Despite the technical and scientific advances in the field of dentistry, particularly in relation to dental procedures, in which it is possible to make use of preventive and curative therapeutic measures that allow us to maintain the tooth in the oral cavity, we note that it is still quite common to come across situations in which tooth extraction is the most appropriate treatment option.
It is important to emphasise that this less conservative management often involves the poorer and more undernourished segment of the population because their socio-economic status does not allow them adequate food intake(Reference Shahar, Shai and Vardi1), oral hygiene practices or the use of therapeutic resources to prevent the loss of their teeth, because in most cases, they are treated in public health services, which do not possess sufficient resources to perform atraumatic surgical techniques and which conform to the principles of an aseptic chain(Reference Adeyemo2). Thus, these patients often suffer considerable post-operative pain and can even develop a severe condition known as alveolitis(Reference Papa3–Reference Resende5). The signs and symptoms most commonly found in this disease are exposed alveolar bone, absence of tissue healing, purulent secretion, oedema, hyperaemia, severe lymphoadenopathy, throbbing pain and fetid and persistent odour(Reference Nusair and Younis4–Reference Ricieri, Aranega and Takahashi6).
Malnutrition takes on major proportions in Brazil and is present in all regions and areas, although the population most affected lives in the north and northeast(Reference Monteiro7). The author in question argues that undernourished individuals manifest clinical signs of inadequate quantity (energy) or quality (nutrients) from the diet or as a result of diseases that cause poor biological utilisation of the food ingested. Thus, malnutrition was identified as a major cause of secondary disability in the response of the organism to infectious agents, favouring even the progression of a localised infection to a systemic one(Reference Chandra8).
The oral cavity presents one of the most concentrated and varied microbial populations, harbouring more than 500 species of bacteria(Reference Dufour and Svoboda9). Most of these micro-organisms are to be found in the oral cavity of healthy individuals constituting the endogenous microbiota, which is also known as normal microbiota(Reference Rodrigue and Lavoie10). This may benefit the host through the development of the immune system(Reference Marcotte and Lavoie11) or the microbial antagonism, thereby preventing the excessive growth of harmful micro-organisms(Reference Paster, Boches and Galvin12, Reference Ye, Harty and Chapple13). The occurrence of an imbalance between this microbiota and the host favours the onset of inflammatory and/or infectious processes(Reference Ye, Harty and Chapple13). In these cases, the micro-organisms belonging to the normal microbiota are referred to as opportunistic pathogens(Reference Pelczar, Chan, Krieg, Pelczar, Chan and Krieg14). It is important to emphasise that these micro-organisms cause host cells to produce pro-inflammatory cytokines, including IL-1, IL-6, TNFα and platelet-activating factor, which may induce destructive processes that attack the soft and/or hard tissues of the oral cavity(Reference McManus and Pinckard15–Reference Deo and Bhongade17).
Many micro-organisms normally considered non-pathogenic have the ability to produce infection and disease. This property depends not only on their virulence factor, but also on the defence mechanisms of the host, which may be weakened in some situations, such as nutritional deficiency, immunosuppressive drugs, acquired immunodeficiency syndrome, diabetes mellitus, cancer, infections, among others(Reference Souza and Scarcelli18).
The risk of post-operative complications increases considerably in immunocompromised or undernourished patients, owing to their diminished resistance. This includes greater vulnerability to disease, delayed wound healing and high rates of infection(Reference Neumann, Friedmann and Roy19, Reference Ohyanagi20). These, in turn, may lead to septicaemia, whose mortality risk is high(Reference Yeh21, Reference Griffiths22).
Oral diseases usually exhibit a self-limiting behaviour. Thus, clinical procedures such as periodontal probing, dental extraction or even a thorough brushing can cause the introduction and spread of bacteria from the flora of the oral cavity into the bloodstream triggering transient bacteraemia. This, in a normal organism, is asymptomatic, of short duration (eliminated in a few minutes by the reticuloendothelial system of the host) and has no major clinical significance, because the inoculum is small and the virulence of the micro-organisms involved is low(Reference Papa3, Reference Rocha Barros, Ito and Azevedo23, Reference Siviero, Kanegane and Bispo24).
Bacteraemia has been defined as the presence of viable bacteria in the blood(Reference Salles, Sprovieri and Bedrikow25). This allows the installation of bacteria in different body sites(Reference Bhatawadekar and Bhardwaj26). However, the clinical importance of bacteraemia is determined by several factors, such as the site of infection, severity of primary disease, type of pathogen involved and the patient's immunity(Reference Lam, Jan and Sándor27, Reference Lockhart, Brennan and Sasser28). Extraction is the dental procedure most likely to cause bacteraemia(Reference Bruno29, Reference Tsolka and Katritsis30).
The present study analysed the bacteriological aspects during the process of alveolitis, relating it to a higher incidence of bacteraemia in adult rats undernourished during the suckling period and that recovered thereafter until adulthood. The study of these aspects in animals is important from the experimental and clinical standpoints, due to nutritional aggression during the neonatal period. This represents a stage of great vulnerability, due to the formation of various organic systems, particularly the defence of the host against infection.
Materials and methods
Ethical considerations
The present study was approved by the Ethics Committee on Animal Experimentation of the Center for Biological Sciences of the Federal University of Pernambuco (Pernambuco, Brazil; protocol no. 008283/2007 – 34).
Animals
A total of forty albino male Wistar rats from the breeding colony of the Department of Nutrition of the Federal University of Pernambuco were used. These animals were kept in a bioterium at a temperature of 23 ± 2°C in 12 h light–12 h dark cycles (light, 6–18 h; dark, 18–6 h).
Nutritional manipulation
After birth, the male pups were kept with their mothers in groups of six as this number helps to maintain the nutritional pattern(31). Thus, when necessary, we carried out a reduction or complementation of the offspring through a random selection of male pups. It is worth noting that in the case of complementation, this was conducted using other litters of the same age. Then, on the basis of the dietary regimen employed, we obtained the following groups, each of which comprised twenty animals: nourished (N) pups whose mothers were fed with 17 % casein during the period of lactation; undernourished (UN) suckled rats whose mothers were fed through a nutritionally deficient diet, 8 % casein, during lactation. After weaning, the mothers of both groups were euthanised.
Somatic growth
The nourished and malnourished animals were breast-fed until the 21st day after birth, corresponding to the lactation period(Reference Harkness and Harkness32). During this period, a daily record (digital electronic weighing scale – Marte, model S-4000 with a sensitivity of 0·1 g; Marte Balanças e Aparelhos de Precisão Ltda, São Paulo, Brazil) of the body weight of each animal was maintained to monitor weight during nutritional manipulation. From the 22nd day of life, the animals were separated from their mothers, kept in collective cages containing three animals each and fed ad libitum water and the standard diet of the bioterium (Labina – Purina Brazil S/A, São Paulo, Brazil), containing 23 % mixed protein until the end of the experiment. Their body weight was measured once a week, thus allowing their nutritional recovery to be monitored.
Induction process of alveolitis
After 90 d, all forty animals (nourished and undernourished) were submitted to the alveolitis process.
To implement this process, the animals were anaesthetised with ketamine hydrochloride (10 mg/kg) and xylazine hydrochloride (0·5 mg/kg), mixed in the same syringe and administered intramuscularly. Next, the upper right incisor was extracted, using the technique proposed by Okamoto & Russo(Reference Okamoto and Russo33), in which the instruments used were similar to those employed in the extraction of primary teeth. After extraction, alveolar ischaemia was induced by the insertion of a small sterile cotton pad soaked in 1:1000 adrenaline for 15 min. Following the removal of this pad, the animals remained under observation for 15 min, with the aim of verifying the absence of blood clot formation inside the alveolus, and after 48 h the animals were examined clinically, by the separation of the lips, which allowed us to observe the development of alveolitis by the presence of local oedema, hyperaemia, abscess formation, purulent secretion and fetid odour(Reference Poi, Carvalho and Carvalho34–Reference Araújo, Castro and Severo36).
From the time of extraction to the day of euthanasia, the animals were kept separately in individual polypropylene cages covered with zinc wire, following which both the N and UN rats were randomly separated into the following groups:
(1) (1) Group N-21 – nourished animals that were euthanised on the 21st day after the clinical verification of alveolitis.
(2) (2) Group UN-21 – undernourished animals that were euthanised on the 21st day after the clinical verification of alveolitis.
(3) (3) Group N-28 – nourished animals that were euthanised on the 28th day after the clinical verification of alveolitis.
(4) (4) Group UN-28 – undernourished animals that were euthanised on the 28th day after the clinical verification of alveolitis.
It should be noted that each group comprised ten animals.
Bacteriological analysis
For this analysis, the animals were anaesthetised using the same technique as that employed for the induction of alveolitis. The operating table was covered with a sterile surgical drape and the animal was positioned in the dorsal decubitus position. This position facilitated the mouth opening and separation of the lips, thus allowing the collection of the oral microbiota from the perialveolar region of the upper right incisor, according to the technique advocated by Araújo(Reference Araújo35) and Araújo et al. (Reference Araújo, Castro and Severo37), using a swab soaked in 40 μl of 0·9 % sterile NaCl. The swab was then placed in a sterile tube containing 460 μl of brain heart infusion-enriched liquid medium that allows bacterial growth. The samples were homogenised, and 100 μl of each were removed and transferred to another sterile tube containing 900 μl brain heart infusion. This 1000 μl solution was again homogenised and, with the aid of a 1 μl calibrated loop, Petri dishes containing Agar-blood and Agar-Levine were seeded for the isolation of Gram-positive and Gram-negative bacteria. These plates were incubated in a bacteriological incubator at 37°C for 48 h, and the resulting colony-forming units (CFU) were counted and their percentage was calculated. An enterobacteria kit (Laborclin/Interlab Distribuidora de Produtos Científicos Praça Isaac Oliver, São Paulo, Brazil) was used for the identification of the Gram-negative bacteria, and Staphcillin, Novobiocin, Optochin, Bile Esculin agar and 6·5 % NaCl were used for the identification of the Gram-positive bacteria. In addition, slides were prepared from dry swabs at room temperature. These were fixed by heat using the flame of a Bunsen burner, with subsequent staining by Gram's method, which enabled the identification of the bacterial forms and arrangements.
This procedure was performed before extraction of the upper right incisor, 5 min after the extraction, on the 21st day after the clinical verification of alveolitis for groups N-21 and UN-21 and on the 28th day after the clinical verification of alveolitis for groups N-28 and UN-28.
Blood culture
Venous blood (100 μl) was collected from the tail of each animal to perform the blood culture before the extraction of the upper right incisor, 5 min after extraction, on the 21st day after the clinical verification of alveolitis for groups N-21 and UN-21 and on the 28th day after the clinical verification of alveolitis for groups N-28 and UN-28. The blood was collected in tubes containing 900 μl sterile brain heart infusion medium and hermetically sealed. Then, the tubes were placed in a bacteriological incubator at 37°C for 7 d. The cultures were seeded in Petri dishes containing agar–blood every 24, 48, 72, 120 and 168 h. Blood cultures were regarded as positive in the first of these intervals in which the presence of bacteria was observed. This was identified through tests similar to those previously mentioned for carrying out the bacteriological analysis of normal oral microbiota from the perialveolar region. Blood cultures were considered negative when, after 7 d of observation, no micro-organisms were present in the seedings.
Statistical analysis
The data were analysed using the ‘Jandel SigmaStat Statistical Software’ (Systat Software Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test for normality was applied to all the variables, but only weight presented a normal distribution (P = 0·072). Thus, to analyse weight, the t test was used in a daily comparison between the groups (N and UN) and the paired t test in the analysis before extraction and after alveolitis in each of the groups. Also, in relation to the analysis of weight behaviour, repeated-measures ANOVA was applied from the 1st to the 90th days of life of the animal. With regard to CFU, Mann–Whitney non-parametric tests were used in the comparison between the groups, and the Wilcoxon test was used in the comparison of the measurements before and after extraction and after alveolitis in each group. Means and standard deviations were used as representatives of the measures of the central tendency and variation for the analysis of the variable weight, and the median and interquartile interval were used for the analysis of the CFU and Gram-positive and Gram-negative bacterial species. Statistical significance was set at the 5 % level in all cases.
Results
After the body weight of the nourished and undernourished animals had been recorded, a weight curve was constructed for each of these groups (Fig. 1). The ANOVA showed a significant increase in weight over time in both groups, as well as a significant difference between them, which started on the animal's 4th day of life (P < 0·05).

Fig. 1 Body weight of the nourished (![]() ) and undernourished (
) and undernourished (![]() ) animals from the 1st to the 21st day of life and on the 30th, 60th and 90th days of life. * Mean value was significantly different from that of the nourished animals (P < 0·05).
) animals from the 1st to the 21st day of life and on the 30th, 60th and 90th days of life. * Mean value was significantly different from that of the nourished animals (P < 0·05).
Body weight was also examined before extraction and after alveolitis in all groups (Fig. 2). Within each group, only the N-21 group showed an increase in weight after alveolitis (P < 0·05). However, when making a comparison between groups N-21 × UN-21 and N-28 × UN-28 both before extraction and after alveolitis, it was observed that the weight of the undernourished animals was always lower than that of the nourished animals (P < 0·05).

Fig. 2 Body weight of animals belonging to groups N-21, UN-21, N-28 and UN-28 (see Materials and methods) before extraction (□) and after alveolitis (![]() ). Significant difference (P < 0·05).
). Significant difference (P < 0·05).
The CFU found in the perialveolar region of the upper right incisors of rats were calculated before extraction, after extraction and after alveolitis in all groups (Fig. 3). In group N-21, when the times before and after extraction were compared, it was found that there was a significant reduction in the number of CFU after extraction and a significant increase when the times after extraction and alveolitis were compared (P < 0·05). In group UN-21, when the times before and after extraction were compared, it was found that there was a significant reduction in the number of CFU after extraction and a significant increase after alveolitis when compared with the periods before and after extraction. In group N-28, there was a decrease in CFU when the times before extraction were compared with the times after extraction and alveolitis. In group UN-28, there was a significant difference between all periods, an increase in CFU being observed after alveolitis. In view of the comparison between groups N-21 × UN-21 and N-28 × UN-28 after alveolitis, a greater number of CFU were found in the undernourished group (P < 0·05). On the other hand, when groups N-21 × N-28 and UN-21 × UN-28 were compared after alveolitis, a decrease in the number of CFU was found in both the nourished and undernourished groups (P < 0·05).

Fig. 3 Frequency of colony-forming units observed in the peri-alveolar region of the upper-right incisors of animals belonging to goups N-21, UN-21, N-28 and UN-28 (see Materials and methods) before extraction, after extraction and after alveolitis. Significant difference (P < 0·05).
The Gram-positive and Gram-negative bacterial species observed in the perialveolar region of the upper right incisors of rats were recorded before extraction, after extraction and after alveolitis in all groups (Table 1). When groups N-21 × UN-21 were compared before and after extraction, it was found that there were fewer Gram-positive bacteria in the undernourished animals than in the nourished animals (P < 0·05). However, this situation was reversed after alveolitis, when there were more Gram-positive bacteria in undernourished animals than in nourished animals (P < 0·05). Also, in relation to this comparison, it is important to emphasise the presence of Gram-negative bacteria in only one undernourished animal after alveolitis. When groups N-28 × UN-28 were compared before extraction, it was observed that the number of Gram-positive bacteria in the undernourished animals was lower than in the nourished animals (P < 0·05). However, this picture was reversed after alveolitis, when more Gram-positive (P < 0·05) and Gram-negative bacteria were found in the undernourished animals than in nourished animals. However, again with regard to Gram-negative bacteria, it was found that after alveolitis, 50 % of the undernourished animals presented a colonisation formed by one or two of these bacteria.
Table 1 Number of Gram-positive and Gram-negative bacterial species found in the peri-alveolar region of the upper-right incisors of animals belonging to groups N-21, UN-21, N-28 and UN-28 before extraction, after extraction and after alveolitis †
(Medians, 25th and 75th percentiles)
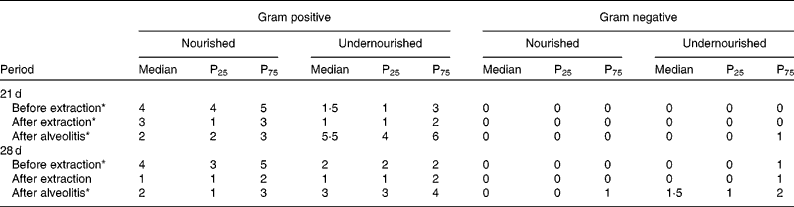
* Significant difference (P < 0·05). Comparing nourished with undernourished for gram positive.
† For details of groups, see Materials and methods.
The percentage of positive blood cultures before extraction, after extraction and after alveolitis was determined in all animals in groups N-21, UN-21, N-28 and UN-28 (Fig. 4). When comparing groups N-21 × UN-21 and N-28 × UN-28 after alveolitis, it was observed that the percentage was higher in the undernourished animals than in nourished animals.

Fig. 4 Percentage of positive blood cultures in the animals of groups N-21, UN-21, N-28 and UN-28 (see Materials and methods) before extraction (□), after extraction (■) and after alveolitis (![]() ).
).
The bacteria in the blood cultures of groups N-21, UN-21, N-28 and UN-28 before and after extraction and after alveolitis are shown in Table 2. In view of the comparison between the groups N-21 × UN-21 and N-28 × UN-28 after alveolitis, a larger number of positive cases were found in the undernourished animals. However, in group UN-21, Staphylococcus aureus and S. saprophyticus were the most frequently encountered species, with S. saprophyticus being the most frequent species in group UN-28.
Table 2 Distribution of the bacteria found in the blood cultures of the groups N-21, UN-21, N-28 and UN-28 before extraction, after extraction and after alveolitis*
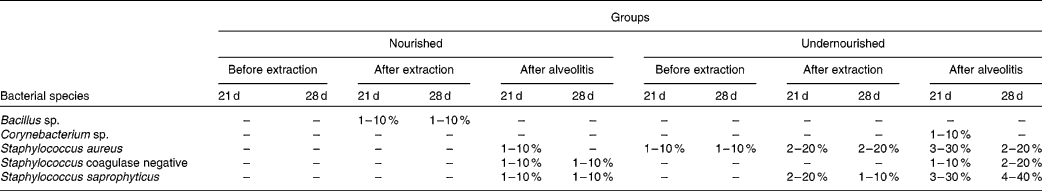
* For details of groups, see Materials and methods.
Discussion
Reduced blood supply impairs the healing of any wound and facilitates the onset of an infectious process(Reference Menezes, Gaujac and Trento38). This was demonstrated during the course of the present study: after extraction of the upper right incisor of rats, alveolar ischaemia was induced by the insertion of a small sterile cotton pad soaked in 1:1000 adrenaline for 15 min, and after its removal no blood clot formation was observed inside the alveolus; after 48 h, however, the development of dental alveolitis was observed in all animals.
In the present study, neonatal malnutrition triggered by an 8 % casein low-protein diet led to a deficit in body weight by inducing a delay in weight gain during the lactation period from the 4th day of life. These data are consistent with those of Porto et al. (Reference Porto, Araújo and Melo39, Reference Porto, Viana and Silva40) and Severo(Reference Severo41), who used the same diet and were able to show similar effects from the 5th and 4th days of life, respectively. Another experimental model of malnutrition was used by Wanderley(Reference Wanderley42), Barros et al. (Reference Barros, Manhães-De-Castro and Lopes-De-Souza43) and Andrade et al. (Reference Andrade, Miranda and Lucena44), which was applied to a regional basic diet with 7·87 % protein(Reference Teodósio, Lago and Romani45). It is worth noting that the effects of both diets were proven, since they adversely affected the protein content of the breast milk, thereby producing their deleterious effects on the offspring. However, in the present study, 8 % casein was used, owing to the fact that its administration is advocated internationally as a standard experimental diet capable of inducing malnutrition.
With respect to neonatal malnutrition, the influence of a low-protein diet on the body weight of animals until adulthood – 30, 60 and 90 d of life – was also verified in the present experiment, because even with the administration of a balanced diet – Labina (standard bioterium diet containing 23 % mixed protein) after weaning, the body weight of the undernourished animals remained lower than that of the nourished ones. These results corroborate those of other studies(Reference Porto, Araújo and Melo39–Reference Andrade, Miranda and Lucena44, Reference Barreto-Medeiros, Feitoza and Magalhães46), in which the body weight of undernourished animals remained low in adulthood, even when they were submitted to nutritional recovery.
Quantitative and qualitative changes in oral microbiota were observed in the present study when comparing the times before extraction, after extraction and after alveolitis within each group and between groups. Thus, with respect to the quantitative aspects, in making a comparison between the periods before and after extraction within each group, it was observed that there was a reduction in CFU after extraction; this was probably due to the mechanical removal during the process of inducing alveolitis. These results are consistent with those cited by Araújo(Reference Araújo35). A comparison between groups N-21 × UN-21 and N-28 × UN-28 both before and after extraction revealed that there were fewer CFU in undernourished animals. However, this situation was reversed after alveolitis, because there was a larger number of CFU in these animals. These data enable us to make an analogy with reports from Porto et al. (Reference Porto, Viana and Silva40) that indicate that when the usual number of resident micro-organisms is greatly reduced, opportunistic invaders establish themselves more easily. As for the qualitative aspects, after alveolitis, when the nourished and undernourished animals were compared, a greater quantity of Gram-positive and Gram-negative bacteria was observed in the undernourished animals. However, the presence of Gram-negative bacteria was observed in group UN-21 and a considerable increase in their number in group UN-28, since 50 % of these animals presented one or two species. These changes were consistent with those reported by Chow(Reference Chow, Mandell, Bennett and Dolin47) and Darby & Curtis(Reference Darby and Curtis48), in which diet and infectious processes are able to produce changes in the composition of oral microbiota. It is worth noting that the Gram-negative bacteria are the ones with a higher pathogenic potential(Reference Rodrigues, Newman, Cardoso and Gonçalves49–Reference Socransky and Haffajee51).
Oral infections are typically local, but they can serve as a focus for systemic infections(Reference Daniel, Granato and Grando52, Reference Spolidorio, Estrela and Bedran53). There are three possible mechanisms by which oral infections can trigger infections at a distance: metastatic infection due to the translocation of micro-organisms; metastatic injury as a result of the movement of toxins from oral micro-organisms; metastatic inflammation caused by immunological injury induced by oral micro-organisms(Reference Gonçalves54). The presence of these micro-organisms in the tissues leads to contact with the immune effector cells, such as residual macrophages, by inducing a localised inflammatory response with the production of pro-inflammatory cytokines, enzymes, mediators derived from the metabolism of arachidonic acid, oxygen-free radicals, among others, leading to tissue damage and the onset of the disease. At the same time, anti-inflammatory cytokines are released, regulating and resolving the local process(Reference McManus and Pinckard15, Reference Deo and Bhongade17). In the event of a decrease in immunological competence, this control may be disrupted, producing a dissemination of micro-organisms, a variety of clinical signs and symptoms and a worsening of the inflammatory/infectious process, all of which may lead to bacteraemia and result in septicaemia(Reference McManus and Pinckard15). The diagnosis of bacteraemia is obtained through the detection of viable bacteria in the bloodstream, and obtaining blood cultures has been recommended as the most sensitive method for identifying the condition(Reference Baitello, Neto and Filho55). Thus, in the present study, the development of bacteraemia in nourished and undernourished rats was confirmed by positive blood cultures. However, bacteraemia may be transient, that is, when its duration does not exceed 1 h and micro-organisms are rapidly destroyed by the defences of the host, or are permanent, that is, when the bacteria are not eliminated from the body and remain in the blood stream(Reference Cury, Joly and Araújo56, Reference Silva, Marceliano and Souza57). The latter is usually associated with predisposing factors in the patient, one of which is malnutrition(Reference Krebs and Taricco58, Reference Morais, Silva and Avi59). In the present study, after the onset of alveolitis, bacteraemia was detected in 30 % of the animals in the N-21 group, 80 % of those in the UN-21 group, 20 % of those in the N-28 group and in 80 % of those in the UN-28 group. This result therefore shows a higher percentage of positive blood cultures in animals subjected to neonatal malnutrition, probably due to their compromised immune system.
The oral microbiota from the perialveolar region of rats is composed of the following bacteria: Bacillus sp., S. aureus, Streptococcus viridans, Corynebacterium sp., Staphylococcus coagulase negative, Enterococcus sp., Escherichia coli, S. saprophyticus, Klebsiella oxytoca, K. pneumoniae and Serratia liquefaciens (Reference Araújo, Castro and Severo37). The micro-organisms normally found in the oral cavity are responsible for a large number of bacteraemias(Reference Ghizoni, Sant'ana and Taveira60). The present findings bear this out, since all the bacterial species present in the blood cultures (Bacillus sp., Corynebacterium sp., S. aureus, Staphylococcus coagulase negative and S. saprophyticus) were part of the endogenous microbiota from the perialveolar region of the upper right incisor of the rat.
The bacterial species S. aureus and Staphylococcus coagulase negative were identified in most of the diagnoses of bacteraemia(Reference Krebs, Pedroso and Diniz61). This was clearly demonstrated in the present study, because 50 % of the positive blood cultures from the animals in groups UN-21 and UN-28 harboured these bacteria after alveolitis.
An epidemiological study of sepsis and causative agents in children showed that malnutrition was a risk factor for the onset of a septic condition in childhood, because 71·1 % of children who had episodes of sepsis were undernourished and the predominant bacterium in the blood cultures was S. aureus (Reference Ribeiro and Moreira62). These findings were also observed in the present experiment because, after alveolitis, the animals subjected to neonatal malnutrition showed a higher percentage of positive blood cultures, S. aureus being one of the most frequently encountered bacteria.
The development of sepsis in burned patients was caused mostly by Gram-positive bacteria and the most common of these was S. aureus (Reference Macedo, Rosa and Macedo63). These findings may be related to those of the present study, since bacteraemia was triggered only by Gram-positive bacteria, S. aureus again being one of the bacterial species most commonly found in the blood cultures of undernourished animals.
Neonatal malnutrition can cause sequelae in the body's defences, thus representing a high risk factor for the development of bacteraemia, and Staphylococcus coagulase negative is its main aetiological agent(Reference Brito, Soares and Abdallah64). In the present study, neonatal malnutrition was able to trigger a higher percentage of positive blood cultures after alveolitis, and Staphylococcus coagulase negative was one of the causative agents.
Conclusion
Thus, based on the present results and a review of the literature, it was possible to confirm the importance of the present study for the area of dentistry, since it clearly and decisively demonstrated the influence of neonatal malnutrition in the process of dental alveolitis, both the composition of the microbiota from the perialveolar region and the development of bacteraemia, thereby revealing the existence of a direct relationship between oral and systemic diseases. In future work, we intend to assess the influence of neonatal malnutrition in controlling or failing to control the local process of dental alveolitis by quantifying pro-inflammatory mediators in the exudate of the alveolitis and serum.
Acknowledgements
The present study was supported by Laboratory of Immunopathology Keizo-Asami, Federal University of Pernambuco (LIKA-UFPE) and Coordination of Further Training of Higher Education Personnel (CAPES). The survey was designed by F. R. G. d. A. and C. M. M. B. d. C. All the authors contributed to the collection and interpretation of the data as well as to the drafting of the manuscript. The authors have no conflicts of interest to declare.










