Neurofibromatosis type 1 (NF1) is an autosomal dominant neurocutaneous disease. Individuals with NF1 can develop benign and malignant tumors, with the hallmark lesions being cutaneous neurofibromas. Other common neoplasms are plexiform neurofibroma, optic pathway glioma, or other low-grade gliomas. Reference Ferner and Gutmann1
Cutaneous glomus tumors have been associated with NF1. Reference Stewart, Sloan and Yao2–Reference Brems, Park and Maertens4 They are tumors of contractile neuromyoarterial receptors called glomus bodies, which are involved in thermoregulation and have high concentrations in the fingertips. These tumors are usually benign and solitary but there have been reports of malignant and multifocal disease. They are often subungual. Reference McDermott and Weiss5
A combination of magnetic resonance imaging (MRI) and clinical examination is used to support the diagnosis. These tumors can cause exquisite focal pain from contraction of the glomus cells. Surgical removal of the glomus tumor is effective and can be curative. However, inadequate resection often leads to tumor recurrence. Reference McDermott and Weiss5
We present a case of a 28-year-old man with multiple surgically refractory painful fingertip glomus tumors whose pain responded to targeted chemotherapy. He had a history of genetically-confirmed NF1 and had no known family history of NF1. He had a childhood optic pathway glioma treated with surgical resection and chemotherapy, with resultant panhypopituitarism and blindness. He had a remote leiomyosarcoma of the right temporal lobe treated with surgery and chemoradiotherapy at the age of 14.
He began having episodes of fingertip pain 6 years prior to presentation to our clinic. Each episode lasted 5–10 minutes and could include one or several fingers. These pain crises were rated up to 9/10 in severity. Triggers included touch, cold, heat, or pressure. Over the course of 5 years, he had multiple surgical resections of the subungual lesions providing partial relief of pain. Five of the six resected lesions were glomus tumors, and one was a neurofibroma. He tried several medications for the pain including naproxen, gabapentin, and topical ketamine which were not helpful. On examination, he had no visible abnormalities of his fingertips, even with trans-illumination. There was no tenderness to palpation. A left-hand MRI showed a subungual lesion consistent with glomangioma in the 3rd digit and post-operative changes in the 4th digit. (Figure 1, left panel). In the right hand, there were lesions consistent with glomangioma in the 1st, 3rd, and 4th digits (Figure 1, right panel).
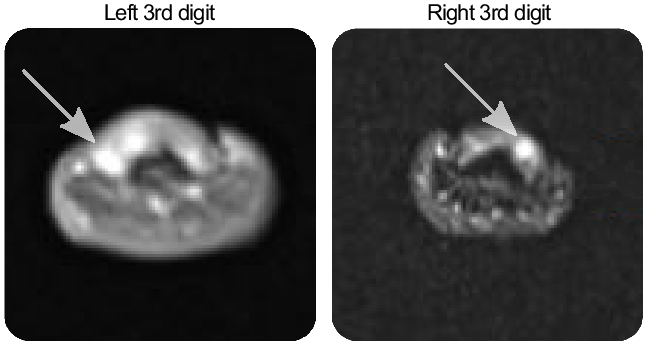
Figure 1: Glomangioma (arrows) are visible on axial hand MRI scans.
We treated him with trametinib 2 mg orally daily. He recorded his pain crises both before and after starting the drug (Figure 2). In the month leading up to the start of trametinib, he recorded 31 painful episodes, 5 of which were marked as severe (i.e. worse than 5/10). The total time in severe pain that month was 37 minutes. A month after starting trametinib, he recorded 26 painful episodes, 2 of which were marked as severe. He was in severe pain for 15 minutes that month. He developed an acneiform eruption due to trametinib and was started on oral and topical antibiotics. His trametinib was paused and was restarted 8 weeks later at a dose of 0.5 mg daily. His rash is now mild and manageable. A month after restarting trametinib, his painful episodes have reduced in frequency and intensity. He had 12 painful episodes, none of which were marked as severe. A repeat MRI of his hands after 6 months showed stable glomangioma. He continues to take trametinib.
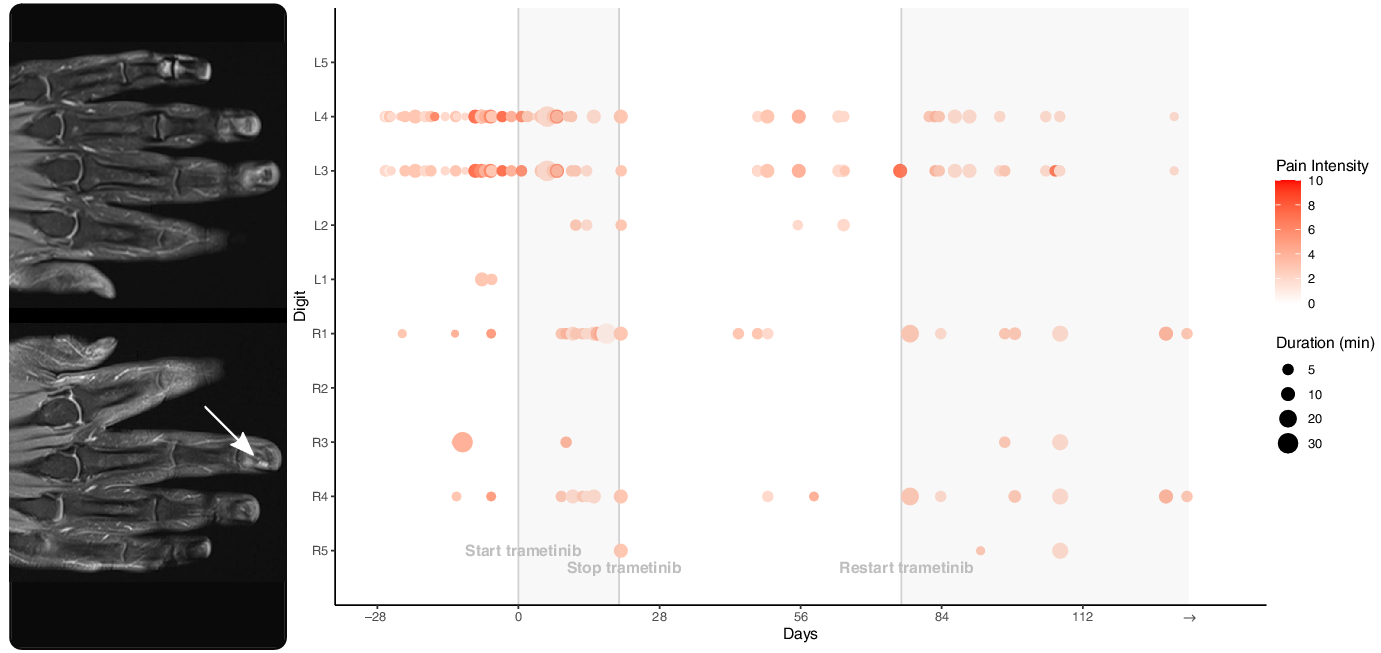
Figure 2: Coronal T1-weighted post-gadolinium MRI of the hands showing small, enhancing glomangiomas, best seen on the right distal 3rd digit (arrow) and on the left 3rd digit (arrow), better seen on axial images. The associated pain scores and durations for each digit are shown in the adjacent graph. The patient recorded his pain crises before and after starting trametinib. Day 0 represents the first day taking trametinib 2 mg daily.
It is increasingly recognized that NF1 tumors are largely driven by mitogen-activated protein kinase (MAPK) pathway upregulation. Loss of neurofibromin in NF1 results in activation of Ras/MAPK signal cascade leading to cell proliferation and tumor formation. Inhibitors of this pathway can successfully treat certain NF1-associated tumors.
The SPRINT phase II clinical trial of selumetinib therapy in NF1-associated plexiform neurofibroma demonstrated 72% partial response with improvement of tumor-related pain and motor impairment. Reference Gross, Wolters and Dombi6 A retrospective cohort study showed a 44% response rate in trametinib-treated low-grade glioma, half of which were NF1-driven. Reference Selt, van Tilburg and Bison7 The average time to best overall response was 4 months. Reference Selt, van Tilburg and Bison7 In NF1-associated glomus tumors, there is evidence of MAPK upregulation when compared to sporadic glomus tumors. Reference Brems, Park and Maertens4 There is thus reason to believe that trametinib, or other MEK inhibitors, can be used to treat recurrent or refractory glomus tumors.
The most common toxicities associated with MEK inhibition were acneiform dermatitis, diarrhea, peripheral edema, and fatigue. These were managed by dose interruptions or dose reduction. Rare but serious toxicities of trametinib were seen in a study including left ventricular dysfunction/decreased ejection fraction, interstation pulmonary disease, and ocular toxicities (central serous chorioretinopathy). Reference Falchook, Lewis and Infante8 Routine monitoring with physical examination, vital signs, ophthalmologic examination, laboratory testing, and echocardiogram are recommended. Our case shows the fine balance of the MEK inhibitor dose intensity for clinical efficacy and drug adverse effects. Even with the dose reduction of the MEK inhibitor, there were fewer episodes of pain and there was improvement of skin toxicity.
We herein describe the first known case of a MEK inhibitor used to treat subungual glomus tumors. Our patient has surgically and medically refractory NF1-related subungual glomus tumor pain. His pain significantly improved after starting trametinib. This case report adds to the body of literature of NF1-driven tumor types responsive to MEK inhibitors.
Funding
The company Novartis provided a compassionate supply of trametinib to our patient. They had no role in this research publication.
Disclosures
The authors have no conflicts of interest to declare.
Statement of authorship
SAC and RCR designed and conceptualized the study, analyzed the data, and wrote the manuscript. DJA revised the manuscript for intellectual content. WPM helped acquire the data, interpreted the data, and revised the manuscript for intellectual content.






