Introduction
Diagnostic criteria for mild cognitive impairment (MCI) in Parkinson’s disease (PD) were developedReference Litvan, Goldman and Tröster 1 because PD-MCI is common; it is present in 10% to 64%, that is approximately 40%, of non-demented PD patients (although exact estimates differ from study to study).Reference Lawrence, Gasson and Loftus 2 - Reference Hobson and Meara 6 This condition is characterized by the presence of cognitive impairment that does not significantly alter daily living. In PD-MCI, attentional/executive, visuo-spatial and memory functions are usually the most impaired, although other domains are also often altered, especially as the disease progresses.Reference Litvan, Goldman and Tröster 1 , Reference Hobson and Meara 6 - Reference Yarnall, Breen and Duncan 9 Identifying a way to slow down or prevent the cognitive decline in PD-MCI is crucial, as longitudinal studies suggest that around 11% of PD-MCI patients annually convert to Parkinson’s disease with dementia (PDD)Reference Hobson and Meara 6 and that most new PD-MCI cases could progress to PDD within as little as 5 years.Reference Pigott, Rick and Xie 10
The progressive degeneration of dopaminergic neurons in the nigrostriatal and mesocorticolimbic pathways and the presence of Lewy bodies in midbrain neurons are the neuropathological substrates of early clinical symptoms in PD and PD-MCI. Changes in the cholinergic, noradrenergic and serotoninergic systems also appear during the evolution of PD,Reference Bohnen, Kaufer and Hendrickson 11 - Reference Ye, Altena and Nombela 15 and contribute to motor and cognitive symptoms. As for dopamine (DA), nigrostriatal damage results in DA depletion in the caudate nucleus, which progresses from its dorso-lateral to ventro-medial segments. These areas are involved in frontostriatal loops. This phenomenon partly reflects the usual evolution of cognitive impairment in PD: an early presence of a dysexecutive syndrome (dorso-lateral loop) and the later development of difficulties with reward-based control of behavior (orbitofrontal loop).Reference Cools 16 , Reference Poletti and Bonuccelli 17 In parallel, neurodegeneration also occurs in the DA mesocorticolimbic system, which innervates many structures involved in cognitive functioning, such as the prefrontal cortex and the hippocampal formation.Reference Agid, Javoy-Agid and Ruberg 18 , Reference Gotham, Brown and Marsden 19
As the earliest and most important pathological mechanism of PD affects DA transmission, prescribed medications are principally dopaminergic agents. Although DA depletion does not explain all cognitive impairments, it nevertheless alters cognition.Reference Jellinger 20 , Reference Matsumoto 21 Therefore, dopaminergic agents, by compensating DA depletion, are likely to affect cognition in PD. The effects of antiparkinson medications on cognition have been investigated by several scientists since the advent of levodopa (L-D). However, the results of this work have never been reviewed in a systematic manner. In 2013, the first review tackling this questionReference Poletti and Bonuccelli 22 was more a narrative than a systematic review. Although this work provided some insight into the subject, few details were reported about the selection process and the characteristics of the studies. In addition, neither methodological quality of the articles nor effect sizes of the results were reported.
Therefore, the objective of the present article was to study the impact of clinically relevant dopaminergic antiparkinson medications on cognitive functioning in mild-to-moderate non-demented PD patients with compromised or intact cognition, by systematically reviewing the literature and providing quantitative data to assess the significance of the reported effects. Among all the antiparkinson drugs, this review covers 71% of treatments that are currently approved for clinical use. More precisely, antiparkinsonians recommended by the Canadian Neurological Sciences Federation (CNSF) Guidelines,Reference Grimes, Gordon and Snelgrove 23 namely L-D (with or without concomitant dopa decarboxylase inhibitor [DDCI] and/or catechol o-methyltransferase [COMT] inhibitor), pramipexole (PRX), ropinirole, selegiline (SEL) and rasagiline (RAS), were chosen to be reviewed.
Method
Search Strategy and Selection Criteria
A systematic search of the English- and French-language literature published between January 1969 and November 2017 was undertaken using the electronic databases MEDLINE, PsychNET, EMBASE and EBSCO (see Table 1 for keywords and search strategy). A manual search in the references of the selected articles was also made.
Table 1 Search strategy and keywords used in the MEDLINE, EMBASE, PsychNET and EBSCO databases

Eligible studies had to be randomized trials or have a non-randomized design (e.g., pre-post or off-on studies) with a control group or within-group comparisons, with or without placebo (Pb). Designs that allowed the isolation of the effect of one selected medication on at least one cognitive measure were accepted. Participants had to be diagnosed with PD according to the Queen Square Brain Bank (QSBB)Reference Gibb and Lees 24 or the National Institute of Neurological Disorders and StrokeReference Gelb, Oliver and Gilman 25 criteria. Studies published before the QSBB criteria had to specify that a neurologist made the diagnosis and had to minimally use the Hoehn and Yahr (H&Y) stagesReference Hoehn and Yahr 26 , Reference Goetz, Poewe and Rascol 27 to document the evolution of the disease. Parkinson’s disease severity had to be mild to moderate, defined by H&Y stages ≤3 and a PD duration ≤10 years at baseline. Medication dosage had to be indicated and results had to be reported on at least one validated cognitive measure before and after intervention. Exclusion criteria were as follows: cohort and case-control studies; case reports and animal model studies; trials including participants presenting with major psychiatric or neurologic disorder besides PD; and PDD per the DSM 28 , 29 criteria or an Mini-Mental State Examination (MMSE)Reference Folstein, Folstein and McHugh 30 score <26, as recommended by the Movement Disorder Society (MDS).Reference Dubois, Burn and Goetz 31
Article Selection and Data Extraction
Figure 1 illustrates the three phases of article selection. Phases two and three were conducted by two independent reviewers; all discrepancies were solved by consensus. The methodological quality analysis was made using the Cochrane Collaboration Depression, Anxiety and Neurosis Review (CCDAN) quality assessment scale,Reference Moncrieff, Churchill, Drummond and McGuire 32 which is a 23-criteria scale, with each criterion worth up to 2 points, for a maximum score of 46. This score was converted to percentage to obtain the methodological quality (MQ) score, and it was interpreted as follows: 0%-44%=very low quality; 45%-64%=low quality; 65%-84%=medium, acceptable quality with some risk of bias; and 85%-100%=high quality with little or no risk of bias. As the authors of this tool did not provide any interpretation method for their scores, we devised these benchmarks based on common sense. It was done in order to facilitate the interpretation of data, to help comparisons between articles and to provide rough estimates of the quality of the articles for each medication. As these interpretation benchmarks are not validated, the results section contains the main limitations for the group of articles associated with each medication in an effort to clarify the reasons why they were classified as they were.
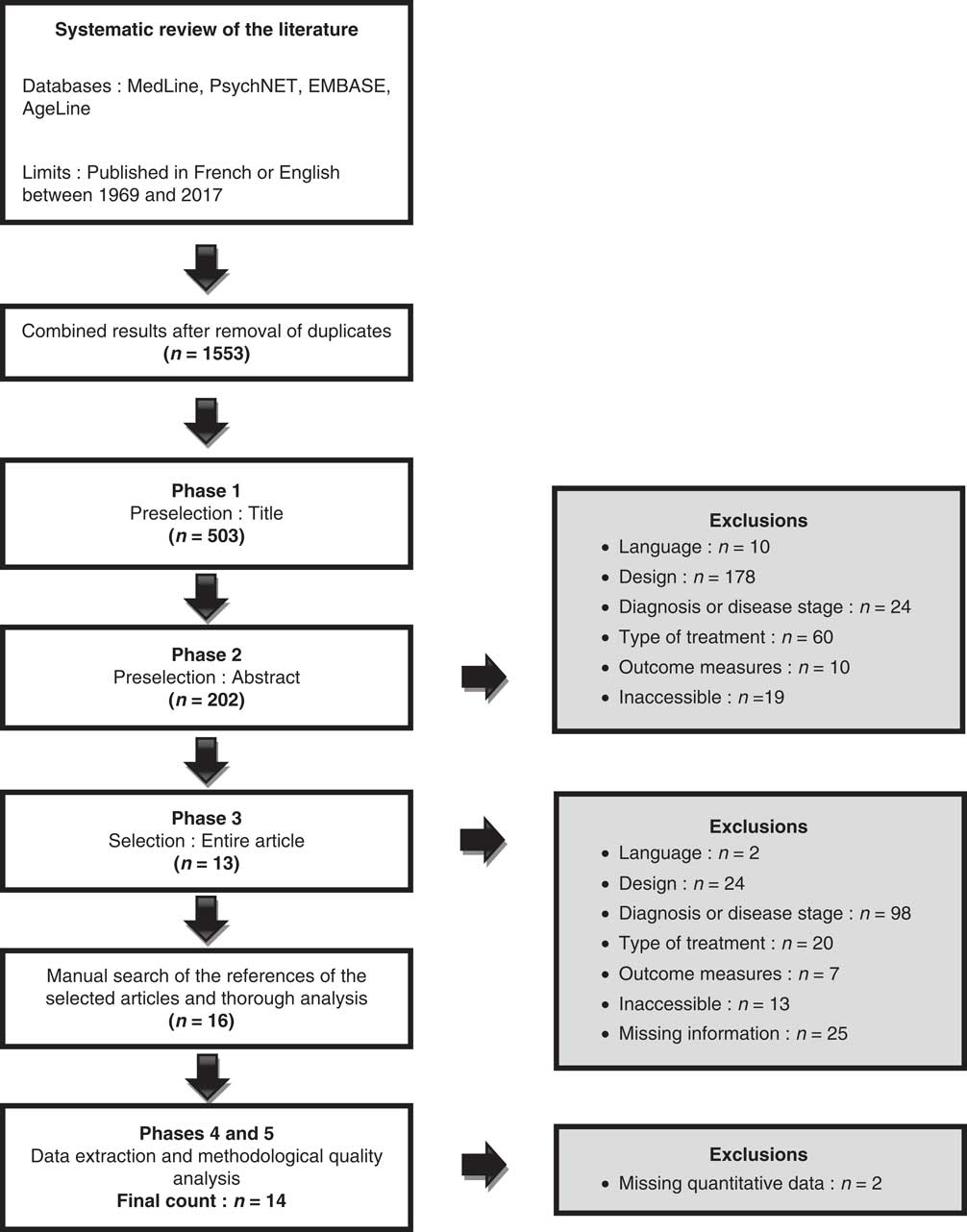
Figure 1 Flowchart.
Statistical Analysis
It was impossible to conduct a meta-analysis of our data. Data on several cognitive outcome measures were needed to achieve our objective of assessing the effects on all cognitive domains, making it hard to pool the data in a relevant way. The second best option was thus chosen, namely a systematic review of the literature with unpooled statistical analyses. Effect size calculations were conducted whenever available data allowed the comparison of cognitive performance before and after treatment. Hedges’ g Reference Hedges 33 was used, as it provides a correction to Cohen’s d, which overestimates the effect size when small samples are involved. For matched-sample comparisons, the formula took into consideration the covariance of the patients’ performance. Whenever there were insufficient data to estimate the covariance, it was computed to be of medium size (r=0.3).Reference Cohen 34 , Reference Lakens 35 Cohen’s d benchmarks were used for interpretation, as Hedges’g and Cohen’s d can be interpreted the same way. Values of g=0.2, 0.5 and 0.8 were, respectively, interpreted as small, medium and large effect sizes. Student’s t-tests were also performed. Results were considered statistically significant with p<0.05.
Results
Figure 1 presents the flowchart. Fourteen articles meeting the selection criteria studied L-D with and without DDCI, PRX, SEL and RAS. No article was found regarding the effects of ropinirole or COMT inhibitors on cognition. No study used an extensive neuropsychological assessment to differentiate patients with intact versus compromised cognition (except for oneReference Hanagasi, Gurvit and Unsalan 36 ), nor did they use MCI or PD-MCI clinical criteria to categorize cognitively altered patients. The global scores of the MMSE/Montreal Cognitive Assessment (MoCA) were used to exclude PDD patients. Tables 2A-2D show the studies’ characteristics. Table 3 describes the cognitive outcome measures administered in the studies.
Table 2A Characteristics of participants and medications in studies on the effects of levodopa on cognition

% ♂=mean percentage of male participants in the group; %Max=percentage of maximal daily dose recommended; Age=mean age of participants in years; d.=days; Dur.=mean disease duration in years; Educ.=mean duration of formal education expressed in years; Gr.=group; H&Y=mean stage of Parkinson’s disease progression on the Hoehn & Yahr scale; HCS=healthy control subjects; iPD=pharmacologically treated participants with idiopathic Parkinson’s disease; L-D=levodopa; L-D+DDCI=levodopa+dopa decarboxylase inhibitor; mg/day=mean daily dose expressed in milligrams per day; MMSE=mean total score on the Mini-Mental State Examination; mth.=months; MQ=Methodological Qualiy score; n=number of participants; n/a=not applicable; nil=information not available; Pb=placebo; PER=pergolide; PP-WG=pretest-posttest within-group design; PP-WG-C=pretest-posttest within-group controlled design; PP-WG-PC=pretest-posttest within-group placebo-controlled design; PRX=pramipexole; RCT=randomized controlled trial; RCO=randomized cross-over trial; Ref.=reference; Rx=medication; UPDRS=Unified Parkinson’s Disease Rating scale; wks.=weeks.
All numerical data are expressed in mean (SD) or mean [range], except for n and study duration.
* de novo patients at baseline.
** Authors specify that the score was obtained on the UPDRS section 3 (UPDRS-III) only.
Table 2B Characteristics of participants and medications in studies on the effects of pramipexole on cognition

% ♂=mean percentage of male participants in the group; %Max=percentage of maximal daily dose recommended; Age=mean age of participants in years; Dur.=mean disease duration in years; Educ.=mean duration of formal education expressed in years; Gr.=group; H&Y=mean stage of Parkinson’s disease progression on the Hoehn & Yahr scale; HCS=healthy control subjects; iPD=pharmacologically treated participants with idiopathic Parkinson’s disease; L-D=levodopa; mg/day=mean daily dose expressed in milligrams per day; MMSE=mean score on the Mini-Mental State Examination; MoCA=Montreal Cognitive Assessment; mth.=months; MQ=Methodological Qualiy score; n=number of participants; n/a=not applicable; nil=information not available; PER=pergolide; PP-WG=pretest-posttest within-group design; PRX=pramipexole; RCT=randomized controlled trial; RCO=randomized cross-over trial; Ref.=reference; Rx=medication; UPDRS=Unified Parkinson’s Disease Rating Scale; wks.=weeks.
All numerical data are expressed in mean (SD) or mean (range), except for n and study duration.
* Authors specify that the score was obtained on the UPDRS section 3 (UPDRS-III) only.
** Authors specify the patients were measured in “Off” state.
*** de novo patients at baseline.
Table 2C Characteristics of participants and medications in studies on the effects of selegiline on cognition

% ♂=mean percentage of male participants in the group; %Max=percentage of maximal daily dose recommended; Ø Rx=untreated participants with idiopathic Parkinson’s disease; Age=mean age of participants in years; Dur.=mean disease duration in years; Educ.=mean duration of formal education expressed in years; Gr.=group; H&Y=mean stage of Parkinson’s disease progression on the Hoehn & Yahr scale; HCS=healthy control subjects; mg/day=mean daily dose expressed in milligrams per day; MMSE=mean score on the Mini-Mental State Examination; mth.=months; MQ=Methodological Qualiy score; n=number of participants in study group; n/a=not applicable; nil=information not available; Pb=placebo; PP-C=pretest-posttest controlled design; RCO-P=randomized cross-over placebo-controlled trial; Ref.=reference; RPC=randomized placebo-controlled trial; Rx=medication; SEL=selegiline; UPDRS=Unified Parkinson’s Disease Rating Scale; wks.=weeks.
All numerical data are expressed in mean (SD) or mean (range), except for n and study duration.
* de novo patients at baseline.
** Data presented in IU.
*** Authors specify that the score was obtained on the UPDRS section 3 (UPDRS-III) only.
**** Authors specify the patients were measured in “Off” state.
Table 2D Characteristics of participants and tested medications in studies on the effects of rasagiline on cognition

% ♂=mean percentage of male participants in the group; %Max=percentage of maximal daily dose recommended; Age=mean age of participants in years; Dur.=mean disease duration in years; Educ.=mean duration of formal education expressed in years; Gr.=group; H&Y=mean stage of Parkinson’s Disease progression on the Hoehn & Yahr scale; mg/day=mean daily dose expressed in milligrams per day; MMSE=mean score on the Mini-Mental State Examination; mth.=months; MQ=Methodological Quality score; n=number of participants in study group; n/a=not applicable; nil=information not available; Pb=placebo; RAS=rasagiline; RPC=randomized placebo-controlled trial; Ref.=Reference; Rx=medication; UPDRS=Unified Parkinson’s Disease Rating scale; wks.=weeks.
All numerical data are expressed in mean (SD) or mean [range], except for n and study duration.
** Authors specify that the score was obtained on the UPDRS section 3 (UPDRS-III) only.
Table 3 Cognitive measures of efficacy
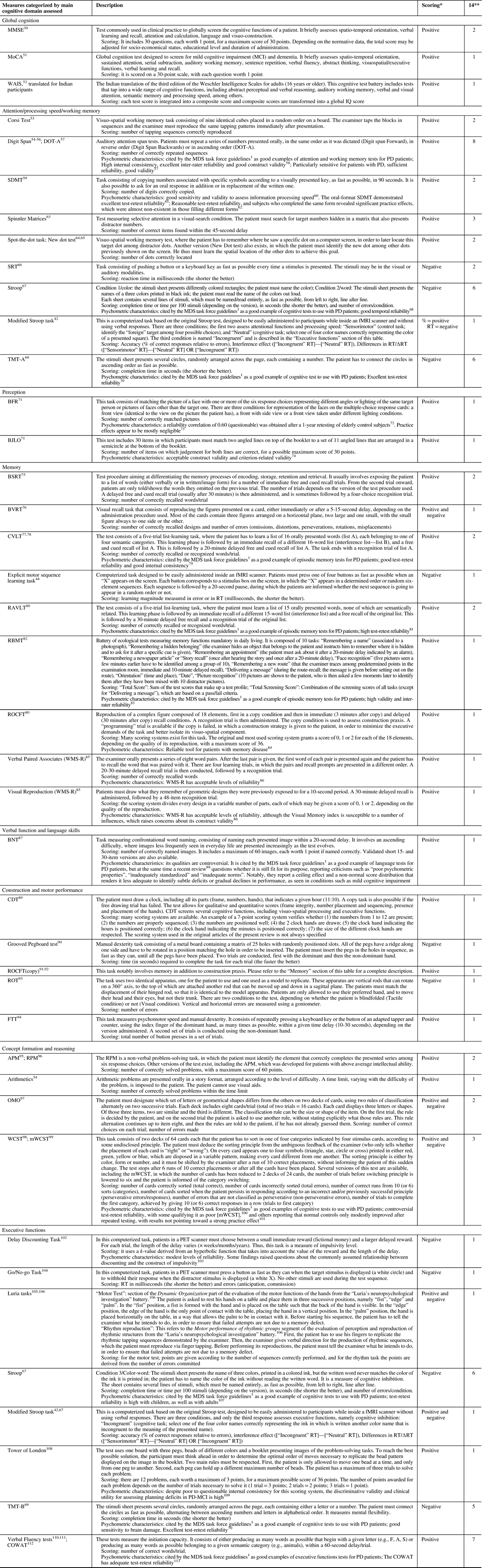
APM=Advanced Progressive Matrices; BFR=Benton Facial Recognition; BJLO=Benton Judgment of Line Orientation; BNT=Boston Naming Test; BSRT=Buschke Selective Reminding Test; BVRT=Benton’s Visual Retention Test; CDT=Clock Drawing Test; COWAT=Controlled Oral Word Association Test; CVLT=California Verbal Learning Test; DOT-A=Adaptive Digit Ordering Test; FTT=Finger Tapping Test; MDS=Movement Disorders Society; MMSE=Mini-Mental State Examination; mWCST=Modified Wisconsin Card Sorting Test; MoCA=Montréal Cognitive Assessment; OMO=Odd Man Out; RAVLT=Rey Auditory Verbal Learning Test; RBMT=Rivermead Behavioral Memory Test; ROCFT=Rey-Ostterieth Complex Figure Test; ROT=Rod Orientation Test; RPM=Raven’s Progressive Matrices; RT=Reaction time; SDMT=Symbol Digit Modalities Test; SRT=Simple Reaction Time; TMT=Trail Making Test; WAIS=Weschler Adult Intelligence Scale; WCST=Wisconsin Card Sorting Test; WMS-R=Wechsler Memory Scale Revised.
Cognitive domain classification is based on Lezak, Howieson et al.Reference Lezak, Howieson, Bigler and Tranel 114 Some descriptions are based on Strauss et alReference Strauss, Sherman and Spreen 115 and Lezak et al.Reference Lezak, Howieson, Bigler and Tranel 114
* Positive scoring implies that the higher the score is, the better the performance is, whereas negative scoring implies that the higher the score is, the worse the performance is.
** Number of selected original articles using the described cognitive task as an outcome measure.
Of the 14 studies that were finally included in this review, three (21%) did not report any statistically significant result on cognitive variables (“negative” studies): two were on PRX and one was on SEL. In total, 11 studies (79%) reported significant cognitive results (“positive” studies), together with some non-significant results.
Levodopa
Table 2A presents the characteristics of the seven studies of very low to medium MQ on L-D. A small sample size, the lack of blinding for the participants, as well as for the assessors, the absence of a power calculation and the lack of concealment of subject allocation were the most common limitations of the low- and very low-MQ studies on L-D. The main limitations of the medium MQ studies were the absence of power calculation, of concealment of allocation and of blinding of assessors. The L-D studies described the effects of 358-540 mg/day of L-D and of 200-281 mg/day of L-D and concomitant dopa decarboxylase inhibitor (L-D+DDCI). One study was a randomized controlled trial (RCT),Reference Kulisevsky, Garcia-Sanchez and Berthier 39 three were randomized cross-over trialsReference Brusa, Bassi and Stefani 40 , Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 , Reference Brusa, Tiraboschi and Koch 43 and three were quasi-experimental studies.Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 , Reference Fera, Nicoletti and Cerasa 42 , Reference Kwak, Müller, Bohnen, Dayalu and Seidler 44 All of the quasi-experimental studies used a pretest-posttest design and within-group comparisons, with two of them having a healthy control group.Reference Fera, Nicoletti and Cerasa 42 , Reference Kwak, Müller, Bohnen, Dayalu and Seidler 44 The trials lasted between <5 days and 24 months.
The number of participants enrolled in the patient groups varied from 10Reference Kulisevsky, Garcia-Sanchez and Berthier 39 to 387,Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 with 5/7 studies having sample sizes ≤20. Mean age and disease duration ranged, respectively, from 56.0 to 67.3 and from 1.2 to 3.9 years. A study selected only de novo patients.Reference Kulisevsky, Garcia-Sanchez and Berthier 39 The means of total scores on the MMSE/MoCA at baseline (from 28.4 to 28.9/30) suggest that the majority of PD patients had either mildly impaired or intact cognitive functioning. Table 4A presents the results from the L-D studies.
Table 4A Effects of levodopa on cognitive outcome measures

↑=Statistically significant improvement post-treatment/better performance in the experimental group; ↓=statistically significant deterioration post-treatment/worse performance in experimental group; ∆RT=differences (∆) in RT associated with the task conditions (incongruent and neutral) after subtraction of the sensorimotor RT; BL=baseline; BSRT=Buschke Selective Reminding Test; BVRT=Benton’s Visual Retention Test; CVLT=California Verbal Learning Test; FTT=Finger Tapping Test; g=Hedges’ g effect size; Gr.=group; HCS=healthy control subjects; LD-FR=long delay-free recall; MMSE=Mini-Mental State Examination; mth=months; MoCA=Montreal Cognitive Assessment; nil=information not available; ns=non-significant; OMO=Odd Man Out; RAVLT=Rey Auditory Verbal Learning Test; Ref.=reference; ROCFT=Rey-Osterrieth Complex Figure Test; RPM=Raven’s Progressive Matrices; RT=reaction times; Rx=medication administered to the experimental group; SDMT=Symbol Digit Modalities Test; SRT=Simple Reaction Time; TMT=Trail Making Test; WAIS=Wechsler Adult Intelligence Scale; mWCST=modified Wisconsin Card Sorting Test.
Every study presented some statistically significant results on their cognitive outcome measures. Of a total of 185 results, 32 (17.3%) indicated a statistically significant effect; 12.5% of these effects were deleterious and 87.5% were beneficial. Most significant effects were found on measures of executive functions (53.1%) and episodic memory (28.1%). Half of the significant effects were reported by the two studies with medium MQ, whereas the other half were reported by the five studies with low to very low MQ. Within each cognitive domain, results from studies with lower and higher MQ scores were generally convergent.
Global Cognition
Only one studyReference Kwak, Müller, Bohnen, Dayalu and Seidler 44 reported the L-D effects on global cognition using the MMSE and the MoCA. The comparison between the “On” (L-D-200 mg/carbidopa-100 mg) and “Off” treatment states of the 17 patients showed no significant difference on the two measures.
Attention/Processing Speed/Working Memory
A study (n=10)Reference Kulisevsky, Garcia-Sanchez and Berthier 39 reported a deterioration of large effect size on the Stroop-Word condition (information processing speed; p=0.028; g=0.754) at 12 months of treatment. The results for months 3, 6, 18 and 24 were in the same direction and had medium effects sizes, but did not reach statistical significance (p from 0.063 to 0.116; g from 0.503 to 0.614). There were no significant differences reported on the SRT and the Stroop Color condition.
This study,Reference Kulisevsky, Garcia-Sanchez and Berthier 39 along with most other studies that administered the Digit Span test (n=20;Reference Brusa, Bassi and Stefani 40 40;Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 387Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 ), did not report any significant effects for this task. However, another study (n=20)Reference Brusa, Tiraboschi and Koch 43 reported an improvement of medium size (p=0.027; g=0.515) on the Digit Span Backward after 2 months of treatment with 450 mg/day of L-D.
No significant change was observed after treatment on most tests of visuo-spatial attention and working memory (Trail Making Test (TMT)-A, New Dot, Corsi and Spinnler Matrices tests).Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 , Reference Kulisevsky, Garcia-Sanchez and Berthier 39 , Reference Brusa, Bassi and Stefani 40 , Reference Brusa, Tiraboschi and Koch 43 However, a small benefit of L-D (p<0.001; g=0.174) was obtained on the Symbol Digit Modalities Test (SDMT) after 6 months of treatment.Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37
Episodic Memory
Three different verbal episodic memory tests were administered in seven L-D studies: the Buschke Selective Reminding Test (BSRT),Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 the California Verbal Learning Test (CVLT)Reference Brusa, Bassi and Stefani 40 , Reference Brusa, Tiraboschi and Koch 43 and the Rey Auditory Verbal Learning Test (RAVLT);Reference Kulisevsky, Garcia-Sanchez and Berthier 39 , Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 however, significant differences were only observed on the RAVLT. Improvements on the RAVLT-trials 1-5 (verbal encoding and learning) were reported after 3,Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 12 and 24 monthsReference Kulisevsky, Garcia-Sanchez and Berthier 39 of treatment with medium (p=0.016; g=0.568 at 3 months) to large effect sizes (p=0.016 and g=0.859 at 12 months; p=0.020 and g=0.819 at 24 months). On the delayed recall trial (retention over time), no significant effect was reported.
Regarding visual episodic memory, L-D did not have any effect on the BVRT scores, but the performance on the Rey-Ostterieth Complex Figure Test (ROCFT) improved. Improvements of large effect sizes (p from 0.006 to 0.016; g from 0.852 to 1.029) were recorded at 6, 12 and 24 months post baseline evaluations on the ROCFT delayed recall.Reference Kulisevsky, Garcia-Sanchez and Berthier 39
Construction and Motor Performance
The trend toward beneficial effects of treatment increased at each post-baseline evaluation on the FTT (left hand), reaching statistical significance and large effect sizes at months 18 and 24 (from p=0.024 and g=0.783 at month 18 to p=0.009 and g=0.961 at month 24).Reference Kulisevsky, Garcia-Sanchez and Berthier 39 No change was reported on the ROCFT-Copy and on the Grooved Pegboard tests.Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 , Reference Kulisevsky, Garcia-Sanchez and Berthier 39
Concept Formation and Reasoning
A small beneficial effect was obtained on the first of two administrations of the odd man out (OMO) test (p=0.002; g=0.165) after 6 months of treatment.Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 L-D treatment yielded no significant effect on Raven’s Progressive Matrices (RPM), Weschler Adult Intelligence Scale (WAIS)-Arithmetics and modified Wisconsin card sorting test (mWCST) performances.Reference Brusa, Bassi and Stefani 40 , Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 , Reference Brusa, Tiraboschi and Koch 43
Executive Functions
Three studiesReference Brusa, Bassi and Stefani 40 , Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 , Reference Brusa, Tiraboschi and Koch 43 yielded deleterious effects of medium to large effect size (p from <0.001 to 0.002 and g from 0.779 to 1.736) exclusively on the Stroop Color/Word (inhibition) condition, with the administration of 358-458 mg/day of L-D during 18 to 24 weeks. A divergent result came from one study (n=12)Reference Fera, Nicoletti and Cerasa 42 reporting an improvement on the inhibition condition of a modified version of the Stroop (p<0.03) using an “On-/Off-treatment” paradigm with 250 mg/day of L-D+DDCI.
Treatment was most frequently associated with beneficial effects in four studies using the verbal fluency test.Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 , Reference Brusa, Bassi and Stefani 40 , Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 , Reference Brusa, Tiraboschi and Koch 43 Two studiesReference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 , Reference Brusa, Tiraboschi and Koch 43 showed beneficial effects of medium size on the Lexical Fluency task (p=0.031; g=0.501 at 2 monthsReference Brusa, Tiraboschi and Koch 43 and p=0.046 and g=0.457 at 3 monthsReference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 ). The performance on the Category Fluency task also improved with small,Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 mediumReference Brusa, Tiraboschi and Koch 43 and largeReference Brusa, Bassi and Stefani 40 effect sizes (p from 0.003 to 0.046 and g from 0.140 to 0.738 after 2 or 6 months of treatment).
On the Luria tasks, large beneficial effects of treatment were reported on the motor tasks from months 3 to 24 (p from <0.001 to 0.005; g from 1.071 to 1.636). There was no significant effect of treatment for the rhythm reproduction task.
On the Tower of London test (procedural learning; mental flexibility), patients’ performance improved with medium effect size (p=0.024; g=0.528) after 3 months of treatment.Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 Other beneficial effects were reported on the TMT-B (alternance and attention/processing speed; large effect; p<0.001; g=0.898) and TMT B-A (alternance only; medium effect; p=0.008; g=0.641).Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41
Acute Versus Chronic Effects of Treatment
As the study duration for the seven L-D articles varied greatly, we compared the results of the two studiesReference Fera, Nicoletti and Cerasa 42 , Reference Kwak, Müller, Bohnen, Dayalu and Seidler 44 assessing the acute effects (On/Off paradigm) and the five studiesReference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 , Reference Kulisevsky, Garcia-Sanchez and Berthier 39 - Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 , Reference Brusa, Tiraboschi and Koch 43 reporting the chronic effects (longer study duration) of L-D on cognition. Significant acute effects were only reported in a test of cognitive inhibitionReference Fera, Nicoletti and Cerasa 42 and a test of motor sequence learning,Reference Kwak, Müller, Bohnen, Dayalu and Seidler 44 whereas chronic effects were obtained on multiple tests across five cognitive domains. However, the studies on acute effects were of poor quality and only reported the results of four tests divided across four cognitive domains. Furthermore, only one test (modified Stroop task) is comparable to one of the different tests used in the studies on chronic effects, and the result is incongruent with those. This might indicate that acute and chronic treatments have opposing effects on cognitive inhibition (acute being beneficial and chronic being deleterious), but it is currently impossible to conclude anything on that matter considering the scarce data on acute effects of L-D in the context of this review.
Pramipexole
Table 2B presents the characteristics of four articles with very low to low MQ reporting the effects of 1-3.9 mg/day of PRX on cognition. The main limitations of these studies were the same as the L-D studies with low or very low MQ. Two trials were randomized controlled,Reference Costa, Peppe, Dell’Agnello, Caltagirone and Carlesimo 45 , Reference Relja and Klepac 46 one used a randomized cross-over designReference Brusa, Bassi and Stefani 40 and the last one used a pretest-posttest within-group design.Reference Antonelli, Ko and Miyasaki 47 Study duration ranged from <1 week to 6 months.
Sample sizes varied from 7 to 30 patients. Mean age and disease duration ranged, respectively, from 57.0 to 63.0 years old and from 2.5 to 6.8 years. A study exclusively enrolled de novo patients.Reference Costa, Peppe, Dell’Agnello, Caltagirone and Carlesimo 45 The MMSE/MoCA mean scores (from 28.1 to 28.8) suggest that the majority of PD patients had either slightly altered or intact cognition before treatment. Table 4B shows the results of the PRX studies.
Table 4B Effects of pramipexole on cognitive outcome measures
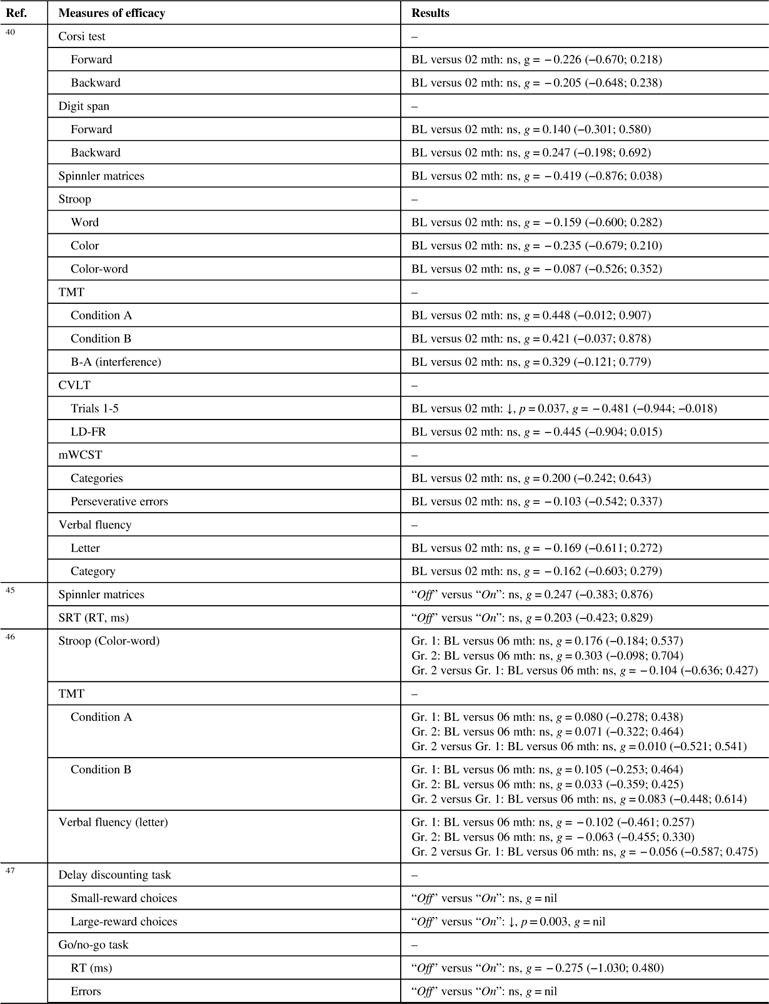
↓=Statistically significant deterioration post-treatment/worse performance in experimental group; BL=baseline; CVLT=California Verbal Learning Test; g=Hedges’ g effect size; Gr.=group; LD-FR=long delay-free recall; mth=months; mWCST=modified Wisconsin Card Sorting Test; ns=non-significant; Ref.=reference; RT=reaction times; SRT=simple reaction time; TMT=Trail Making Test.
Two of the four PRX studies presented some statistically significant results on their cognitive outcome measures. On a total of 35 results, 2 (5.7%) indicated a statistically significant effect, both of those effects being deleterious. With these few significant results, it was not possible to appreciate the possible convergence of the effects.
Attention/Processing Speed/Working Memory
No significant effects of PRX were found on tests of visual and verbal attention/working memory (Spinnler Matrices,Reference Brusa, Bassi and Stefani 40 , Reference Costa, Peppe, Dell’Agnello, Caltagirone and Carlesimo 45 Stroop test,Reference Brusa, Bassi and Stefani 40 , Reference Relja and Klepac 46 TMT A-B,Reference Brusa, Bassi and Stefani 40 , Reference Relja and Klepac 46 SRT,Reference Costa, Peppe, Dell’Agnello, Caltagirone and Carlesimo 45 Corsi test,Reference Brusa, Bassi and Stefani 40 Digit SpanReference Brusa, Bassi and Stefani 40 ) after <1 to 24 weeks of treatment.
Episodic Memory
Only one study (n=20)Reference Brusa, Bassi and Stefani 40 reported data on the effects of PRX on memory, using the CVLT. A detrimental effect of small to medium effect size (p=0.037; g=0.481) was reported after 2 months of treatment with 3.9 mg/day of PRX on the CVLT-trials 1-5 score (verbal encoding and learning). The difference in performance for the CVLT-delayed recall (retrieval and retention over time) did not reach statistical significance (p=0.052; g=0.445).
Concept Formation and Reasoning
There was no significant difference in performance on the mWCST after 2 months of treatment.Reference Brusa, Bassi and Stefani 40
Executive Functions
The performance of the seven participants involved in an On/Off-treatment paradigm deteriorated on the large reward choices condition of a delay discounting task (p=0.003), but did not change on a go/no-go task (both tests assess impulsivity/inhibition).Reference Antonelli, Ko and Miyasaki 47 No significant effect of PRX was found on verbal fluency tasks (n=20Reference Brusa, Bassi and Stefani 40 ; 30Reference Relja and Klepac 46 ) after 2 and 6 months of treatment.
Acute Versus Chronic Effects of Treatment
There was one study on acute effects and three studies on chronic effects for PRX. Out of the two tests administered in the acute effects study, both measuring inhibition/impulsivity, one yielded a significant deleterious effect. However, as no studies on chronic effects administered tests of impulse control or cognitive inhibition, it is impossible to compare the impact of acute and chronic administration of PRX.
Selegiline
Table 2C presents the characteristics of three studies with very low to high MQ that evaluated the effects of 10 mg/day of SEL on cognition. The main limitations of the studies with low-very low MQ were the same as the L-D studies in the same categories. As for the high-MQ study, it only lost a few points for the lack of description of side effects and for not detailing the reasons for patient withdrawals. One study was a randomized, double-blind, placebo-controlled trial,Reference Kieburtz, McDermott and Como 48 another was a randomized, double-blind, cross-over, placebo-controlled trialReference Dalrymple-Alford, Jamieson and Donaldson 49 and the last one was a pretest-posttest controlled trial.Reference Dixit, Behari and Ahuja 50 Study durations ranged from 3 to 24 months.
Group sample sizes varied from 9 to 187 patients. Mean age and disease duration ranged from, respectively, 55.4 to 66.9 years old and from 1.3 to 3.9 years. Only one study enrolled de novo patients and provided MMSE data before treatment (mean score=28.8),Reference Kieburtz, McDermott and Como 48 indicating mildly impaired or intact cognitive functioning. Table 4C shows the results of the SEL studies.
Table 4C Effects of selegiline on cognitive outcome measures

↑=Statistically significant improvement post-treatment/better performance in the experimental group; ↓=statistically significant deterioration post-treatment/worse performance in experimental group; APM=Advanced Progressive Matrices; ARoC=Annual Rates of Change; BL=baseline; BSRT=Buschke Selective Reminding Test; COWAT=Controlled Oral Word Association Test; g=Hedges’ g effect size; Gr.=group; MMSE=Mini-Mental State Examination; mth=months; ns=non-significant; OMO-Odd Man Out; PGIMS=PGI Memory Scale; RBMT=Rivermead Behavioral Memory Test; Ref.=Reference; ROT=Rod Orientation Test; RT=reaction times; SDMT=Symbol Digit Modalities Test; WAIS=Weschler Adult Intelligence Scale; WCST=Wisconsin Card Sorting Test.
Two of the three SEL studies presented some statistically significant results on their cognitive outcome measures. On a total of 124 results, 11 (8.9%) indicated a statistically significant effect, 36.4% of these effects being deleterious and 63.6% being beneficial. Most significant effects were found on measures of concept formation and reasoning (45.5%). In all, 55% of the significant effects were reported by the high-MQ study, whereas the remaining 45% were reported by the low-MQ study. For the cognitive domains that were assessed by more than one study, results tended to be divergent between studies.
Global Cognition
Performance in the SEL group was maintained, whereas the Pb group’s performance improved, leading to a small deleterious effect of SEL when comparing the two groups (p=0.010; g=0.274) in the annual rate of change (ARoC) on the MMSE (n=187).Reference Kieburtz, McDermott and Como 48 Regarding the WAIS-IQ score, no significant difference was reported between the SEL and the untreated PD groups after 3 months of treatment (n=17).Reference Dixit, Behari and Ahuja 50
Attention/Processing Speed/Working Memory
There was no significant change after SEL treatment on the Digit Span, the Spot-the-Dot task and the SDMT.Reference Kieburtz, McDermott and Como 48
Episodic Memory
A robust studyReference Kieburtz, McDermott and Como 48 obtained a detrimental effect of SEL treatment on the ARoC on the BSRT-delayed recall, but with a small effect size (p=0.018; g=0.173). However, this effect was no longer significant when compared with Pb. In another study,Reference Dixit, Behari and Ahuja 50 the performance of the SEL group compared with the untreated group on the PGIMS total score was in the direction of an amelioration, but did not reach statistical significance (p=0.052; g=0.699). Finally, a third studyReference Dalrymple-Alford, Jamieson and Donaldson 49 reported no significant difference on the Rivermead Behavioral Memory Test (RBMT) after 2 months of treatment, except for a large beneficial effect on the screening score (p=0.044; g=0.717) for one of the two groups (n=17 and 11) when compared with baseline, which was no longer significant when compared with Pb.
Construction and Motor Performance
No significant change on the Rod Orientation Test (fine manual dexterity) was observed after 2 months of SEL treatment.Reference Dalrymple-Alford, Jamieson and Donaldson 49
Concept Formation and Reasoning
The performance of patients deteriorated when they switched from a 2-month Pb trial to a 2-month SEL trial on the WCST, with an increase of the perseverative responses (p=0.012; g=1.129) and errors (p=0.035; g=0.926).Reference Dalrymple-Alford, Jamieson and Donaldson 49 On a similar test, the OMO, there was no difference compared with Pb. The OMO is generally easier to perform than the WCST, because it involves fewer concepts to discover and also less trials to complete.
Executive Functions
Both the SEL-treated (p<0.001; g=0.588) and Pb (p<0.001; g=0.629) groups improved on the ARoC of the Controlled Oral Word Association Test (COWAT) (verbal fluency). However, no significant difference was found between the ARoC of the two groups. Thus, the improvement probably indicates a practice effect, rather than a real treatment effect of SEL.
Rasagiline
Table 2D presents the characteristics of the only studyReference Hanagasi, Gurvit and Unsalan 36 on RAS that met our inclusion criteria. As a study with low MQ, it has the same limitations as the L-D studies with low-very low MQ, except for the blinding of assessors and subjects. In this randomized, double-blind, placebo-controlled trial, a dose of 1 mg/day of RAS was given to 23 participants for a 3-month period. Mean age and disease duration of the RAS and Pb groups were, respectively, 65.2 and 67.6 years old and 4.1 and 4.0 years. The mean MMSE score at baseline was unavailable. However, according to the authors, patients had to be cognitively impaired, but not demented, to be enrolled in the study. The authors defined cognitive impairment as having a performance 1.5 SDs below normative scores on the screening neuropsychological tests for two out of the four cognitive domains they assessed. These criteria are similar to the PD-MCI definition published later.Reference Litvan, Goldman and Tröster 1 Its main methodological limitations were same as those of the SEL studies. Table 4D shows the results on the cognitive outcomes.
Table 4D Effects of rasagiline on cognitive outcome measures

↑=Statistically significant improvement post-treatment/better performance in the experimental group; ↓=statistically significant deterioration post-treatment/worse performance in experimental group; BFR=Benton Facial Recognition; BJLO=Benton Judgment of Line Orientation; BL=baseline; BNT=Boston Naming Test; CDT=Clock Drawing Test; DOT-A=Adaptive Digit Ordering Test; g=Hedges’ g effect size; Gr.=group; mth=months; ns=non-significant; Ref.=reference; TMT=Trail Making Test; WAIS=Weschler Adult Intelligence Scale; WMS-R=Weschler Memory Scale-Revised.
Of a total of 25 cognitive results, two (8%) indicated a statistically significant effect, both of them being beneficial. With these few significant results, it was not possible to appreciate the possible convergence of the effects.
Attention/Processing Speed/Working Memory
The performance remained unchanged on the Digit Span forward (attention span) after 3 months of treatment, but improved on the Digit Span backward (verbal working memory), with a medium-size effect (p=0.042; g=0.594). On the DOT-A, a beneficial change of medium effect size was also reported, although it did not reach statistical significance (p=0.051; g=0.569). No significant difference was obtained on the TMT-A (attention/processing speed). Altogether, these results suggest a positive, but selective, effect of RAS on the Central Executive System of Baddeley’s Working Memory model.Reference Baddeley and Hitch 116 , Reference Baddeley 117
Perception, Episodic Memory, Naming and Construction Performance
No significant effects of RAS were registered on the tests of naming, visual perception and construction, verbal and visual episodic memory after 3 months of treatment.
Executive Functions
Patients treated with RAS ameliorated performances on the verbal fluency total score with medium effect size (p=0.038; g=0.608). However, when the results of each fluency task (category and lexical fluency) were analyzed individually, the improvement was not significant on the lexical (p=0.155; g=0.411) and the category (p=0.124; g=0.445) fluency tasks. No change occurred on performing the Stroop test and the TMT-B.
Discussion
The present systematic review aimed at studying the impact of currently relevant antiparkinson medications on cognition in mild-to-moderate PD. In total, 14 studies involving non-demented PD patients with intact or compromised cognition were analyzed. Overall, quality of evidence was poor; 11/14 studies had low or very low MQ scores. On average, SEL studies were of higher quality (mean MQ score=57.2%), followed by RAS (52.2%), L-D (49.7%) and PRX (40.8%). Nevertheless, all studies used well-validated cognitive tests, and most studies administered an exhaustive neuropsychological battery. However, some cognitive domains such as language, praxis performances and perception were under-represented in the reviewed studies. Therefore, we lack data to clearly assess the effects of antiparkinson medication on these cognitive domains.
Regarding L-D, medium to large deleterious effect sizes were almost exclusively obtained on a well-validated measure of inhibition capacity (Stroop). On the other hand, beneficial effects were mainly reported on processes such as memory encoding and retrieval (medium to large effect sizes), planning/organization (medium effect size), flexibility (medium to large effect sizes), verbal fluency (small to large effect sizes) and concept formation (small effect size). Results were less consistent for attention/processing speed and for working memory, sometimes with a beneficial effect of L-D (small to medium effect sizes) and sometimes with deterioration or no change.
These results are mostly in accordance with the dopamine overdose hypothesis,Reference Gotham, Brown and Marsden 19 , Reference Gotham, Brown and Marsden 118 stating that in mild PD clinically effective doses of L-D could compensate for the DA depletion in the dorso-lateral frontostriatal loop, while at the same time overdosing relatively intact circuits such as the orbitofrontal loop. As suggested in the inverted U-shaped model Reference Cools 16 of dopaminergic stimulation, insufficient and excessive levels of DA in a loop’s structures could alter the cognitive processes associated with these structures. The present findings, in line with these hypotheses, suggest that L-D principally improves executive processes associated with the dorso-lateral prefrontal cortex (DLPFC), while also altering the inhibition capacity associated with the orbitofrontal cortex (OFC) in mild-to-moderate PD.
Yet, as mentioned earlier, some contradictory results were also reported, with some benefits on inhibition and deterioration in information processing speed. The large deterioration on the Stroop-Word was recorded by the only study that had recruited de novo patientsReference Kulisevsky, Garcia-Sanchez and Berthier 39 and used the highest mean daily dosage of L-D (540 mg/day). The combination of milder disease and higher dosage might partly explain the negative effects, possibly via overdosing the less-damaged DA loops. The improvement of the inhibitory processes was reported by the only studyReference Fera, Nicoletti and Cerasa 42 that used an LD+DDCI treatment, a Stroop test modified for MRI administration, and an On/Off-treatment paradigm. In addition, the participants had longer disease durations but the UPDRS scores were comparable to other studies using the Stroop.Reference Kulisevsky, Garcia-Sanchez and Berthier 39 - Reference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 , Reference Brusa, Tiraboschi and Koch 43 Thus, these patients possibly deteriorated more slowly and/or had better motor response from antiparkinson medication compared with patients from other studies. Taken together, these methodological differences might explain the contradictory results.
Cognitive results following PRX treatment suggest that PRX negatively affects verbal learning capacities (medium effect) at high dosage (3.9 mg/day) and impulse control at low dosage (1 mg/day). On a brighter note, there were no indications that PRX induced changes in other cognitive tasks (attentional/executive).
However, many factors must be taken into consideration regarding the PRX results. First, the four PRX studies were of very low to low MQ, exposing them to bias and limiting their capacity to detect or exclude important effects. Second, the two significant results were each reported solely on one condition of one test, making it impossible to verify whether the significant effects tend to converge or diverge within each cognitive domain. Thus, there is insufficient evidence to conclude that PRX affects cognition, but it is impossible to exclude that there might be some effects either. At best, these results could indicate that episodic memory and impulse control might be more susceptible to be affected by this medication. Some support for impulse control already exists, as other studies report an increased incidence of impulse-control disorders following PRX treatment in PD.Reference Müller 119 , Reference Poletti, Logi and Lucetti 120
Regarding SEL, the three studies administered the maximum recommended dose (10 mg/day) and their results suggest that SEL negatively affects concept formation and reasoning (large effect), as well as global cognition (small effect). Some divergent results were obtained on episodic memory, although no change in performance ended up being statistically significant. These conflicting results seem to be best explained by methodological differences between studies (i.e., low vs. high MQ, presence of a Pb group or not) and from differences in group characteristics within studies (i.e., disease stage, severity of symptoms, scolarity), than by an effect of SEL treatment.
The detrimental effect on global cognition was reported on the MMSE (small effect size) by a robust longitudinal studyReference Kieburtz, McDermott and Como 48 (MQ=84.8%) when SEL was compared with Pb. Considering that the MMSE is not particularly sensitive to cognitive changes in PD,Reference Riedel, Klotsche and Spottke 121 , Reference Hoops, Nazem and Siderowf 122 the fact that a significant deterioration was caught, albeit small, likely suggests that SEL could have some longer-term deleterious effect on global cognition.
Data on RAS came from only one studyReference Hanagasi, Gurvit and Unsalan 36 of low MQ that included exclusively cognitively impaired, but not demented, patients. Three months of RAS treatment at maximum recommended doses (1 mg/day) did not alter these patients’ cognitive abilities. It might even have benefited the central executive component of working memory (medium effect) and verbal fluency (medium effect).
Nevertheless, it is impossible to conclude that, overall, RAS has the potential to benefit certain cognitive functions in mild-to-moderate PD, nor is it possible to exclude this possibility. Although the studyReference Hanagasi, Gurvit and Unsalan 36 had many strengths (e.g., randomized, double-blind, placebo-controlled trial, extensive cognitive battery), its results were obtained on a small sample of cognitively altered PD patients on a short period of time, which alters the validity and generalizability of the reported effects. It might nonetheless indicate that working memory and verbal fluency might be more susceptible to be affected by RAS. However, two recent studies that did not quite meet our selection criteria seem to support the absence of cognitive effects for RAS in cognitively altered or cognitively intact PD patients. The first studyReference Weintraub, Hauser, Elm, Pagan, Davis and Choudhry 123 tested the effects of 24 weeks of RAS treatment on cognition, motricity and activities of daily living (ADL) on 170 PD-MCI patients (n=86 RAS; 84 Pb) using a multicenter, randomized, double-blind, placebo-controlled design. The authors report beneficial effects on motricity and ADL, but not on cognition. Although this studyReference Weintraub, Hauser, Elm, Pagan, Davis and Choudhry 123 can neither support nor invalidate the possibility that certain cognitive functions might be more susceptible to be affected by RAS in PD-MCI, given that global cognitive measures were administered instead of a detailed neuropsychological battery, it does support that RAS does not seem to worsen cognition in this population. The second studyReference Frakey and Friedman 124 was also a randomized, double-blind, placebo-controlled trial, but did not restrict its sample to PD-MCI patients and used a neuropsychological battery covering multiple domains, including attention, executive functions, language, visuo-spatial perception and memory. The effects of 26 weeks of RAS treatment were tested on cognition and motricity using a sample of 45 non-demented patients with mild-to-moderate stage PD (n=23 RAS; 22 Pb). It concludes that RAS is an effective treatment for motor symptoms, but has no effect on cognition in this population.
When compared with one another, L-D had the most beneficial and deleterious effects on cognition; PRX showed indications toward a few negative effects, but did not affect cognition overall; SEL mildly deteriorated global cognition over time and altered concept formation; and RAS principally showed no change, but might induce a few benefits. In general, beneficial effects were principally reported on DLPFC-related attentional/executive functions, and deleterious effects were mostly obtained on OFC-related inhibitory processes and impulse control. The results are thus mostly in accordance with the predictions of the DA overdose hypothesis in mild-to-moderate PD, which is especially true for L-D, as data on other drugs were scarce. These findings are also in accordance with the conclusions of a narrative review on antiparkinsonian effects in mild PD.Reference Poletti and Bonuccelli 22 However, the present work analyzed the effects of more treatments (MAO-B inhibitors) and presented more exhaustive and quantitative data.
A more puzzling conclusion to be made is with regard to the effects of antiparkinson medications on episodic memory. The findings in this domain differed with each treatment and ultimately did not converge toward a general tendency.
Psychometric Qualities of the Cognitive Tests
In total, 42 different cognitive tests were used through the 14 selected studies. The majority of those tests were well-known measures of their respective cognitive domain, such as the Digit Span, the Stroop test, the TMT, the SDMT, the CVLT, the RAVLT, the ROCFT, the RBMT, the WCST or the COWAT (see Table 3 for more details). The most frequently used tests by far were the Digit Span, the Stroop test, the TMT and the verbal fluency tests. These five tests, along with the RAVLT, concurrently yielded most of the significant results. The vast majority of the above-listed tests are cited by the MDS task force guidelinesReference Litvan, Goldman and Tröster 1 as good examples of tests to administer for cognitive assessment in PD. This ensures their validity to detect difficulties with attention, executive function, episodic memory and verbal fluency for this population. In addition, they have also shown good or acceptable test-retest and/or inter-rater reliability with various populations, which is an important factor in clinical trials in which the measures are administered by different examiners repetitively over a short period of time.
However, one test raised questions among the present authors regarding its psychometric qualities. The Delay Discounting Task, used in one study,Reference Antonelli, Ko and Miyasaki 47 is reported to have modest levels of reliability and questionable construct validity.Reference Smith and Hantula 103 Considering we already advised caution in the interpretation of the significant result obtained on this task following PRX treatment, these psychometric concerns reinforce our statement.
Limitations
Many factors must be taken into account for the interpretation of the present data. First, samples were generally small, with most studies including around 15 to 20 treated PD patients, with the exception of the two DATATOP studies (n>180 treated participants). Small sample sizes result in lower statistical power, which can in turn result in a failure to detect subtle treatment effects. Moreover, given that PD patients have a heterogeneous cognitive decline, large sample sizes are mandatory to ensure external validity in studies investigating the impact of medications on cognition.
Second, methodological designs varied widely across the studies, affecting the internal validity of the results. Only five of the 14 reviewed studies were RCTs.Reference Growdon, Kieburtz, McDermott, Panisset and Friedman 37 , Reference Kulisevsky, Garcia-Sanchez and Berthier 39 , Reference Relja and Klepac 46 , Reference Kieburtz, McDermott and Como 48 , Reference Dalrymple-Alford, Jamieson and Donaldson 49 Randomized controlled trials are the most robust research designs, yet most studies used only within-group comparisons. It is thus impossible to know whether a placebo effect or the disease progression contributed to some results. Nine studies were not blinded, making them vulnerable to patient and investigator biases. Furthermore, when using an On-/Off-treatment design, studies are more vulnerable to be contaminated by a learning effect, because of short test-retest periods. Nevertheless, the utilization of tests that show low vulnerability to learning effects at retest in the reviewed studies should have minimized these effects.
Third, only two studiesReference Kulisevsky, Garcia-Sanchez and Berthier 39 , Reference Kieburtz, McDermott and Como 48 lasted more than 6 months, leaving long-term effects of treatment on cognition mostly unknown. When looking closely at the results of the longest studies, one notices that the effects of some medications vary significantly over time. Hence, it is mandatory to realize more longitudinal studies to understand the possible variations of the antiparkinson medications’ effects on cognition over time.
Fourth, the present review selected studies involving patients taking the relevant drugs in monotherapy or with an adjuvant medication for which the dosage was not always reported by the authors. Thus, an interaction effect cannot be excluded for some results, making the comparison of effects between studies more complex. However, only studies with a design that isolated the effect of the selected drug were included in this review. Furthermore, the adjuvant medications administered in the studies are those usually given to PD patients in clinical settings, thus supporting external validity of the present results.
Fifth, it was difficult to control for the baseline cognitive functioning of the patients. Only studies including non-demented patients were selected, but it was not always possible to distinguish cognitively compromised from cognitively intact patients at baseline, notably because the clinical criteria for PD-MCI are relatively recent.Reference Litvan, Goldman and Tröster 1 Furthermore, some patients could have had co-pathology, especially with Alzheimer’s disease, which would have an impact on cognition irrespective of the PD. Without the use of biomarkers, it is difficult to exclude this possibility. Thus, comparability between studies could be hindered. Nonetheless, global scores from MMSE or MoCA, when available, indicated that patients from different studies had overall similar scores.
Sixth, there are some concerns regarding the methodological quality assessment. The scale had some limitations during its validation: it was developed for psychiatric studies, and our MQ score is not validated. The principal limitation listed by the CCDAN authors is the portion of subjectivity that affects the score, as suggested by the lower-than-expected inter-rater reliability of 0.5.Reference Moncrieff, Churchill, Drummond and McGuire 32 To minimize the impact of this limit, the two evaluators of the present review held meetings before and after they independently performed the quality assessment. The goals of these meetings were to reach consensus on the operationalization of more subjective criteria before starting the assessment of the studies (CCDAN authors’ suggestions were used when available), and to solve all discrepancies following the evaluation of the studies. Whenever a consensus could not be reached, an experienced researcher in the domain (MS) was consulted. Regarding the psychiatric purposes of the CCDAN, our rationale was that the literature on cognitive effects of antiparkinson medications shared some similar limitations to psychiatric studies (e.g., designs of highly variable quality, small sample sizes). To better differentiate our studies and avoid a floor effect, we aimed to find a scale that allowed for a detailed and systematic consideration of the various aspects of trials’ quality. As for our MQ score, actions have been taken to ensure transparency of the process (see Method section). We advise caution regarding over-interpretation of the scores.
Finally, the choice of the antiparkinson medications to be reviewed in the present paper, based on the CNSF guidelinesReference Grimes, Gordon and Snelgrove 23 (Level A quality of evidence) to initiate dopaminergic treatment in PD, might be perceived by non-Canadian readers as being too specific. However, American Academy of Neurology (AAN)Reference Miyasaki, Martin, Suchowersky, Weiner and Lang 125 and National Institute for Health and Care Excellence (NICE) 126 guidelines have generally the same Level A treatment recommendations to initiate PD treatment. The only differences between the AAN and NICE versus CNSF recommandations is the addition of cabergoline (AAN) and rotigotine (NICE). There was only one studyReference Brusa, Pavino, Massimetti, Bove, Iani and Stanzione 41 on the cognitive effects of cabergoline and rotigotine together with L-D, and it has been reviewed in this paper. The authors of this study report that the three drugs did not significantly modify their patients’ cognitive performance compared with an Off-treatment condition.
Future Research
Even with low MQ, the data extracted from the reviewed studies provided interesting information regarding the possible association between dopaminergic antiparkinson medications and cognitive changes in patients with mild-to-moderate PD without dementia. However, one should keep in mind that some studies showed no impact at all, whereas some of the observed effects were of questionable validity. Thus, to better understand this association, especially during longer periods of time, studies with significantly higher MQ are required. To achieve this, future studies will have to use the most robust research designs (double-blind randomized placebo-controlled trials), use sample sizes of 100 or more, as indicated by the FDA for phase 2 clinical trials, 127 and have a study duration of ≥6 months to clearly assess the evolution of effects, because the results of studies with longer follow-ups have shown that some effects are only detectable after 2 to 6 months of treatment, whereas other effects seem to vary over time. This is also supported by a trialReference Molloy, Rowan, O’Brien, McKeith, Wesnes and Burn 128 that compared the cognitive effects of L-D in demented and non-demented PD patients, whose results suggest that some effects differed more than others between those two milestones of PD progression.
Regarding the cognitive evaluations, the current problems reside in the tendency of using only a few tests to measure several cognitive functions and of using several tests to assess only one or two cognitive domains. For instance, domains such as attention and executive functions were almost always assessed, whereas domains such as language, perception or visuo-constructive praxis were neglected. Although attention and executive functions are the cognitive functions most likely to be affected by DA antiparkinsonians, other domains might also be affected (after all, visuo-construction praxis and syntax involves some executive functioning, and can be both impaired by alterations of basal gangliaReference Bocanegra, García and Pineda 129 ). Furthermore, some antiparkinson medications, notably monoamine oxidase inhibitors, do not exclusively affect dopaminergic neurotransmission, which could in turn affect cognitive domains underlied by different brain regions. Therefore, all cognitive domains should be assessed minimally with one test, whereas the cognitive domains hypothesized to be more responsive to treatment should be thoroughly evaluated (>one test).
As the cognitive effects of the antiparkinson medications seem to vary over time and with disease progression, it is important to better differentiate the patient’s cognitive status before treatment. Interestingly, the only study that recruited exclusively cognitively compromised patientsReference Hanagasi, Gurvit and Unsalan 36 reported some of the most beneficial results. Thus, differentiating PD-MCIReference Litvan, Goldman and Tröster 1 from non-MCI patients would provide better insight regarding the most favorable contexts to prescribe the different drugs for healthcare providers.
Acknowledgments
The authors thank Mr Gaétan Daigle, Statistician, Mathematics and Statistics Department at Laval University, for his help with the statistical analyses performed in this study. The authors are also thankful to Ariane Giguère-Rancourt, Éva Racine, Laila El-Amrani, Marika Plourde, Marianne Couture, Élodie Thériault and Valérie Coulombe for their assistance in the preparation of the manuscript. Part of the results were presented at the 13th International Conference on Alzheimer’s and Parkinson’s Diseases and Related Neurological Disorders (AD/PDTM 2017).
Funding
None.
Disclosures
M-AR has nothing to disclose. MD reports grants from Parkinson Society Canada and grants from Canadian Institutes of Health Research—Institute of Neurosciences, Mental Health and Addiction (201210), outside the submitted work. JT-C reports grants from Canadian Institutes of Health Research, outside the submitted work. ND reports grants from Quebec Parkinson Network and grants from Actelion Pharmaceuticals, outside the submitted work. MS reports grants from Canadian Institutes of Health Research-Big Data on Dementia and grants from Alzheimer Society Research Program/Pacific Alzheimer Research Foundation, outside the submitted work.
Statement of Authorship
M-AR carried out conception, organization, and execution of the research project. He was also responsible for data extraction, including article selection and analysis, and tables; statistical analysis including design and execution; and writing of the drafts. MD contributed to data extraction, including article selection and analysis. JT-C contributed to data extraction for the preparation of tables. ND contributed to the conception of the research project. MS was responsible for the supervision of the research project; resolving discrepancies in article selection; and for the study design. MD, JT-C, ND, and MS carried out critical review of the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2018.21















