, physiological, metabolic and behavioural responses. Changes in the central nervous system (CNS) result in fever, activation of the hypothalamic–pituitary–adrenal axis, and such behavioural changes as malaise, anorexia, hypo- or hypersomnia and depression, which are collectively referred to as “sickness behaviour.”Reference Dantzer 1 The short-term consequences of sepsis include confusion, delirium and coma (septic encephalopathy), and long-term consequences include persistent neurocognitive deficits and psychiatric disorders.Reference Zhang, Sheng and Yao 2 Innate immune cells are activated by pathogen-associated molecular patterns, such as the binding of lipopolysaccharide (LPS) released by Gram-negative bacteria to Toll-like receptor 4 on monocytes and macrophages. This triggers a cascade of intracellular signalling events that result in the generation of such cytokines as tumour necrosis factor (TNF), interleukin (IL)-1β and IL-6. Peripheral administration of LPS to human volunteers as well as to experimental animals resulted in behavioural and cognitive changes.Reference Reichenberg, Yirmiya, Schuld, Kraus, Haack, Morag and Pollmächer 3 , Reference Godbout, Chen, Abraham, Richwine, Berg and Kelley 4 LPS administration to mice resulted in activation of central microglia, the resident macrophages of the brain.Reference Semmler, Okulla, Sastre, Dumitrescu-Ozimek and Heneka 5 A study of postmortem tissue from human subjects with sepsis demonstrated an increase in CD68-positive microglia compared to normal control subjects.Reference Lemstra, Groen in’t Woud, Hoozemans, van Haastert, Rozemuller and Eikelenboom 6 These central microglia can then generate cytokines and chemokines that contribute to the pathophysiology of central responses to sepsis. For instance, administration of endotoxin to rats induced IL-1 expression in activated microglial cells in the CNS.Reference van Dam, Brouns, Louisse and Berkenbosch 7 Apart from the local generation of both pro- and anti-inflammatory cytokines, significant contributors to brain dysfunction during sepsis have included disruption of the blood–brain barrier, cerebral ischemia, imbalance of neurotransmitters, excitotoxicity and oxidative stress.Reference Papadopoulos, Davies, Moss, Tighe and Bennett 8 , Reference Wilson and Young 9 The final common pathway that results in brain dysfunction has been proposed to be deregulation of neurotransmitters, particularly acetylcholine.Reference van Gool, van de Beek and Eikelenboom 10
Little is known about the expression of cytokines or chemokines in the human brain during sepsis. There is interest in utilizing these and other molecules as biomarkers of sepsis in clinical practice. Therefore, most human studies have assessed cytokines and chemokines as blood or serum biomarkers.Reference Pierrakos and Vincent 11 Studies with IL-6,Reference Patel, Deen, Youngs, Warwick and Keighley 12 IL-18,Reference Emmanuilidis, Weighardt, Matevossian, Heidecke, Ulm and Bartels 13 macrophage inhibitory factor (MIF),Reference O’Grady, Tropea, Preas, Reda, Vandivier and Banks 14 monocyte chemotactic protein (MCP)-1/CCL2Reference Bacher, Meinhardt, Lan, Mu, Metz and Chesney 15 and TNFReference Riche, Panis, Laisné, Briard, Cholley and Bernard-Poenaru 16 distinguished between survivors and non-survivors, but the general clinical utility of most biomarkers is limited, because many are not specific for sepsis and can show variable and unpredictable expression during the course of disease. To our knowledge, there are no comprehensive descriptions of cytokine and chemokine expression in the human autopsy brain in cases of sepsis. We are aware of one study that compared autopsy cases of delirium to normal controls and found an increased brain expression of IL-6 as well as enhanced microglial activation.Reference van Munster, Aronica, Zwinderman, Eikelenboom, Cunningham and de Rooij 17 Brain tissue profiling may identify particular factors that contribute to CNS dysfunction and determine the local balance of pro- and anti-inflammatory factors. Our study describes the expression of 36 cytokines and chemokines in autopsy-derived brain tissue from three cases of human sepsis. We have identified factors that were increased in all three cases and discuss these in the light of the literature on animal models of sepsis and human biomarkers.
MATERIALS AND METHODS
Case Reports
Case 1
A 38-year-old female with a previous diagnosis of primary sclerosing cholangitis presented to the emergency room in January of 2013 with right upper quadrant pain, diagnosed as ascending cholangitis. This was treated with intravenous antibiotics and endoscopic retrograde cholangiopancreatography (ERCP), and stent placement was performed. On initial admission, her temperature was normal, which was attributed to prior therapy with the TNF blocking agent adalimumab. No other anti-inflammatory agents were being taken. Two days later, she presented with recurrent cholangitis and fever (39oC), and ERCP revealed stent migration. In March, a blood culture grew Acinetobacter. During this admission, her temperatures continued to spike. Between March and June, her circulating leucocyte counts were normal. Right upper quadrant pain persisted, and significant peripheral oedema developed. In early April, cellulitis developed in the right groin, abdomen and breast. In mid-May, there was a decline in renal function associated with confusion and asterixis so that hepatic or renal encephalopathy was suspected. Although the cholangitis and cellulitis were treated, the significant oedema, right upper quadrant pain and confusion persisted. Brain imaging was not recorded. The patient was not considered suitable for dialysis or liver transplant, and palliative measures were instituted. The patient subsequently died in June of 2013. Complete autopsy was performed and confirmed primary sclerosing cholangitis with severe biliary cirrhosis. Gram-positive and Gram-negative rods and cocci were identified in the biliary tree.
Case 2
A 65-year-old male presented to the emergency room with acute shortness of breath, a two-day history of diarrhoea and a two-month history of general malaise and fatigue. These were the only neurological symptoms recorded. His admission temperature (36.7oC) and circulating leucocyte count were normal. There was no prior history of anti-inflammatory drug use. He was diagnosed with acute renal failure and hyperkalemia. He was treated in the emergency room for hyperkalemia and transfused one unit of blood. Brain imaging was not carried out. After transfer to a nephrology unit, he developed hypotension and bradycardia and went into cardiac arrest with pulseless electrical activity. Resuscitation efforts were unsuccessful, and the patient died on the same day. Pre-mortem blood and urine cultures were positive for Escherichia coli. A complete autopsy was performed and showed acute pyelonephritis secondary to urinary tract obstruction from an enlarged prostate extensively involved by a previously undiagnosed prostatic adenocarcinoma.
Case 3
A 37-year-old female presented in May of 2014 to the emergency room with several days of cough, fever and dyspnoea, and was diagnosed with right lower lobe pneumonia and acute respiratory distress syndrome. She had a viral prodrome of “feeling unwell” prior to admission but no specific neurological symptoms. She was not on any regular anti-inflammatory medications. On admission, her temperature was elevated (39.7oC), and the circulating leucocyte count was elevated two days after admission (at 16.19×109/L [normal range=4.5–11×109/L]). Sputum and blood cultures were positive for methicillin-resistant Staphylococcus aureus. Brain imaging was not recorded. She subsequently developed hypoxemia, tachycardia and hypotension requiring intubation and vasopressors. Four days after admission, she was treated with extracorporeal membranous oxygenation. The following day she went into cardiac arrest, and although circulation was restored, the prognosis was poor and comfort measures instituted. The patient died on the same day. A complete autopsy confirmed the presence of extensive necrotizing bronchopneumonia.
Brain Sampling
A complete autopsy was carried out on all three cases, which included removal of the brain. Complete autopsy and brain removal was also carried out in an additional 10 control cases, in which sepsis was not diagnosed and CNS disease was not suspected. The profile of these cases is listed in Table 1. These cases were donated to the Maritime Brain Tissue Bank (Halifax, Nova Scotia, Canada), and ethics approval for this study was obtained from the Nova Scotia Health Research Ethics Board. A portion of the right frontal pole was removed from the fresh brain in each case and frozen at –80oC. The left hemisphere was donated to the brain bank. The rest of the right hemisphere for each case was fixed in formalin for two weeks and then dissected and sampled in a routine fashion by a neuropathologist. Tissue blocks were processed to obtain histologic slides. Each case was subsequently reported as “normal brain, without pathologic abnormalities” by a neuropathologist. Specifically, all of the cases (sepsis and controls) did not show morphologic markers of injury. Ischemic changes were not found, including red cytoplasmic alteration in cortical neurons (red neurons) and foci of acute or chronic infarction. Minor perivascular collections of CD3-positive mononuclear inflammatory cells are commonly found in normal cases, and were noted here, but significant inflammation was not found, there were no features of putrefaction and no collections of neutrophils compatible with microabscess formation. Cases were also processed for immunohistochemistry to detect the astrocytic marker GFAP (glial fibrillary acidic protein) and the microglia/macrophage markers CD68 and CD45.
Table 1 Case demographics for non-septic controls and cases of sepsis (PM refers to postmortem interval; pathologic findings combine clinical history and autopsy findings)
Protein Extraction and Multiplexed Enzyme-Linked Immunosorbent Assay
A Bio-Plex Pro™ premixed human cytokine kit (Bio-Rad, kit #171-AK99MR2, Hercules, CA, USA) was used to analyse tissue from the 3 cases of sepsis and 10 controls. Samples of frozen right frontal pole were thawed on ice, weighed and subsequently homogenized for 2 min in sterile ice-cold phosphate buffered saline (PBS) containing a protease inhibitor (50 mM sodium fluoride; Sigma Aldrich, St. Louis, MO, USA) using a handheld homogenizer (Pro Scientific Inc., Oxford, CT, USA). Leptomeninges were removed from the brain tissue, which included both grey and white matter. Protein concentrations were determined using a Bradford protein assay (Bio-Rad) and tissue samples diluted to a final concentration of 2 mg/ml. A standard curve was constructed using a fourfold dilution series of beads with a known fluorescence spectrum unique to each marker. Magnetic anti-cytokine conjugated beads (50 µl/well) were added to the wells of a 96-well plate and washed twice with 100 µl of assay buffer. Each sample was measured in duplicate wells by adding 50 µl of sample to each well. The plate was covered and incubated in the dark at room temperature (RT) on a shaker set at 850 rpm for 30 min followed by three washes. Next, detection antibody was added to each well and incubated in the dark at RT on a shaker set at 850 rpm for 30 min followed by three washes. Lastly, streptavidin PE (50 µl) was added to the wells and incubated in the dark at RT on a shaker set at 850 rpm for 10 min. Beads were then washed three times, re-suspended in assay buffer and analysed with the Bio-Plex® 200 suspension array system. The data were acquired using Bio-Plex Manager software version 6.1 with five-parameter logistic regression curve fitting (Bio-Rad).
Real-Time Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was extracted from the frontal pole of six controls and the three sepsis cases using an Aurum™ total RNA fatty and fibrous tissue kit (Bio-Rad) according to the manufacturer’s instructions. This tissue contained both grey and white matter, but leptomeninges were stripped away prior to processing. In order to measure the concentration, quality and overall purity of isolated total RNA, an Experion bioanalyser equipped with an RNA StdSens Analysis Kit was utilized (Bio-Rad). Following quality control, total RNA was diluted to 2 μg and synthesized into cDNA using SuperScript II (Invitrogen, Burlington, ON, Canada). The CFX connect real-time PCR machine (Bio-Rad) was used for qPCR, using GoTaq qPCR Master Mix (Promega, Madison, WI, USA) according to the manufacturer’s instructions for amplification and quantification. Gene-specific primers for human CCL13, CCL22, CXCL10, CXCL12, CXCL8, HDAC6 and EIF 4A2 were purchased from Invitrogen (see Table 2). The data from the qPCR were collected and analysed using Livak and Schmittgen’s 2−ΔΔCT method.Reference Livak and Schmittgen 18 Fold change in messenger RNA (mRNA) expression was calculated by first normalizing the cycle threshold (ct) of the indicated gene against cytochrome C followed by a comparison against the respective controls.
Table 2 Primer sequences used for real-time qPCR
Data Analysis
Sections of GFAP, CD68 and CD45 staining from the right superior frontal gyrus of five controls and the three sepsis cases were scanned into an Aperio ScanScope (Leica Biosystems, Buffalo Grove, IL, USA) and images obtained at ×200 (GFAP, CD45) or ×400 magnification (CD68). The higher magnification for CD68 was used to improve cell resolution. White matter regions (three from each case) at these magnifications were selected at random, while blinding the observer to case identity, and analysed for percentage area of positive staining using NIH-Image J software (National Institutes of Health, Bethesda, MD, USA) to determine GFAP, CD68 and CD45 expression. Statistical analysis was performed with GraphPad Prism software (San Diego, CA, USA). The type of analysis is indicated in the text.
RESULTS
The expression of 36 human chemokines and cytokines is shown in Figure 1 and Table 3. Figure 1 plots the expression of each factor in each of the three cases with sepsis (Figure 1A–C). Mean values were obtained for the 10 control cases, and values for each sepsis case plotted as a percentage of this mean. Table 3 shows the concentration of each factor in pg/ml and highlights values in each of the three cases of sepsis that are either elevated or (in the case of GM-CSF) reduced. Elevated expression is defined when it exceeds the control mean plus two standard deviations, and reduced when lower than this mean minus two standard deviations. The range of normal values is also shown in Table 3. In most instances, elevated values were above the normal range, exceptions applying to single elevated values for CCL22, CCL27 and IL-10. Of the 36 chemokine/cytokines measured, 24 were elevated in at least one case of sepsis, while GM-CSF was the only factor to be reduced (in one of the three cases). No changes in ligand expression were noted for the following 11 factors: CXCL6, CXCL9, CXCL16, CCL3, CCL15, CCL16, CCL19, CCL23, CCL25, CX3CL1 and interferon (IFN)-γ. Each case had a different but overlapping profile of elevated (or reduced) expression. In case 1, 18 factors were elevated: CXCL2, CXCL8, CXCL10, CXCL12, CCL1, CCL2, CCL7, CCL8, CCL13, CCL20, CCL21, CCL22, CCL27, interleukin (IL)-2, IL-4, IL-16, IL-1β and tumour necrosis factor (TNF). This case also showed a reduction in GM-CSF. In case 2, eight factors were elevated: CXCL1, CXCL8, CXCL10, CXCL12, CXCL13, CCL13, CCL22 and CCL26. In case 3, 18 factors were elevated: CXCL1, CXCL2, CXCL8, CXCL10, CXCL12, CXCL13, CCL1, CCL2, CCL8, CCL11, CCL13, CCL20, CCL22, IL-6, IL-10, IL-16, IL-1β and TNF. Five factors were elevated in all three cases—CXCL8, CXCL10, CXCL12, CCL13 and CCL22; while 10 factors were elevated in two of the three cases—CXCL13, CXCL1, CXCL2, CCL1, IL-1β, IL-16, CCL2, CCL8, CCL20 and TNF. The five jointly elevated factors were analysed as average expression for all three cases and compared to average expression in the 10 normal cases. Using an unpaired t test, the expression of each of these five factors was significantly elevated (p<0.05, Figure 1D). Figure 2A shows representative images for expression of GFAP, CD68 and CD45 in sections from white matter in the superior frontal gyrus from a control case (59-year-old female with acute myocardial infarction; see Table 1) and sepsis case 1 (CD45) and sepsis case 3 (GFAP, CD68). All three sepsis cases and five controls were then analysed to determine the area of positive staining in white matter. There was a significant increase in GFAP staining (p<0.05) when comparing the sepsis cases with controls. The highest GFAP expression was found in sepsis case 3. Although CD68 and CD45 expression was not significantly increased in pooled data from the sepsis cases, case 1 showed markedly elevated expression of CD45 compared to the other cases, while case 3 had markedly elevated expression of CD68 (right panels, Figure 2A). When comparing the sepsis cases to normal controls, mRNA expression was unchanged for CXCL8, CXCL10, CXCL12, CCL13 and CCL22. Individual cases of sepsis showed striking elevations in mRNA expression for two additional inflammatory factors: HDAC (histone deacetylase) 6 and EIF (eukaryotic translation initiation factor) 4A2 (Figure 2B).
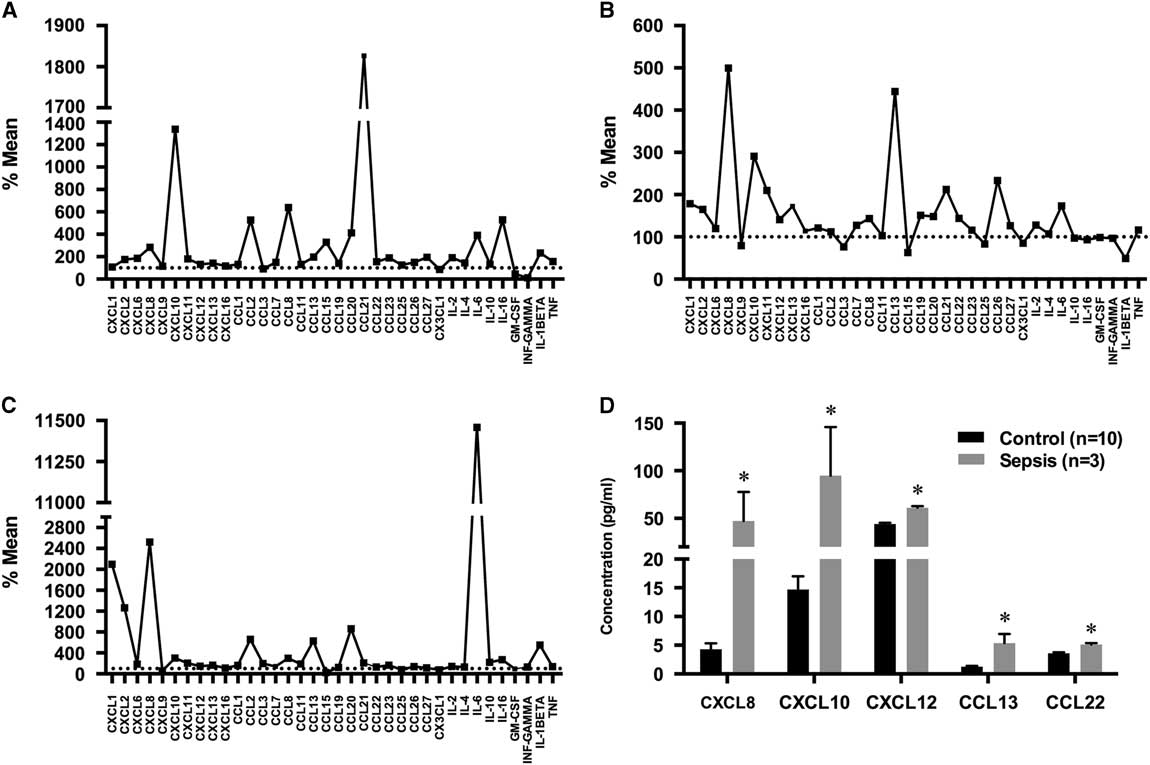
Figure 1 Protein concentrations for brain chemokine and cytokines in sepsis. (A–C) Factor expression in each of the three cases of sepsis is plotted as the percentage of mean expression in 10 controls. (D) Concentration differences between control and sepsis cases for the five most strongly elevated factors. Data are shown as mean ± standard error of the mean (sem), *p<0.05.

Figure 2 Additional inflammatory factor expression in sepsis. (A) Photomicrographs of frontal lobe white matter expression of GFAP, CD68 and CD45 in control and sepsis. Scale bar =100 µm (GFAP, CD45) and 50 µm (CD68). GFAP, CD68 and CD45 expression is shown as percentage area of positive staining for each sepsis case compared to five controls. (B) Fold change in mRNA expression for the five most strongly elevated chemokines (CXCL8/10/12, CCL13/22) and novel factors HDAC6 and EIF 4A2. Data are shown as mean ± sem, *p<0.05.
Table 3 Concentrations (in pg/ml) of brain chemokines and cytokines
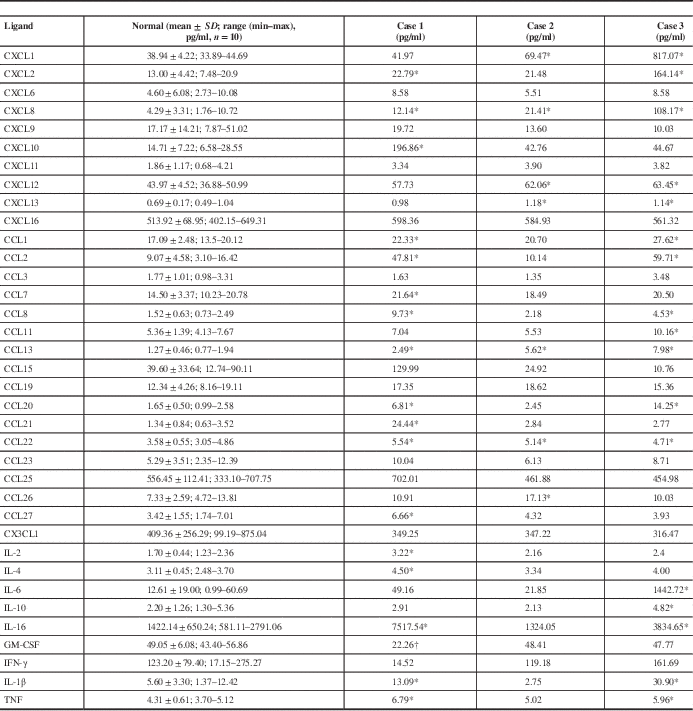
Note: For the control cases, concentrations are mean ± standard deviation (SD) of the mean; the range (minimum–maximum) is also shown. Individual values are shown for each of the three sepsis cases.
* Value greater than the control mean plus two standard deviations.
† Values lower than control minus two standard deviations.
DISCUSSION
Study Limitations
The age range of normals in this series was between 51 and 79 years (average =64.7 years), which was above the age noted in two of the three cases of sepsis. To assess the likelihood that ligand expression could be elevated artifactually as a result of younger patient age, a Pearson regression comparing age to expression was performed for the five factors elevated in all three cases. This showed a weak positive correlation between expression and age, which did not reach statistical significance (r 2 values between 0.10 and 0.25). Therefore, it is unlikely that the increased expression of these factors in the cases of sepsis is due to younger patient age. While just three cases of sepsis were analysed, expression of the five factors was significantly increased (p<0.05, Figure 1).
Other potential limitations include use of anti-inflammatory drugs by cases or controls. Case 1 was on adalimumab to treat primary sclerosing cholangitis, while cases 2 and 3 were not on regular anti-inflammatory medications. Among the 10 controls, 4 were on regular low-dose aspirin (81 mg/day), 1 was on regular naproxen for osteoarthritis, and 1 was prescribed a short-term 3-day course of prednisone (steroid) for exacerbations of chronic obstructive pulmonary disease, taken on an “as-required” basis.
Present Findings
Chemokine/cytokine expression in human brain samples as studied here may be due to elevated circulating blood levels or expression by resident cells such as astrocytes and microglial cells. The methods we employed cannot distinguish between elevated circulating levels or local CNS expression, since CNS tissue and blood vessels are both included in the analysis. However, there was a significant increase in GFAP expression and markedly increased expression of CD68 and CD45 in a subset of the cases (Figure 2A), supporting the notion of resident brain expression. GFAP is upregulated by reactive astrocytes and therefore serves as a surrogate marker for astrocyte activation. CD68 and CD45 are not expressed in resident microglial cells in standard brain sections, because the cells lack extensive processes. However, during activation microglia become ramified and the increased cell processes become detectable with these markers. Therefore, both markers will detect activated microglia. However, although they cannot distinguish between macrophages or perivascular cells, the general morphology suggests that most of the staining seen was microglial.
The most convincing evidence for increased expression in this study was found for five chemokines: CXCL8/IL-8, CXCL10, CXCL12, CCL13 and CCL22 (Figure 1D). The literature suggests that some of these chemokines will promote sepsis while others mitigate its effects. CXCL8/IL-8 is regarded as a pro-inflammatory chemokine with a major role in neutrophil activation. Blood levels of IL-8 increase in patients with sepsis and are associated with a poor prognosis.Reference Marty, Misset, Tamion, Fitting, Charlet and Cavaillon 19 Within the brain itself, endothelial cells represent a major source of IL-8. For instance, cultured human brain endothelial cells generate IL-8 on exposure to E. coli bacteria.Reference Galanakis, Di Cello, Paul-Satyaseela and Kim 20 CCL13 may also be a pro-inflammatory factor,Reference Mendez-Enriques and Garcia-Zepeda 21 but little is known of its expression during sepsis. IL-6 should also be mentioned, because its expression was markedly increased in case 3 (Figure 1C). IL-6 is a pro-inflammatory factor whose blood levels correlate with poor outcomes in cases of human sepsis.Reference Patel, Deen, Youngs, Warwick and Keighley 12 By contrast, the remaining three of five chemokines are likely to limit harmful outcomes in sepsis. CXCL10 or gamma interferon-inducible protein 10 (IP10) showed high sensitivity and specificity as a serum biomarker in cases of human sepsis.Reference Pierrakos and Vincent 11 Similarly, CXCL10 blockade worsened disease outcomes in a mouse model of sepsis.Reference Ness, Hogaboam, Strieter and Kunkel 22 In general, it can be said that interferons protect the host against infection, so CXCL10/IP-10 is part of this protective response. CXCL10 was detected in whole brain homogenates after chronic infection of mice with Toxoplasma gondii.Reference Wen, Kudo, Payne, Wang, Rodgers and Suzuki 23 CXCL12/stromal cell-derived factor 1α and CCL22/macrophage-derived chemokine are also regarded as anti-inflammatory factors. Blood levels of CXCL12 are elevated in human sepsis,Reference Lesur, Roussy, Chagnon, Gallo-Payet, Dumaine and Sarret 24 and human brain expression has been described in several inflammatory diseases, including multiple sclerosis, where expression was detected in astrocytes and endothelial cells.Reference Krumbholz, Theil, Cepok, Hemmer, Kivisäkk and Ransohoff 25 CXCL12 binds to CXCR4 on neutrophils, and blockade of CXCL12 worsened outcomes in a mouse model of sepsis by preventing the egress of neutrophils from bone marrow.Reference Delano, Kelly-Scumpia, Thayer, Winfield, Scumpia and Cuenca 26 Administration of CCL22 was protective in a mouse model of sepsis,Reference Matsukawa, Hogaboam, Lukacs, Lincoln, Evanoff and Kunkel 27 and CCL22 has been detected in human microglial cells with an M2 anti-inflammatory phenotype.Reference Peferoen, Vogel, Ummenthum, Breur, Heijnen and Gerritsen 28 It remains to be seen whether these chemokines are major factors in the pathogenesis of septic encephalopathy in humans. However, it is encouraging that many of the factors expressed are involved in reducing inflammation and promoting repair, which could have therapeutic implications. From a practical perspective, it would be interesting to extend this study to cerebrospinal fluid or blood, in order to correlate expression with clinically obtainable samples. However, we also evaluated mRNA expression for the five most elevated factors in this study, and found no changes (Figure 2B). This is likely a reflection of the timing at which samples were collected. Nevertheless, we report increased mRNA expression for two additional inflammatory factors—HDAC6 and EIF 4A2 (Figure 2B)—both of which have been implicated in sepsis. For example, a specific inhibitor of HDAC6 improved survival in a mouse model of sepsis, where it inhibited cytokine production in the liver.Reference Yoo, Kim, Son, Seo, Baek and Maeng 29 HDAC6 may promote sepsis by stimulating cytokine production from macrophages, since an inhibitor suppressed the ability of LPS to activate cultured mouse macrophages.Reference Yan, Xie, Liu, Ran, Li, Wang and Yang 30 However, HDAC6 also promotes tolerance to LPS in cultured astrocytes, so in CNS sepsis HDAC6 may play a protective role.Reference Beurel 31 There are many different members of the EIF family, and some family members have increased blood levels in patients with sepsis.Reference Johnson, Lissauer, Bochiccio, Moore, Cross and Scalea 32 One study showed that inhibition of EIF 5A improved survival and reduced cytokine levels in a mouse model of sepsis.Reference Moore, Martin, Lee, Taylor, Dondero and Reznikov 33 Gene expression for EIF 4A2 (the family member detected in this study) was increased in samples of the lung taken from baboons 24 h after challenge with E. coli,Reference Zhu, Tang, Ivanciu, Centola, Lupu and Taylor 34 but it has not been studied to date in relation to the CNS. Our study therefore provides information on a range of different factors involved in the complex regulation of human CNS responses during sepsis.
ACKNOWLEDGMENTS
This work was funded by the Nova Scotia Health Research Fund (grant to AE). We gratefully acknowledge the support given by the laboratory of Dr. Patrick Lee of the Department of Microbiology & Immunology at Dalhousie University.
Disclosures
Alexander Easton has the following disclosures: Nova Scotia Health Research Fund: principal investigator, research grant; Dalhousie Medical Research Foundation: researcher, research grant.
Jordan Warford, Anna-Claire Lamport and Barry Kennedy hereby state that they have nothing to disclose.









