Article contents
Whey protein concentrate ameliorates the methotrexate-induced liver and kidney damage
Published online by Cambridge University Press: 23 March 2023
Abstract
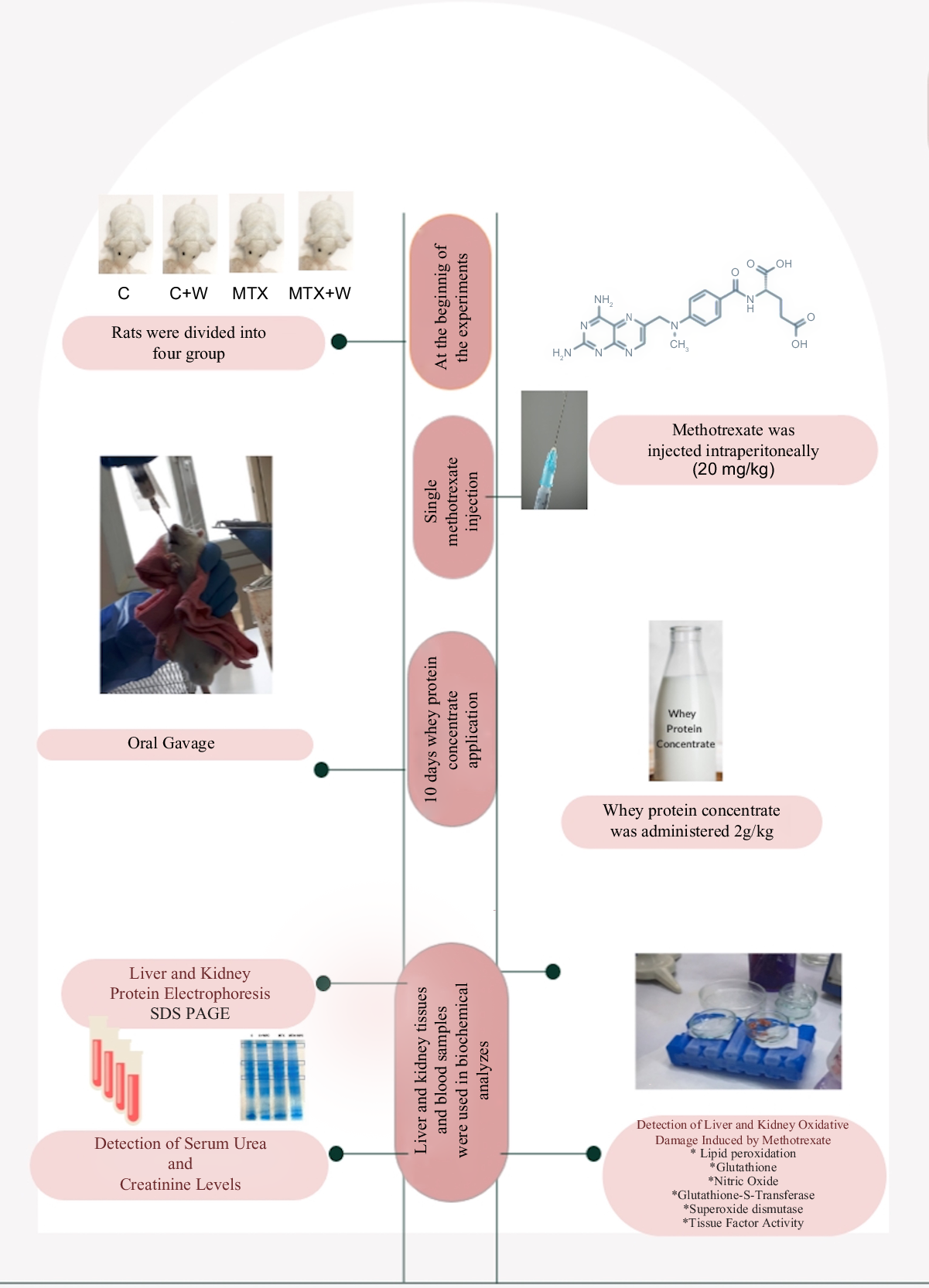
Methotrexate (MTX) is a cytotoxic immunosuppressant that is widely used in the treatment of tumours, rheumatoid arthritis and psoriasis. This study aims to evaluate the effects of whey proteins on MTX-induced liver and kidney damage by focusing on oxidant–antioxidant systems and eating habits. The study was conducted in four groups of thirty Sprague–Dawley rats (control, control + whey protein concentrate (WPC), MTX, MTX + WPC). A single dose of 20 mg/kg MTX was administered intraperitoneally to the MTX groups. Control and MTX groups were given 2 g/kg WPC by oral gavage every day for 10 d. At the end of day 10, blood samples were drawn and liver and kidney tissues were removed. MTX administration increased the lipid peroxidation level and decreased glutathione level, superoxide dismutase and glutathione-S-transferase activities in the liver and kidney. Administration of WPC significantly reduced the damage caused by MTX in the liver and kidney. While a decrease in serum urea level and an increase in serum creatinine level were detected in the MTX group, WPC administration reversed these results up to control group levels. Administration of WPC to the MTX group significantly reversed the histopathological damage scores of the liver and kidney. WPC administration ameliorated the MTX-induced oxidative damage in the liver and kidney tissues due to its antioxidant properties. Liver and kidney damage can be prevented by using whey proteins as a nutraceutical in MTX therapy. In conclusion, whey proteins demonstrated a protective effect against MTX-induced liver and kidney damage.
- Type
- Research Article
- Information
- Copyright
- © The Author(s), 2023. Published by Cambridge University Press on behalf of The Nutrition Society
References
- 1
- Cited by





