Common bean (Phaseolus vulgaris) is a staple food for rural populations in Latin America and Central Africa and represents a valuable source of protein, fibre, starch and minerals. Its consumption is also encouraged in industrialized countries thanks due to beneficial properties on health, such as reducing serum cholesterol levels and risks of CHD and diabetesReference Leterme1.
However, the nutritional value of bean protein is low, due to limited digestibility and marginal deficiency in sulphur-containing amino acids and tryptophanReference Marquez and Lajolo2. The storage globulin phaseolin represents about half of the total protein content of the seed and it is the main source of available methionineReference Gepts and Bliss3. Contrary to other native legume proteins, phaseolin hydrolysis by pepsin and trypsin stops after a limited number of peptides have been cleaved offReference Deshpande and Damodaran4, Reference Jivotovskaya, Senyuk, Rotari, Horstmann and Vaintraub5. However, phaseolin digestion is markedly improved with thermal treatmentReference Deshpande and Nielsen6, Reference Montoya, Lallès, Beebe, Montagne, Souffrant and Leterme7.
Many variants of phaseolin with different subunit patterns have been identified. Type I phaseolin lacks the largest (52 kDa) subunit that the two major phaseolins Sanilac (S) and and Tendergreen (T) haveReference Koening, Singh and Gepts8. Kami and GeptsReference Kami and Gepts9 found that the amino acid sequence of S phaseolin contained an additional methionine as compared to T phaseolin. Moreover, they suggested that the divergence of α and β phaseolin genes predate the divergence between S and T phaseolins. Differences in subunit composition can affect protein hydrolysis among heat-treated S, T and Inca (I) phaseolin types (58, 71 and 71 %, respectivelyReference Montoya, Lallès, Beebe, Souffrant, Leterme and Kharkwal10) and between micro-heterogeneous soyabean storage proteinsReference Fukuda, Maruyama, Kanazawa, Abe, Shimamoto, Hiemori, Tsuji, Tanisaka and Utsumi11. Differences in ileal digestibility between the S and T phaseolins in pigs were observed (57 and 36 %, respectivelyReference Begbie and Ross12). However, in that study phaseolin was provided as whole common bean, thus confounding effects with other bean components.
Bean breeders would like to take advantage of differences in phaseolin composition for developing bean varieties with improved nutritional value. However, there is a need to better understand the kinetics of phaseolin hydrolysis in vivo for two reasons. First, proteolysis by pepsin and trypsin is limited. Therefore, differences in cleavage among phaseolin types could result in differential digestion and in absorbed profile of amino acids. Second, the presence of undigested peptides in the lumen of the small intestine could increase secretionsReference Santoro, Grant and Pusztai13. This would lead to more endogenous proteins (enzymes, antibodies and mucins) lost at the end of the small intestine, and therefore less amino acids available for maintenance and growthReference Le Gall, Quillien, Guéguen, Rogniaux and Sève14, Reference Salgado, Montagne, Freire, Ferreira, Teixeira, Bento, Abreu, Toullec and Lallès15. However, little information on the biochemical aspects of in vivo digestion among native and heat-treated phaseolin types exists.
The objectives of the present work were to study how three types (S, T and I) of purified phaseolin were digested in the rat small intestine, and to characterize both the indigestible phaseolin and some endogenous proteins resulting from the intestinal digestion processes. The study was conducted on unheated and heated phaseolins, since heating was able to reveal difference in the in vitro hydrolysis among phaseolin typesReference Montoya, Lallès, Beebe, Souffrant, Leterme and Kharkwal10.
Experimental methods
Animals and diets
The experiment was conducted in agreement with the guidelines of the National University of Colombia for care and use of laboratory animalsReference Mrad de Osorio and Cardozo de Martinez16. Forty young adult Wistar female rats (generous gift of the Zoo of Cali) with an initial body weight of 255 (sd 14) g, were randomly allocated to one of the eight treatments and placed in individual metabolic cages (Tecniplast 150–300, Buguggiate, Italy) for the whole experimental period. The control diet contained casein as the sole protein source. Phaseolin-containing diets had half of protein supplied by casein and half supplied by the different types of purified phaseolinReference Montoya, Lallès, Beebe, Montagne, Souffrant and Leterme7. Crude protein content (N × 6·25) for casein, and S, T and I phaseolin were 92, 96, 97 and 97 %, respectively. These were incorporated into the diets either untreated or after thermal treatment (121°C for 15 min, 15 psi) (Table 1). The rats were fed the diets for 10 d after which they were killedReference Montoya, Lallès, Beebe, Montagne, Souffrant and Leterme7. Food intake was limited to 10 g/d in order to limit food refusalsReference Montoya, Lallès, Beebe, Montagne, Souffrant and Leterme7.
Table 1 Ingredients and analytical composition of the experimental diets
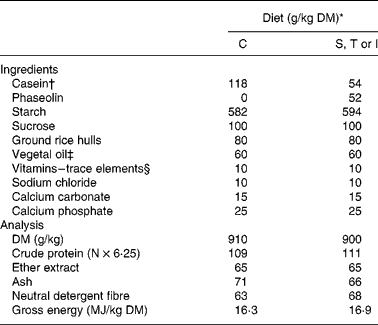
* Diets: C, casein control; S, T and I, diets with Sanilac, Tendergreen and Inca phaseolins (unheated or heated) providing 500 g/kg of the total dietary protein.
† Casein was supplemented with 30 g dl-methionine/kg DM casein.
‡ Soyabean oil–sunflower oil (1:1).
§ Mineral and vitamin mixture supplied per kg diet (control and experimental): 7·5 mg vitamin A; 0·2 mg vitamin D3; 15 mg vitamin E; 6 mg vitamin K; 10 mg vitamin B2; 35 mg calcium pantothenate; 75 mg niacin; 2·5 mg vitamin B6; 0·05 mg vitamin B12; 0·05 mg biotin; 200 mg choline; 150 mg Mn; 500 mg Zn; 40 mg Cu, 200 mg Fe; 2 mg I; 0·5 mg Se, 1 mg Co.
Preparation of ileal digesta and mucosa
Digesta and mucosa were sampled after the killing of the rats on day 11, 3 h after the last mealReference Montoya, Lallès, Beebe, Montagne, Souffrant and Leterme7. Briefly, ileal digesta were collected and immediately frozen and stored at − 20°C before being freeze-dried and ground (1 mm mesh screen). Tissue samples (3 cm in length) of distal ileum were collected, open longitudinally and washed three times in distilled water (4°C) and immediately frozen in liquid nitrogen. Later, the tissue samples were thawed on ice and homogenized in ice-cold saline (0·9 % NaCl; 40 mg tissue/ml). Soluble protein of ileal digesta was extracted in borate buffer (0·1 m-H3BO3, 0·15 m-NaCl, pH 8·0) for 1 h at 4°C (300 mg digesta/ml buffer). Then, the tissue and digesta preparations were centrifuged at 12 000 g for 10 min at 4°C. Supernatants were fractionated in aliquots and stored at − 40°C until electrophoresis and Western blotting analysis. Soluble protein concentration in digesta and mucosa preparations was measured with the Folin phenol reagentReference Lowry, Rosebrough, Farr and Randall17.
Production of hyperimmune plasmas against phaseolin types
Hyperimmune plasmas were prepared in New Zealand White rabbits by injecting an emulsion of SDS–PAGE gel bandsReference Boulard and Lecroisey18 containing one type of phaseolin (S, T or I) and Freund's complete or incomplete adjuvant (F-5881 and F-5506 respectively; Sigma Chemical Co., St Louis, MO, USA). Immunization was conducted with approximately 300 μg pure phaseolin at each of the three injections as reported for other legume grain globulinsReference Salgado, Montagne, Freire, Ferreira, Teixeira, Bento, Abreu, Toullec and Lallès15. Since a high cross-immunoreactivity was found between phaseolin types, a mixture of hyperimmune plasmas against S, T and I phaseolin was prepared and used for Western blotting in the present study.
SDS–PAGE and Western blotting
SDS–PAGE electrophoresis and Western blotting analysis were conducted as previously describedReference Salgado, Montagne, Freire, Ferreira, Teixeira, Bento, Abreu, Toullec and Lallès15. Protein loads were 5 and 30 μg/well for pure protein (phaseolin and casein) and for ileal samples (digesta and mucosa), respectively. Molecular weight (MW) standards (14·4–97·0 kDa; 17-0446-01; Pharmacia, Uppsala, Sweden) were also loaded in a separate well on each gel. After electrophoresis, proteins in one gel were stained by Coomassie brilliant blue while proteins from a similar gel run simultaneously in the same device were electro-transferred to nitrocellulose membranesReference Salgado, Montagne, Freire, Ferreira, Teixeira, Bento, Abreu, Toullec and Lallès15.
Densitometry measurements
The densitometry measurements were made according to Salgado et al. Reference Salgado, Montagne, Freire, Ferreira, Teixeira, Bento, Abreu, Toullec and Lallès15. The gels with Coomassie blue-stained proteins and the membranes were scanned using a phosphor imager (Quantum Appligene, version 2.03; Illkirch, France). Staining density was measured horizontally for each band of interest using image analysis (Image-QuaNT, version 4.2a; Molecular Dynamics, Sunnyvale, CA, USA), in order to generate statistical data for specific bands. These staining densities are homogenous with concentrations since the same amounts of soluble protein for a given type of sample were deposited at the top of the lanes. The MW of each protein band detected visually was determined by linear regression using the MW standards. Densitometry was carried out in both phaseolin peptides and endogenous proteins of digesta samples, while it focused only on phaseolin peptides in the case of ileal tissues.
In-gel trypsin digestion and MS
In-gel digestion with trypsin was carried out according to a methodReference Shevchenko, Wilm, Vorm and Mann19 slightly modified. Briefly, most bands detected visually in SDS–PAGE gels of digesta and mucosa were excised, washed in acetonitrile–0·05 m-ammonium bicarbonate (1:1) and dried in a Speed Vac concentrator (Bioblock, Illkirch, France). Proteins in the sliced gels were reduced with dithiothreitol at 60°C for 40 min and alkylated by iodoacetamide for 30 min in the dark. Digestion by trypsin (sequencing grade; Promega, Charbonnières, France) was carried out at 0·5 μg/sample, in 25 μl 0·05 m-ammonium bicarbonate, at pH 8·0 for 18 h at 37°C. The reaction was stopped by adding 2 μl 5 % trifluoroacetic acid (v/v; Pierce, Touzart & Matignon, Vitry sur Seine, France). The supernatant was analysed by means of matrix-assisted laser desorption ionization–quadrupole time-of-flight MS. Collected peptides (1 μl) were deposited on to the matrix-assisted laser desorption ionization target plate and 1 μl α-cyano-4-hydroxy cinnamic acid matrix at 10 g/l diluted 1:5 (v/v) with solution containing 0·1 % trifluoroacetic acid (v/v) and 70 % acetonitrile (v/v) was added on to the spots dried on the target plate. The plate was introduced into the quadrupole time-of-flight mass spectrometer (Qstar XL; Applied Biosystems, Framingham, MA, USA). oMALDI Xpert2.0 software was used for the matrix-assisted laser desorption ionization MS and MS–MS experiments. Samples were ionized with a laser beam (λ = 337 nm) and each spectrum was an average of 250–500 laser shots. The more representative monocharged ions were automatically submitted to fragmentation with energy of collision of near 0·05 eV/DaReference Wattenberg, Organ, Schneider, Tyldesley, Bordoli and Bateman20. Typically, oMALDI Xpert2.0 software treated each sample well individually and generated an MS peak list. This list was submitted for a peptide mass fingerprinting search and used as a ‘survey scan’ to determine peptide precursors for MS/MS acquisition. All MS and MS–MS data were then used with Modular Approach to Software Construction Operation and Test software (MASCOT, version 1.9) for search into several databases such as Swiss Prot or the National Centre for Biotechnology Information to identify the proteins present in each gel band. The quality of peptide identification was reported using molecular weight search scoringReference Pappin, Hojrup and Bleasby21.
Statistical analysis
Since there were not enough tracks on each gel, only samples corresponding to unheated casein control were analysed together with those coming from phaseolin-fed rats. Indeed, both unheated and heated casein displayed similar in vivo digestibilitiesReference Montoya, Lallès, Beebe, Montagne, Souffrant and Leterme7. Thus, two separate ANOVA of the data were conducted using the General Linear Model procedure of Statistical Analysis Systems statistical software package version 8.0 (SAS Institute Inc., Cary, NC, USA). In the first one, effects of phaseolin type, heat treatment and their interaction were tested. When the F value of the ANOVA was significant (P < 0·05), the means were compared using Duncan's multiple range testReference Duncan22. A second ANOVA was conducted to compare data between casein control and heated phaseolins. Such treatments were usually not different (P>0·10) and data for unheated casein are provided in the tables for information only.
Results
No particular problems were encountered with the rats. They consumed on average 9·2 (sd 1) g food/d with no significant difference among treatments (P = 0·213).
Protein sources
SDS–PAGE. The electrophoresis patterns under reducing condition of casein and S, T and I phaseolin types in native form are presented in Fig. 1. Casein displayed two bands with MW between 29–31 kDa and 31·5–33 kDa. For the phaseolin types, the subunits were observed in MW ranging from 43·1 to 51·5 kDa, three subunits being visible for T phaseolin and two subunits for S and I phaseolins (Table 2).

Fig. 1 SDS–PAGE and Western blotting analysis of casein (C) and Sanilac (S), Tendergreen (T) and Inca (I) phaseolins. Molecular weight (MW) markers are indicated. ▸, bands characterized by MS in Table 2.
Table 2 Molecular weight search (MOWSE) score for MS identification of protein bands in pure unheated phaseolin
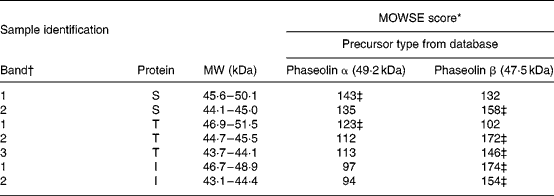
I, Inca phaseolin; S, Sanilac phaseolin; T, Tendergreen phaseolin.
* MOWSE score ≤ 65 is not significant (P >0·05) for MS identificationReference Pappin, Hojrup and Bleasby21.
† Identification of bands as shown in Fig. 1.
‡ Values confirmed by MS–MS.
Western blotting. The antibodies raised against phaseolin did not label casein but recognized all the polypeptide subunits of S, T and I phaseolin types (Fig. 1).
MS. MS identification showed that S and T phaseolins comprised subunits originating from α and β precursors (Table 2). In addition, the third band of T phaseolin and the two bands of I phaseolin originated from β precursor.
Protein patterns in ileal digesta
SDS–PAGE. Representative SDS–PAGE gels of soluble proteins from digesta collected at the end of the small intestine are shown in Fig. 2(A). For the ileal contents from rats fed casein, few faint bands were observed. By contrast, in rats fed diets containing unheated phaseolins, strong bands with MW between 18 and 24 kDa appeared, together with other fainter bands. After thermal treatment, most of these bands disappeared and the remaining visible faint bands were similar to those observed with the control diet.

Fig. 2 SDS–PAGE (A) and Western blotting (B) analysis of ileal digesta of rats fed diets with casein (C) or Sanilac (S), Tendergreen (T) and Inca (I) phaseolins in unheated or heated form. Molecular weight (MW) markers are indicated. ▸, bands characterized by densitometry in Table 3 and by MS in Table 4.
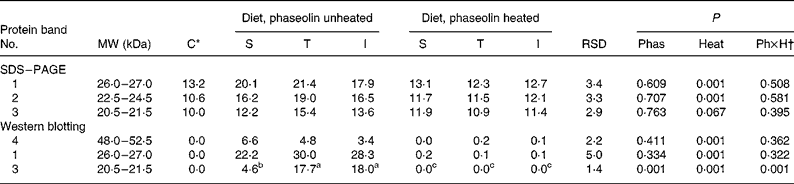
Densities tended (P = 0·10) to be lower with unheated I phaseolin than with the S and T types for bands at MW 26·5–28 and 31·5–33 kDa (Table 3). No other difference due to phaseolin type was seen. Heat treatment was almost always highly significant (P < 0·001) in reducing the intensity of all the bands (Fig. 2(A)).
Table 3 Densitometry analysis (arbitrary density units) of SDS–PAGE and Western blotting patterns of ileal digesta of rats fed with different types of phaseolin either unheated or heated, and casein (C) as control (five rats per treatment)

I, Inca phaseolin; Phas, phaseolin type; RSD, residual standard deviation; S, Sanilac phaseolin; T, Tendergreen phaseolin.
a,b,c Values within a row with unlike superscript letters were significantly different (P < 0·05).
* Control digesta from rats fed the unheated casein diet were not taken into account in the statistical analysis. They were separately compared with the data of heated phaseolins and revealed no significant differences (P>0·05).
† Phaseolin type by heat interaction.
Western blotting. The anti-phaseolin antibodies did not reveal any band in the digesta of rats fed with the casein diet (Fig. 2(B)). By contrast, in the digesta of rats fed unheated phaseolins, a strong immuno-labelling was present at MW ranges of intact phaseolin (44–54 kDa) and phaseolin fragments (19–24 kDa). These bands were seen in SDS–PAGE too. However, all the immuno-reactivity disappeared when digesta from rats fed heated phaseolins were considered. Intensity of the bands was lower at MW 47–50 kDa (P = 0·048) and 44–46·5 kDa (P = 0·112) in digesta of rats fed diets with unheated T and I phaseolin as compared to S phaseolin (Table 3). Intensity of the band at MW 19–21·5 kDa was higher with unheated T phaseolin, as compared to S and I types (P = 0·026). In all cases, thermal treatment reduced band intensities (P < 0·01) with no differences remaining between phaseolin types (P>0·05).
MS. Bands of ileal digesta of untreated phaseolin at MW 18–18·5 and 22–24 kDa for S phaseolin and 22–24 kDa for T phaseolin were shown to originate from α precursor. The rest of the digesta bands in the range of 15·5 to 24 kDa for S, T and I phaseolins were identified as originating from β precursor (Table 4). A smaller polypeptide at MW 11 kDa (Y in Table 4 and Fig. 2(A)) visible with the T phaseolin diet was from β precursor too. On the other hand, three proteins at MW 55–61, 31 (X in Table 4 and Fig. 2(A)) and 26–28 kDa were identified as host enzymes, namely pancreatic α-amylase for the first two bands, and anionic trypsin I for the third band.
Table 4 Molecular weight search (MOWSE) score for MS identification of protein bands in intestinal digesta of rats fed with a diet containing casein or a mixture of unheated casein and phaseolins
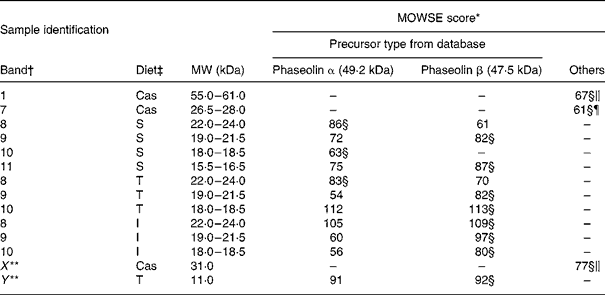
* MOWSE score ≤ 65 is not significant (P >0·05) for MS identificationReference Pappin, Hojrup and Bleasby21.
† Identification of bands on the SDS–PAGE of ileal digesta as shown in Fig. 2(A).
‡ Ileal digesta of rats fed diets differing in protein sources: Cas, casein; I, Inca phaseolin; S, Sanilac phaseolin; T, Tendergreen phaseolin.
§ Values confirmed by MS–MS.
‖ Pancreatic α-amylase precursor, MW 57·8 kDa.
¶ Anionic trypsin I precursor, MW 26 kDa.
** Bands not visually distinguishable in all the rats.
Protein patterns of the ileal mucosa
SDS–PAGE. The electrophoresis of soluble ileal mucosa proteins are presented in Fig. 3(A). In all tracks, several bands were observed. However, three different bands of MW ranging from 18·5 to 26 kDa were detected with high intensity only in rats fed diets containing unheated phaseolins, regardless of phaseolin type (Fig. 3(A)). Density of the first two bands (P < 0·001) and of the third band (P = 0·067) (Table 5) was reduced in rats fed heat-treated phaseolins.
Table 5 Densitometry analysis (arbitrary density units) of SDS–PAGE and Western blotting patterns of ileal mucosa of rats fed with different types of phaseolin either unheated or heated, and casein (C) as control (five rats per treatment)
I, Inca phaseolin; Phas, phaseolin type; RSD, residual standard deviation; S, Sanilac phaseolin; T, Tendergreen phaseolin.
a,b,c Values within a row with unlike superscript letters were significantly different (P < 0·05).
* Control digesta from rats fed the unheated casein diet were not taken into account in the statistical analysis. They were separately compared with the data of heated phaseolins.
† Phaseolin type by heat interaction.
Western blotting. The three SDS–PAGE bands described above and another one at MW 48–52·5 kDa were revealed by Western blotting only in digesta of rats fed diets with unheated phaseolin (Fig. 3(B)). Densitometry indicated an influence of heat treatment (P < 0·001) for the bands at MW 48–52·5 and 26–27 kDa (Table 5). The intensity of the band at MW 20·5–21·5 kDa was higher in the mucosa of rats fed unheated T and I phaseolin, as compared to S phaseolin (P < 0·001). Finally, the band at MW 22·5–24·5 kDa was not consistently revealed by Western blotting.
MS. Most bands of interest in ileal mucosa were shown to be of phaseolin origin (Table 6). However, the patterns substantially differed between phaseolin types. Bands in a decreasing order of MW were from α, α and β precursors for S phaseolin and α, β and α for T phaseolin. In the case of I phaseolin, one band was identified as coming from the phaseolin β precursor while the other one was not phaseolin.
Table 6 Molecular weight search (MOWSE) score for MS identification of protein bands in ileal mucosa of rats fed with a diet containing casein or a mixture of unheated casein and phaseolins
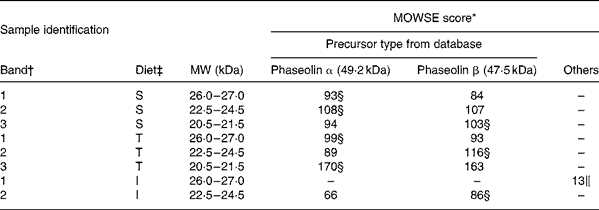
* MOWSE score ≤ 65 is not significant (P >0·05) for MS identificationReference Pappin, Hojrup and Bleasby21.
† Identification of bands on the SDS–PAGE of ileal mucosa as shown in Fig. 3(A).
‡ Ileal mucosa of rats fed diets differing in protein sources: Cas, casein; I, Inca phaseolin; S, Sanilac phaseolin; T, Tendergreen phaseolin.
§ Values confirmed by MS–MS.
‖ Cytokeratin 10.
Discussion
The present data provide evidence for differences in digestion among phaseolin types and add some light on the molecular origin of various protein bands in the ileal digesta and mucosa.
Biochemistry of phaseolin types
The T phaseolin has three bands at MW 53, 47 and 43 kDaReference Hall, McLeester and Bliss23. MS identification of phaseolin subunits confirmed differences in the patterns of precursors among phaseolin types: α and β for S phaseolin, α, β and β for T phaseolin, and β and β for I phaseolin. Phaseolin α and β precursors are known for their high sequence homology. But, they still differ in some amino acids, carbohydrates, phosphate binding sites and contents in solvent moleculesReference Slightom, Drong, Klassy and Hoffman24, Reference Alli, Gibbs, Okoniewska, Konishi and Dumas25.
The sequence of α phaseolin genes showed higher similarity between the S and T phaseolin types than β phaseolin genesReference Kami and Gepts9. Moreover, the sequences between α and β phaseolin genes were divergent. The present observations suggested the divergence between S and T phaseolin typesReference Kami and Gepts9. The gene sequence analysis of one subunit of I phaseolin revealed identical sequences with one subunit for S and T phaseolinsReference Kami, Becerra, Debouck and Gepts26. These authors suggested I phaseolin to be an ancestral phaseolin type. The analysis showed that every phaseolin has a different profile of subunit precursors. Slight differences in the tertiary structure of the monomer cause distinct quaternary structureReference Banerjee, Das, Ravishankar, Suguna, Surolia and Vijayan27, which could be the basis for differences in intestinal digestion.
Phaseolin digestion
Undigested phaseolin polypeptides with MW ranging from 18 to 24 kDa were detected and identified in the rat ileal digesta by both Western blotting and MS. Similar undigested polypeptides were reported in previous in vitro and in vivo studiesReference Jivotovskaya, Senyuk, Rotari, Horstmann and Vaintraub5, Reference Deshpande and Nielsen6, Reference Santoro, Grant and Pusztai13. However, until this work, no evidence was available in the literature about the molecular origin of undigested phaseolin polypeptides. Trypsin cleaves native phaseolin in vitro into nearly two halves of MW 21·3 and 24·7 kDaReference Jivotovskaya, Senyuk, Rotari, Horstmann and Vaintraub5.
Some differences among phaseolin types were found in digestion in the small intestine. Intact polypeptides of MW 44·1–45 and 45·6–50·1 kDa from untreated S phaseolin reached the ileum in concentrations higher than for T and I phaseolins. Also the polypeptide of MW 19–21·5 kDa was apparently less digested in the case of T phaseolin compared to S and I phaseolins, despite a common β origin. However, it is important to consider that T phaseolin has three subunits. The difference in β phaseolin precursors between phaseolin types mentioned earlier could explain the differences in β phaseolin digestion. A study in pigs fed diets containing common beans with S and T phaseolin types reported an ileal digestibility value higher for S phaseolin (57 %) as compared to T phaseolin (36 %)Reference Begbie and Ross12. Such discrepancies may arise from the use of whole beans in the study by Begbie and RossReference Begbie and Ross12 as compared to the present study with purified phaseolin. Also, differences due to animal species and common bean composition cannot be excluded. Two publicationsReference Deshpande and Nielsen6, Reference Bollini and Vitale28 suggest slight differences between phaseolin types after in vitro proteolysis, but these studies based on SDS–PAGE were only qualitative.
Phaseolin and intestinal endogenous protein components
Two endogenous proteins were identified in the ileal digesta: pancreatic α-amylase as a nearly intact molecule at MW 55–61 kDa and a fainter digestion fragment at 31 kDa, and anionic trypsin I at MW 26·5–28 kDa. These two enzymes were also identified with higher concentration and daily flow at the ileum of pigs fed various legume grains as compared to casein as the controlReference Le Gall, Quillien, Guéguen, Rogniaux and Sève14, Reference Salgado, Montagne, Freire, Ferreira, Teixeira, Bento, Abreu, Toullec and Lallès15. The lower number of endogenous bands revealed by SDS–PAGE here could be due to a lower endogenous protein loss in rats as compared to pigsReference Hodgkinson, Souffrant and Moughan29. In the present study, the ileal concentration of intact pancreatic α-amylase was lower with diets containing heated phaseolin, irrespective of phaseolin type. The trypsin concentration tended to be lower with unheated I phaseolin than with the S or T types, emphasizing the idea that I phaseolin is more susceptible to digestion. Intestinal trypsin concentration and flow increase with protein resistance to digestion as shown by guanidination of casein in ratsReference Hara, Nishi and Kasai30. Santoro et al. Reference Santoro, Grant and Pusztai13 have hypothesized that native phaseolin remnants exert a secretagogue activity on the small intestine epithelium (e.g. cell shedding, digestive enzymes, serum proteins, mucus production). Despite this possible secretagogue activity, little effects on small intestine histomorphology were observed in rats fed diets containing unheated phaseolinReference Montoya, Lallès, Beebe, Montagne, Souffrant and Leterme7.
Thermal treatment and phaseolin digestion
Most protein bands in the ileal digesta had intensities drastically reduced in diets containing heated phaseolin, in agreement with improved digestion of phaseolin upon thermal treatment in vivo Reference Marquez and Lajolo2, Reference Montoya, Lallès, Beebe, Montagne, Souffrant and Leterme7 and in vitro Reference Deshpande and Damodaran4, Reference Deshpande and Nielsen6, Reference Montoya, Lallès, Beebe, Souffrant, Leterme and Kharkwal10. Indeed, in vitro trypsinolysis of heated phaseolin quickly generates polypeptides of MW 20–30 kDa which are then rapidly degraded into smaller peptides. Heat treatment influences structural changes and favours enzymatic hydrolysis by decreasing the percentage of α-helices while increasing random structures in the moleculeReference Deshpande and Damodaran4. No differences between heated phaseolin types were observed here. In quantitative sequential pepsin and pancreatin in vitro hydrolysis, we found that T and I phaseolins had a higher degree of hydrolysis than S phaseolin after heat treatment (71, 72 and 58 %, respectivelyReference Montoya, Lallès, Beebe, Souffrant, Leterme and Kharkwal10). The reason for such discrepancies between in vivo and in vitro hydrolysis could be: (1) in vivo digestion of protein is a complex process affected not only by the chemical structure of proteins but also by the biochemistry and physiology of the digestive tract as compared to in vitro digestionReference Savoie31, Reference Savoie, Agudelo, Gauthier, Marin and Pouliot32; (2) the accumulation of in vitro digestion products could affect the efficiency and the rate of hydrolysisReference Savoie31; and (3) in our SDS–PAGE conditions the peptides of low MW ( < 10 kDa) produced after the hydrolysis of heated phaseolin cannot be detected.
Phaseolin and intestinal mucosa
Phaseolin fragments of MW 20·5–21·5, 22·5–24·5 and 26–27 kDa were bound to the ileal mucosa of the rats fed untreated phaseolin, in agreement with earlier observationsReference Santoro, Grant and Pusztai13. This may have resulted from the propensity of undigested phaseolin peptides to aggregateReference Begbie and Ross12. We also found nearly intact phaseolin types on ileal mucosa, in accordance with their presence in ileal digesta. Adsorbed peptide of MW 20·5–21·5 kDa was present in a higher concentration for T and I phaseolins compared to S phaseolin. This could have reflected the higher luminal concentration of a similar peptide of MW 19–21·5 kDa observed with T phaseolin. Reasons for differences between S and I phaseolins for tissue concentration of this peptide are unclear.
In conclusion, the present work highlighted the differences in the phaseolin precursor subunit composition among phaseolin types. Moreover, it provided evidence for differences between phaseolin types in protein digestion in the small intestine of rats. Improvements in phaseolin digestion upon thermal treatment were shown to result from both increased digestion of phaseolin and decreased ileal concentrations of endogenous proteins. Finally, both near-native phaseolin and undigested phaseolin peptides of α and β precursor origins bound to the intestinal mucosa with apparent differences between phaseolin types. Studying a larger number of phaseolin types may help investigate the possible relationships between subunit composition, biochemistry of intestinal digestion, and nutritional value of phaseolin and common bean.
Acknowledgements
Thanks are due to the Volkswagen Foundation (Hannover, Germany), COLCIENCIAS (Bogotá, Colombia), ECOS-Nord (Université de Paris 5, France) and Conseil Régional de Bretagne (Rennes, France) for financial support.














