Human milk contains a large variety of complex oligosaccharides in concentrations ranging from 10 to 20 g/l(Reference Thurl, Munzert and Henker1, Reference Kunz, Rudloff and Baier2). The quantity of these components does not only depend on the lactational stage of the mother but is also affected by the expression of specific glycosyltransferases in the mammary gland(Reference Thurl, Munzert and Henker1–Reference Le Pendu3). Genes encoding for H and Lewis a or x antigens as well as the secretor status determine the presence of α1,2-, α1,3- and/or α1,4-fucosylated core structures of oligosaccharides. In addition, different patterns of sialylation, i.e. the attachment of α2,3- and/or α2,6-linked N-acetylneuraminic acid (NeuAc), increase the variability of human milk oligosaccharide (HMO) to a number of about 115 structures characterised so far(Reference Stahl, Thurl and Zeng4, Reference Urashima, Kitaoka, Terabayashi and Gordon5). The biological significance of HMO for the infant, however, has not been proven yet. Since the 1950s, HMO have been thought to be growth-promoting factors for the so-called ‘bifidus flora’ in the gut of breastfed infants(Reference György, Norris and Rose6). Recently, the analysis of the genome of Bifidobacteria indicated their evolutionary adaptation to preferentially use specific milk components, particularly HMO as their substrate(Reference Sela, Chapman and Adeuya7, Reference Sela and Mills8). However, the bifidogenic effect of HMO themselves and their direct impact on the intestinal microbiota are difficult to demonstrate in vivo. The same applies to other specific in vitro functions of HMO, such as their potential to influence inflammatory and infectious processes via inhibition of the attachment of pathogens to epithelial cells or leucocyte endothelial and neutrophil platelet interactions(Reference Sharon and Ofek9–Reference Bode, Rudloff and Kunz12). Animal and preclinical studies indicate that oligosaccharides like those in human milk may play an important role in many physiological processes such as influencing cell recognition and cell signalling, cell adhesion, the composition of the microbiota or even affecting neurodevelopment(Reference Mysore, Wigginton and Simon13–Reference Fuhrer, Sprenger and Kurakevich15).
To better understand the underlying mechanisms, more information regarding the metabolic fate of HMO in the infants is needed. In a previous study(Reference Rudloff, Obermeier and Borsch16), we demonstrated that the application of stable isotopes to lactating mothers leads to the preferential incorporation of 13C-galactose (Gal) into lactose and milk oligosaccharides. The 13C-enrichment of HMO in this pilot study was high enough to investigate the metabolic fate of HMO in the infants. In an earlier study, we showed in one mother and her infant that the combination of fast atom bombardment-MS (FAB-MS) and isotope ratio-MS (IR-MS) is suitable to address specific questions with regard to the metabolism of HMO in humans(Reference Obermeier, Rudloff and Pohlentz17). In the present study, we report the renal excretion of intact and partly degraded complex oligosaccharides in ten infants after in vivo labelling of HMO by administering 13C-Gal to their mothers.
Subjects and methods
Subjects and study design
Exclusively breastfeeding women (n 10; 3–6 months postpartum) participated in the present study. Three mothers had already participated in our previous study(Reference Rudloff, Obermeier and Borsch16) and seven were newly recruited. Some dietary restrictions, i.e. the avoidance of naturally 13C-rich foodstuffs (e.g. corn, pineapple, fish), were given. The mothers were asked not to drink any milk for breakfast on the study day and to record their daily food intake during the study period. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of the University of Giessen. Written informed consent was obtained from all subjects/patients.
Between 08.00 and 09.30 hours, immediately after breakfast, mothers orally ingested a Gal bolus consisting of 23 g Gal+4 g 13C-Gal dissolved in about 50 ml of drinking water. The purity of 13C-Gal (d-Gal; 1-13C) from Eurisotop (Saint-Aubin Cedex, France) was determined to be higher than 99 %.
Milk and breath sampling of the women
A milk sample (5–10 ml) was collected immediately before the Gal bolus was given (baseline value) and at the beginning of each nursing during the following 36 h(Reference Rudloff, Obermeier and Borsch16). At the same time, mothers took breath samples and collected their urine in 3–4 h fractions.
Sampling of the infants' urine
For urinary collections, diapers consisting purely of cellulose and free of other absorptive material (Procter & Gamble, Frankfurt, Germany) were used. Urine was collected in adhesive bags which were emptied or replaced before each nursing. Diapers were changed before each nursing and immediately frozen at − 20°C. For urine extraction, diapers were thawed and mechanically pressed leading to urine yields of about 70–80 %. In previous experiments, it was verified that the urine collection via diapers and adhesive urine bags did not affect HMO analysis.
Separation of milk fractions and characterisation of milk carbohydrates
The separation of whole milk into fractions with fat, proteins and carbohydrates followed by the removal of lactose was achieved by ultracentrifugation, acetone treatment and gel filtration chromatography of the carbohydrate fraction as described earlier(Reference Kunz, Rudloff and Hintermann18). Then, neutral and acidic HMO were characterised by chromatography and MS (see the following subsections).
High pH anion exchange chromatography with pulsed amperometric detection
High pH anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) on a Carbo Pac PA-1 column (250 × 4·6 mm inner diameter) equipped with a guard column and a Model PAD 2 detector (Dionex, Sunnyvale, CA, USA) were used for characterisation of neutral and acidic HMO at the following conditions: eluent A, 100 mm-NaOH; eluent B, 100 mm-NaOH and 250 mm-sodium acetate. The elution programme started with 3 ml of buffer A followed by a gradient of up to 100 % buffer B in 30 min and a re-equilibration volume of 5 ml buffer A. The flow-rate of 1·0 ml/min was used and 25 μl of 1 or 4 mg/ml solutions were injected. Molar response factors were determined by injecting three to six times equimolar amounts of each oligosaccharide as described previously(Reference Kunz, Rudloff and Hintermann18).
Fast atom bombardment-MS
Oligosaccharides (50–100 μg) were dissolved in 500 μl of pyridine–acetic anhydride (1:1, v/v). The mixture was stirred at room temperature overnight. Then the solvent was evaporated under N2 and the residue was used for mass spectrometric analysis without further purification. The FAB-MS analysis was carried out on a VG-ZAB-T four-sector instrument (Fisons Instruments, Manchester, UK); caesium was used for atom bombardment(Reference Rudloff, Obermeier and Borsch16). The applied acceleration voltage was 8 kV. Thioglycerol–m-nitrobenzylalcohol (1:1, v/v) was used as matrix to which 1 μl of a homogeneous sample solution in chloroform–methanol (1:1, v/v) was added. For spectra recording, positive ions (FAB+) were detected on the second photomultiplier point detector. The spectra were run in a mass range from 100 to 3000 atom mass units with a scan rate of 8 s per decay and up to ten scans were accumulated.
Determination of 13C enrichment by isotope ratio-MS
13C enrichment was determined as δ13CPDB measured in duplicate in whole milk, milk carbohydrates, total urine and urinary carbohydrate fractions by IR-MS (Isoprime; Isoprime Limited, formerly: GV Instruments; Manchester, UK) after total combustion at 1020°C (EuroEA, Eurovector, Milan, Italy)(Reference Rudloff, Obermeier and Borsch16). Breath samples were analysed in a BreathMat IRMS Microgas IRMS, Isoprime Limited). 13C elimination rates were then quantified as described in the following subsection.
13C analysis by isotope ratio-MS
The 13C content of a sample is expressed as delta value (δ13CPDB) (‰) which is the relative difference between the 13C:12C ratio of a sample (Sa) and the international Pee Dee Belemnite (PDB) standard with a (13C:12C)PDB ratio of 0·0112372,
where 13R Sa = (13C/12C)Sa is the isotope ratio of the unknown sample and PDB is Pee Dee Belemnite.
Data evaluation was performed with MassLynx inorganic version 4 software (GV Instruments) taking into account the 17O-content of the sample and the blank correction for the carbon of the tin cups(Reference Rudloff, Obermeier and Borsch16). For an easier comparison of the data, the results were expressed as Δδ13CPDB (corrected for the 13C:12C ratio at baseline). The accepted standard deviation was < 0·2‰ for total urine and isolated carbohydrates and < 0·7‰ for total milk. The accepted deviation for breath sample analyses was < 0·3‰.
Calculation of the cumulative 13C-enrichment in milk and urine
To determine the 13C-enrichment in milk and urine, total organic carbon (TOC) and 13C-content (at %) for each sample were measured. Then, the excreted amounts of 13C were calculated, taking into account the baseline 13C values and the 13C-bolus given.
Using this 13C-at %sa and the TOC of a sample, the absolute amount of 13C of each sample (Sa) was then calculated as follows:
where at % is atom %.
Calculation of the cumulative 13C exhalation
The percentage recovery of the ingested 13C amount in breath (13Ccum) was calculated using the equation shown in the previous subsection. Thereby, the exhaled amount of 13C is defined as
with Δδ13Ccum being the cumulative 13C exhalation.
Determination of galactose and lactose content
Gal and lactose contents of milk and urine were photometrically determined using a colorimetric kit from Boehringer Mannheim (Mannheim, Germany).
Calculation of ingested milk volumes and respective amounts of human milk oligosaccharide
To determine the amount of milk ingested by the infants, the milk volume was determined by weighing the infants on a digital electronic balance before and after each nursing. Oligosaccharides were quantified by HPAEC-PAD analysis. Peak areas of lacto-N-tetraose (LNT) and lacto-N-fucopentaose II (LNFP II) were then calculated as percentage of the total amount of oligosaccharides applied, corrected by the molar response of the amperometric detector(Reference Kunz, Rudloff and Hintermann18).
Results
13C-enrichment of whole milk and 13CO2 exhalation
In all milk samples, there was an immediate increase in 13C-enrichment of whole milk in the first hours after the oral 13C-Gal bolus was given to the mothers followed by a second smaller 13C-peak in six out of ten milk samples from the next morning (Fig. 1). To get an indication of how much Gal was metabolised for energy production, 13C-enrichment of breath was determined by analysing 13CO2. Exhalation over 36 h compared to the 13C:12C ratio in whole milk resulted in a similar course although with a much higher enrichment in most samples (Fig. 1).

Fig. 1 13C-enrichment of whole milk (–◆–) and infants' urine (–◇–) and 13CO2 exhalation by the mothers (–△–) during the first 36 h after the oral intake of a galactose (Gal) bolus consisting of 4 g 13C-Gal+23 g Gal by the mothers. The δ13CPDB (‰) values of each sample are corrected over the baseline values of each respective sample obtained at time point 0, immediately before the Gal intake. The bolus was taken after breakfast which varied between 08.00 and 09.30 hours.
HPAEC-PAD analysis of the neutral and acidic fractions after Sephadex G25 gel filtration chromatography revealed a rather complex mixture of carbohydrates which were then characterised by FAB-MS. As an example, the composition of the major acidic and neutral HMO is given in Table 1. The molecular weight of these oligosaccharides varied between about 500 and 2500 Da.
Table 1 Fast atom bombardment-MS of the major neutral and acidic human milk oligosaccharides from one mother in fractions after Sephadex 25 chromatography

Lac, lactose; Hex, hexose; NAc, N-acetyl; FucLac, fucosyl-lactose; LNT, lacto-N-tetraose; FucLNT, fucosyl-lacto-N-tetraose; FucLNH, fucosyl-lacto-N-hexaose; NeuAcLac, N-acetylneuraminyl-lactose; NeuAcLNT, N-acetylneuraminyl-lacto-N-tetraose.
Determination of the 13C-enrichment of the infants' urine
The 13C:12C ratio of urine fractions collected over 36 h after the Gal bolus was given to their mothers is shown in Fig. 1. The 13C-enrichment follows about the same pattern as in whole milk. Also, the first 13C-appearance in most urine fractions is delayed as compared to the corresponding milk fraction.
To ensure that the higher 13C:12C ratio is not due to the excretion of free 13C-Gal, we determined the total Gal excretion by enzymatic methods. The data which are exemplified for one infant in Fig. 2 revealed that the excretion of free Gal was only high in the first sample after the Gal bolus was given to the mother, and returned to baseline afterwards. In contrast to the total Gal excretion, the 13C-enrichments in the same urine fractions reached their maximum value 10–11 h (fraction 4) after the Gal bolus was given to the mother.
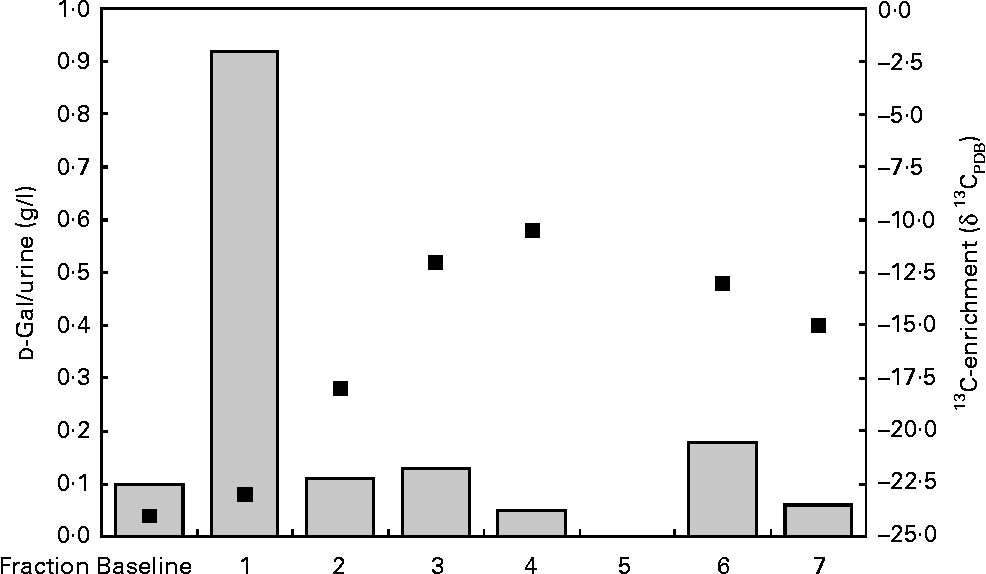
Fig. 2 Excretion of free urinary galactose (![]() ) and 13C-enrichment (■) in fractions of an infant's urine over 24 h.
) and 13C-enrichment (■) in fractions of an infant's urine over 24 h.
To characterise 13C-enriched carbohydrates, urine fractions were subjected to Sephadex gel filtration to obtain components of different molecular sizes and to separate monosaccharides and lactose from oligosaccharides. These fractions were further characterised by HPAEC-PAD, IR-MS and FAB-MS. The major components in these fractions are shown in Table 2. Not only small oligosaccharides such as the lactose derivatives FucLac and Fuc2Lac were detected but also LNT, one of the main core structures in HMO (Table 2). Moreover, the mono-, di- and trifucosylated derivatives of LNT, lacto-N-hexaose, lacto-N-octaoses and lacto-N-decaose could be found. Also, besides neutral components, N-acetylneuraminyl-lactose (NeuAcLac), the major acidic component in HMO and the sialylated derivative of LNT, N-acetylneuraminyl-lacto-N-tetraose, were also present. In addition, fucosyl-lactosamin and fucosyl-lacto-N-triose were identified, with glucose split off from the reducing end of the oligosaccharides (Table 2).
Table 2 Fast atom bombardment-MS of neutral and acidic oligosaccharides from an infant's urine

Lac, lactose; Hex, hexose; FucLacNAc, fucosyl-lactosamin; FucLac, fucosyl-lactose; Fuc1LNTri, fucosyl-lacto-N-triose; LNT, lacto-N-tetraose; NAc, N-acetyl; FucLNT, fucosyl-lacto-N-tetraose; LNH, lacto-N-hexaose; FucLNH, fucosyl-lacto-N-hexaose; FucLNO, fucosyl-lacto-N-octaose; FucLND, fucosyl-lacto-N-decaose; NeuAcLac, N-acetylneuraminyl-lactose; NeuAcLNT, N-acetylneuraminyl-lacto-N-tetraose; NeuAcFucLNH, N-acetylneuraminyl-fucosyl-lacto-N-hexaose.
Quantification of lacto-N-tetraose and lacto-N-fucopentaose II in milk and in the infants' urine
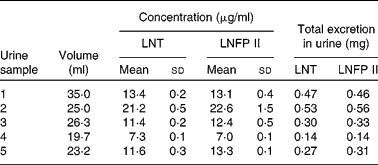
As the milk volume ingested by an infant per suckling was known, we were able to determine in one infant the total amount of LNT and LNFP II taken up with each suckling (Table 3). The total intake of both components varies between 46 and 98 mg for LNT and between 63 and 157 mg for LNFP II per suckling. The determination of the same components in the urine fraction allowed us to quantify the total urinary excretion of these HMO (Table 4).
Table 3 Milk concentration and total intake of lacto-N-tetraose (LNT) and lacto-N-fucopentaose II (LNFP II) per suckling in one infant
(Mean values and standard deviations from five samples of one day)
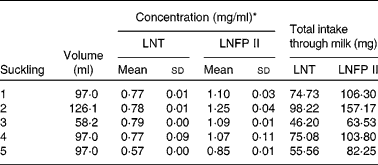
Table 4 Urinary concentration and total excretion of lacto-N-tetraose (LNT) and lacto-N-fucopentaose II (LNFP II) per suckling in one infant
(Mean values and standard deviations from five samples of one day)
Discussion
There is increasing evidence, supported in recent years by animal studies, that HMO have specific beneficial effects on the infant(Reference Wang, Yu and Karim14, Reference Fuhrer, Sprenger and Kurakevich15). Concomitantly with these observations, progress in biotechnology today allows to produce at least some of the major milk oligosaccharides to be potentially added to infant formula at reasonable costs. However, to be able to decide which compound should be added, and in which concentrations or combinations, studies are needed regarding absorption, metabolism and functions in the infants. Previously, we have shown that a major part of a 13C-Gal bolus orally given to lactating mothers was immediately transported to the lactating gland and is directly incorporated into HMO(Reference Rudloff, Obermeier and Borsch16). These results have been confirmed in the present study. Thus, using stable isotopes, an in vivo 13C-labelling of HMO can be achieved to address important questions with regard to their metabolic fate.
Here, we show that in urine of ten infants, HMO are found in all samples collected within 36 h after a 13C-Gal bolus was given to their mothers. The infants' urinary oligosaccharides can only be derived from milk as the biosynthesis of HMO occurs exclusively in the lactating mammary gland. Also, the excretion of intact HMO in the present study with term infants at 3–6 months postpartum verifies our earlier data in preterm infants showing very similar data without using stable isotopes(Reference Rudloff, Pohlentz and Diekmann19).
Besides intact HMO, we also detected cleavage products in which not, as expected, the terminal Gal but the glucose moiety on the reducing end had been split off. At the moment, no plausible explanation can be given for such an unusual metabolic degradation step. There is only one congress contribution from Lundblad's group on the renal excretion of oligosaccharides in a few preterm and full-term infants using mass spectrometric methods for analysis(Reference Chester, Lundbland, Renlund, Yamakawa, Osawa and Handa20). The authors found that small ‘typical milk oligosaccharides’ were excreted by human milk-fed infants. Most of the analysed urine samples contained 2′- and 3-FucLac as well as Fuc2Lac. They could not detect these components in the urine of non-breastfed infants. A further comparison with our data regarding complex oligosaccharides such as LNT and fucosylated and/or sialylated derivatives as well as NeuAcLac is not feasible, since Lundblad and co-workers did not report the analysis of such components.
There are two possible explanations for the occurrence of ‘modified’ oligosaccharides in urine; either they may originate from human milk itself or they are synthesised endogenously from smaller precursors. Although there is no evidence from in vivo studies yet, these modifications may derive from bacterial activity within the gut. Also, if such HMO modifications occur within the colon, the underlying mechanism of the succeeding absorptive process needs to be demonstrated as well.
In the present study, we also determined the amount of HMO an exclusively breastfed infant may receive with each suckling. We showed that the intake of individual oligosaccharides by the infant ranges from 50 to 150 mg per suckling. This large amount of oligosaccharides which ‘rinse’ the whole digestive tract emphasises the potential of anti-adhesive or cellular effects shown for individual carbohydrates in many in vitro studies(Reference Kunz, Rudloff and Baier2, Reference Sharon and Ofek9, Reference Coppa, Zampini and Galeazzi11, Reference Kuntz, Rudloff and Kunz21–Reference Donovan24). However, to exert systemic effects, HMO must be absorbed and transported in the peripheral blood to specific cells where they might also be metabolised. Although HMO are considered to be indigestible(Reference Gnoth, Kunz and Kinne-Saffran25), we detected specific components like LNT and LNFP II in the infants' urine indicating intestinal absorption. As the renal excretion was in the range between 1 and 3 mg/d, the actual intestinal absorption must be much higher, and hence, larger amounts of these HMO must have been circulating in the infant's blood. Therefore, in addition to local functions of HMO within the gastrointestinal tract, systemic effects such as the adhesion of leucocytes to endothelial cells or the interaction of platelets with neutrophils may be influenced(Reference Bode, Rudloff and Kunz12, Reference Bode, Kunz and Muhly-Reinholz23). Recently, an impact of HMO on brain glycoconjugate composition has also been discussed. Carlson & House(Reference Carlson and House26) compared an intra-peritoneal administration to an intra-gastrical application of NeuAc on rat brain composition, and found that both oral and systemic routes resulted in significantly more cerebral and cerebellar glycolipid and glycoprotein NeuAc than did glucose injections. Compared to free NeuAc, orally given NeuAcLac, the major acidic oligosaccharide in human milk, was even more effective on brain composition. These data supported an earlier observation by Witt et al. (Reference Witt, von Nicolai and Zilliken27) comparing radiolabelled free NeuAc and NeuAcLac; the authors showed a preferential incorporation of 14C-NeuAcLac in rat brain gangliosides. Our data suggest that the absorption of HMO in breastfed infants supports the possibility that sialic acid from HMO is utilised as a substrate for the biosynthesis of components including gangliosides or glycoproteins (e.g. for the brain).
In conclusion, we have shown that the application of 13C-labelled HMO alleviates investigations regarding the metabolic fate of HMO in human subjects. The intake of HMO by the infants ranges within several hundred mg per suckling. Some of these components are excreted as intact oligosaccharides or as slightly modified metabolites within the infants' urine. Therefore, HMO have the potential for not only local but also systemic effects. Such effects, however, remain to be demonstrated in future studies. The enormous biotechnological progress in recent years will enable necessary studies in vivo.
Acknowledgements
The authors appreciate the contribution of all mothers who participated in this study which was supported by the German Research Foundation DFG (Ku 781/8-3 and Ru 529/7-3). The authors have no conflict of interest. The authors' responsibilities were as follows: S. R. designed the study, supervised the analyses and wrote the paper; G. P. performed FAB-MS; C. B. performed IR-MS; M. J. L. recruited the subjects; C. K. designed the study, recruited the subjects and wrote the paper.










