Intra-uterine growth restriction is considered to be major health problem for both humans and animals. Indeed, more than 5 % of infants suffer from IUGR because of inadequate food intake, disease, environmental stress or dysfunction of the placenta, endometrium or uterus(Reference Wu, Bazer and Wallace1, Reference McMillen and Robinson2). It has been shown that the intestinal weight, as well as length, wall thickness, villous height and crypt depth, in neonates with IUGR is reduced relative to body weight(Reference Baserga, Bertolotto and Maclennan3, Reference Xu, Mellor and Birtles4). D'Inca et al. (Reference D'Inca, Kloareg and Gras-Le Guen5) reported that IUGR may induce alterations in the developmental pattern of the intestinal barrier, which are possibly related to increased morbidity associated with IUGR.
Amino acids (AA) originating from dietary proteins are used for protein synthesis and partly metabolised by the enterocytes during their transfer from the lumen to the bloodstream. For instance, the principal metabolic fuels for small-intestinal enterocytes are glutamine, glutamate and aspartate, which have been shown to be extensively oxidised by the enterocytes in the process of transfer from the luminal content to the bloodstream(Reference Blachier, Boutry and Bos6). Other AA such as cyst(e)ine are also known to be substantially catabolised by the enterocytes(Reference Coloso and Stipanuk7, Reference Yin, Huang and Li8). Although highly converted to ornithine and urea in the enterocytes, arginine isolated from weaned pigs is not degraded in neonate piglets but, instead, produced from different AA precursors(Reference Shoveller, Stoll and Ball9). Lysine is not degraded by pig enterocytes irrespective of the developmental stages(Reference Blachier, M'Rabet-Touil and Posho10–Reference Chen, Li and Wang12). AA absorption requires numerous transport systems that differ in their substrate specificity and efficiency. The system b0,+, which consists of a heavy subunit (rBAT) and a light subunit (b0,+AT), is characterised by Na+-independent AA transport(Reference Chen, Yin and Jobgen11, Reference Feliubadaló, Font and Purroy13). The system b0,+ is an antiporter that takes up cationic AA and cystine in exchange for neutral AA(Reference Chen, Li and Wang12–Reference Dello Strologo, Pras and Pontesilli15). The b0,+AT protein, encoded by the SLC7A9 gene, is known to be fully functional in the absence of the heavy subunit rBAT(Reference Munck14–Reference Flynn, Meininger and Haynes19). This AA transporter mediates the apical uptake of basic AA, such as lysine, arginine and the S-containing compound cystine. Among these AA, lysine is essential and arginine is considered as essential in the period of mammal development(Reference Verrey, Closs and Wagner17, Reference Reig, Chillarón and Bartoccioni18, Reference Wu, Fang and Yang20).
The Huanjiang mini-pig, mainly bred in southern China, especially in the Guangxi Province(Reference Flynn, Meininger and Haynes19, Reference Zhao21–Reference Yang, Li and Kong26), has received increasing attention from researchers due to its small body size and thus easy to handle during experiments. The body fat content of the Huanjiang mini-pig is relatively low and its meat is characterised by high phosphatidylcholine and glutamine contents(Reference Wu, Fang and Yang20, Reference Zhao21, Reference Zhang, Jing and Cui27, Reference Yu, Hua and Xu28). In addition, the pig represents a useful experimental model because its intestinal physiology and metabolism are not very different from those in humans(Reference Yang, Fu and Shao22–Reference He, Ren and Kong24, Reference Zhang, Liu and Luo29–Reference Luo, Zhang and Liu31).
A low growth rate of piglets with IUGR represents a serious agronomical problem(Reference He, Ren and Kong32). Previous studies have emphasised the uptake of AA on the placental surface in humans and experimental animals, with few studies focusing on AA intestinal absorption during the intense developmental period from birth to weaning(Reference Li, Li and Yang25–Reference Luo, Zhang and Liu31). Therefore, the present study aimed at investigating the ontogenic expression of the protein subunit b0,+AT of the intestinal apical AA transporter b0,+ in suckling piglets born with normal body weight (NBW) and small body weight due to IUGR. In addition, circulating blood plasma concentrations of AA transported by the b0,+ system were measured in both groups of animals.
Materials and methods
Animals and tissue sample collection
The present study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures involving animal subjects were approved by the Animal Welfare Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences(Reference Liu, Geng and Shu33). Piglets with a birth weight close to mean birth weight ( ± 0·2 sem) g were identified as normal-birth-weight animals (NBW, control), and those with mean minus 0·9 sem birth weight ( − 30 %) were defined as piglets with IUGR. A total of twenty litters of Huanjiang mini-pigs were spontaneously delivered from sows at term (approximately 114 d of gestation). At birth, one IUGR piglet and one NBW piglet were selected from each of the twenty litters and weighed immediately. On day 0, five IUGR and five NBW piglets were paired from the same five litters, and these piglets were not allowed to suckle milk from sows until euthanasia. The rest of the selected piglets (fifteen IUGR and fifteen NBW) were positioned in the second teat pairs for suckling from their own mother and used at different time points at days 7, 14 and 21. Suckling piglets were helped to fix to the nipple to prevent fighting during suckling. The piglets then got used to fixing to a given nipple during the suckling period. These suckling piglets were used 1 h after the last suckling for blood recovery and euthanasia. Blood samples (10 ml) were obtained from the jugular vein into heparinised tubes, centrifuged (3000 g for 10 min at 4°C) and the supernatants (plasma samples) were collected and immediately stored at − 20°C for biochemical analyses(Reference Tang, Yin and Nyachoti34). Immediately after blood sampling, pigs were euthanised with an intraperitoneal injection of sodium pentobarbital (50 mg/kg body mass). The intestine and liver tissue samples were collected(Reference Yao, Yin and Chu35). The entire intestine was then rapidly removed and dissected free of mesenteric attachments and placed on a cold surface tray. A 30 cm segment in the middle of the small intestine was taken as the jejunal tissue sample. The isolated intestinal segments were immediately opened lengthwise following the mesentery line and flushed with ice-cold saline (154 mm-NaCl, 0·1 mm-PMSF, pH 7·4) and divided into 15 cm segments(Reference Yin, Baidoo and Schulze36). Each tube, containing approximately 15 g tissue, was tightly capped and frozen in liquid N2 immediately and stored at − 80°C until biochemical analysis.
Body weight and determination of plasma concentrations of amino acids
Body weights of piglets were measured 1 h after the last suckling before undergoing euthanasia. Plasma AA concentrations were determined using a Hitachi L-8800 Amino Acid Analyzer (Shimadzu), as described previously(Reference Kong, Yin and He37).
RNA extraction and complementary DNA synthesis
Intestinal tissue sample was pulverised under liquid N2. Total RNA was isolated from 100 mg of the homogenate using the TRIzol reagent (Invitrogen) and treated with DNase I (Invitrogen) according to the manufacturer's instructions. RNA quality was checked by 1 % agarose gel electrophoresis, stained with 10 μg/ml of ethidium bromide. It was duly verified whether RNA had an OD260:OD280 ratio between 1·8 and 2·0, where OD is the optical density. Synthesis of the first-strand complementary DNA (cDNA) was performed with oligo(dT)20 and Superscript II reverse transcriptase (Invitrogen)(Reference Wang, Gu and Tang38).
Quantification of mRNA levels by real-time RT-PCR analysis
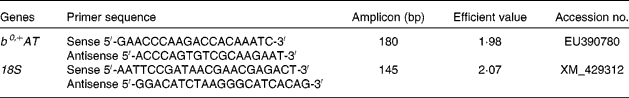
Primers for b0,+AT and 18S rRNA were designed with Primer Express software 5.0 (PE Applied BioSystems) based on the b0,+AT cDNA sequence of the Huanjiang mini-pig to produce an amplification product (Table 1). 18S rRNA was used as an internal reference gene to normalise target gene transcript levels. Real-time PCR was performed using a SYBR Green detection kit, containing MgCl2, dNTP and Hotstar Taq polymerase. An aliquot (2 μl) of cDNA template solution was added to a total volume of 25 μl containing 12·5 μl SYBR Green mix, and 1 μl each of forward and reverse primers. The following protocol was used: (1) pre-denaturation programme (10 s at 95°C); (2) amplification and quantification programme, repeated forty cycles (5 s at 95°C, 20 s at 60°C); (3) melting curve programme (60–99°C with a heating rate of 0·1°C/s and fluorescence measurement). The identity of each product was confirmed by dideoxy-mediated chain termination sequencing at Sangon Biotechnology, Inc. We calculated the relative expression ratio (R) of mRNA using the following equation: R= 2(CT(18s) − CT(test)), where 18s is the reference. All primers were optimised for efficiency by generating a cDNA dilution series (100, 50, 20, 10, 5 and 1 %) from tissue RNA and by using real-time PCR based on the same dilutions. C t values were then exported to QGene and efficiency values for each primer pair were acquired according to the equation 10( − 1/slope) and were found to be consistent between target mRNA and 18S rRNA(Reference Muller, Janoviak and Miserez39, Reference Wang, Shi and Zhang40). Negative controls were performed using water instead of cDNA.
Table 1 Primers used for real-time PCR
Protein immunoblot analysis
Frozen samples were powdered under liquid N2 using a mortar and pestle. The powdered tissue was homogenised in seven volumes of buffer (20 mm-HEPES, pH 7·4, 100 mm-KCl, 0·2 mm-EDTA, 2 mm-ethylene glycol tetraacetic acid, 1 mm-dithiothreitol, 50 mm-NaF, 50 mm-β-glycerolphosphate, 0·1 mm-phenylmethanesulphonylfluoride, 1 mm-benzamidine, 0·5 mm-sodium vanadate, and 1 mm-microcystin, leucine and arginine). The homogenate was centrifuged at 10 000 g for 10 min at 4°C. The supernatant was aliquoted into microcentrifuge tubes, and its protein content was quantified using a detergent-compatible protein assay kit (Bio-Rad). Samples obtained from the intestine were loaded onto 10 % polyacrylamide gel. The liver sample of the NBW group obtained at day 0 was used as the positive control, which was used to express the b0,+AT protein(Reference Muller, Janoviak and Miserez39). Aliquots of 10 μg protein for each sample were mixed with a one-fifth volume of the sample buffer (0·35 m-Tris–HCl, pH 6·8, 10 % SDS, 30 % glycerol, 9·3 % dithiothreitol and 0·175 mm-bromophenol blue). The samples were boiled for 5 min and cooled on ice before being used for Western blot analysis. The separated proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore) overnight at 4°C and then incubated with a blocking solution (0·05 % Tween 20, 50 mm-Tris, pH 8·0, 150 mm-NaCl and 5 % powdered non-fat milk) overnight at 4°C. The membranes were incubated for 2 h at room temperature with a polyclonal SLC7A9/b0,+AT antibody diluted at 1:1000 dilution (MBL). The membranes were incubated with an appropriate peroxidase-labelled secondary antibody prepared in PBS-Tween 20. The membranes were then washed and incubated for 2 h at room temperature with a goat anti-rabbit secondary antibody (Zhongshan Goldbridge Biotechnology) at 1:5000 dilution. The same procedure was followed with a goat polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase antibody (Santa Cruz) diluted at 1:1000, and then with a horseradish peroxidase-conjugated rabbit anti-goat secondary antibody (Zhongshan Goldbridge Biotechnology). Primary antibody binding was visualised using an enhanced chemiluminescence kit (Pierce) and Hyperfilm-MP (Amersham International). The intensities of proteins on the membranes were quantified using the Alpha Innotech 8800 image station equipped with FluorChem software. The relative amounts of b0,+AT in the different samples were determined based on the band optical density, and the b0,+AT:glyceraldehyde 3-phosphate dehydrogenase optical density ratio was then calculated.
Bioinformatic analysis
BLAST was used to identify homologous sequences in GenBank databases. Sequences were aligned in the multiple alignment program Clustal V.
Statistical analysis
All results are reported as mean values with their standard errors. Differences between the groups were determined using SAS (version 6.12; SAS Institute). Data were analysed using a mixed model for repeated measures, taking into account age, body weight, sow and day × size interactions with body weight (NBW v. IUGR) and day (0, 7, 14 and 21) as independent fixed effects and sows as a random factor (SAS Institute). Differences with P values < 0·05 were considered as statistically significant.
Results
Body weight and plasma amino acids
In the present study, IUGR piglets from birth until 21 d of age had a 43–50 % lower body weight than NBW piglets (P< 0·05). The data on the body weight of pigs and relative body weight using the body weight of IUGR piglets on 0 d as reference are summarised in Table 2. The plasma concentrations of arginine, cyst(e)ine and lysine of NBW or IUGR piglets from days 0 to 21 are shown in Table 3. The plasma concentrations of these three AA decreased over time both in IUGR and NBW piglets (P< 0·05). Lysine also decreased in both groups (P< 0·05), as well as arginine in NBW piglets (P< 0·01). During the suckling period, the concentration of arginine in IUGR piglets was significantly lower from days 0 (P= 0·017) to day 7 (P= 0·039) compared with NBW piglets, while from days 14 to 21, the difference in arginine levels was not significant between the IUGR and NBW piglets. Overall, in IUGR piglets, plasma arginine concentration represented 46 % of the value in the control animals. Inversely, plasma cyst(e)ine levels were not significantly different between these two groups throughout the entire suckling period. Plasma lysine concentration in IUGR piglets was similar to that in NBW piglets from days 0 to 14; however, at day 21, lysine concentration was significantly lower in IUGR piglets than in NBW piglets (P< 0·01).
Table 2 Body weight as a function of age for normal-body-weight (NBW) and intra-uterine growth restriction (IUGR) piglets† (Mean values with their standard errors, n 5 piglets per group)
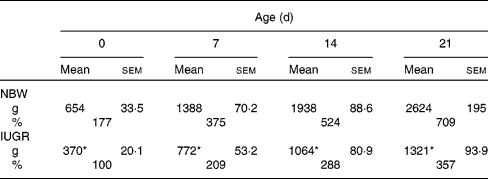
* Mean values of the body weight in IUGR piglets were significantly lower than the body weight of NBW piglets at all time points (P< 0·05).
† Body weights (g) of piglets were measured at birth (day 0) and at different periods of time during the suckling period. Relative growth (%) was calculated using the body weight of IUGR piglets at 0 d as 100 %.
Table 3 Plasma concentrations (μmol/l) of amino acids in Huanjiang mini-pigs with normal body weight (NBW) and intra-uterine growth restriction (IUGR)* (Mean values with their standard errors, n 5 piglets per group)
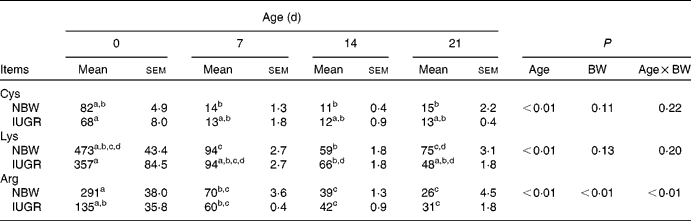
BW, body weight; Cys, cyst(e)ine.
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Plasma concentrations of the amino acids were measured in piglets at birth and at different suckling periods.
PCR efficiency analysis
Fig. 1 shows the 18S rRNA expression at each time point during the developmental stage as measured by real-time PCR. There was globally no significant difference in 18S expression between the NBW and IUGR groups.
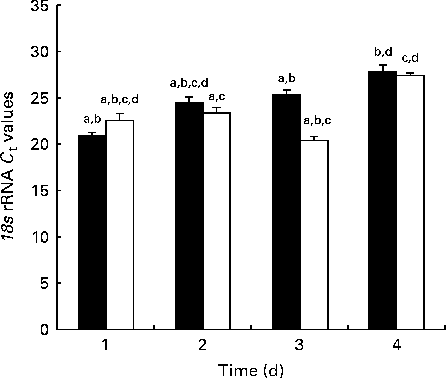
Fig. 1 Reference gene expression during development measured by real-time PCR in the jejunum of piglets. C t values are means (n 5 piglets), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P< 0·05). ■, Normal body weight; □, intra-uterine growth restriction.
b0,+AT mRNA relative abundance during ontogenesis in the intestine
The developmental changes in b 0,+AT mRNA expression in Huanjiang pigs are shown in Fig. 2. In the statistical analysis, using body weight (NBW v. IUGR) and day (0, 7, 14 and 21) as independent fixed effects and sows as a random factor, we found that for mRNA expression in piglets, the interaction of age and body weight was highly significant (P< 0·01). At birth, b 0,+AT mRNA expression was markedly higher in NBW piglets than in IUGR piglets. Then, in the suckling period, decreased b0,+AT expression in both NBW and IUGR piglets was measured (linear, P< 0·01), but with a marked decrease between days 0 and 7 (P< 0·01). b 0,+AT mRNA expression was significantly lower in IUGR piglets when compared with that in the NBW animals throughout the experimental period (P< 0·05), except for day 14 (P= 0·289). On days 14 and 21, b0,+AT expression in NBW piglets remained at the same level than in the day 7 piglets, while on day 21, it had a trend of recovery. In IUGR piglets, a marked decrease was observed during the same period and b0,+AT expression on day 21 was found to be close to the limit of detection.
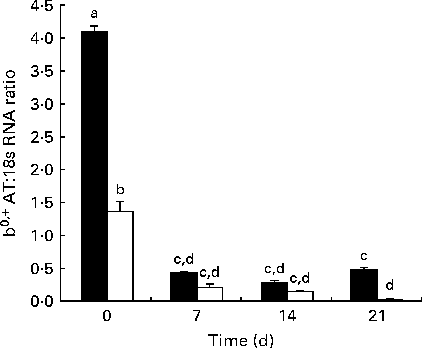
Fig. 2 Expression of mRNA corresponding to b 0,+AT in the jejunum of normal-body-weight (NBW, ■) and intra-uterine growth restriction (IUGR, □) Huanjiang piglets. b 0,+AT mRNA abundance was measured in the intestine of NBW and IUGR piglets at birth and at different times of the suckling period. All samples were normalised using 18S rRNA expression as an internal control in each real-time PCR. Relative levels of b 0,+AT mRNA were analysed by the 2( − ΔC t) method. Values are means (n 5 piglets), with their standard errors represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P< 0·05).
b0,+AT protein relative abundance during ontogenesis in the intestine
The b0,+AT protein expression of Huanjiang pigs is shown in Fig. 3. The pattern of expression is not very different from that of mRNA expression. During lactation, the amount of b0,+AT protein was markedly lower in IUGR piglets compared with the NBW animals (Fig. 3). The amount of b0,+AT decreased significantly from day 7 in both groups of animals, although after day 7, the rate of decrease was less significant in NBW piglets. Again on day 21, the b0,+AT protein of the intestine recovered from IUGR piglets was near the limit of detection.

Fig. 3 Protein expression for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and b0,+AT:GAPDH protein ratio in the jejunum of normal-body-weight (NBW, ■) and intra-uterine growth restriction (IUGR, □) of Huanjiang piglets. (A) Protein expression of b0,+AT as measured by Western blot analysis using protein extracts recovered from the jejunum of NBW and IUGR piglets. GAPDH demonstrates equal amounts of protein loaded onto the gel. The positive control is the liver sample of the NBW group obtained at day 0. (B) Densitometric scan ratio of b0,+AT:GAPDH band intensities. Values are means (n 5 piglets per group), with their standard errors represented by vertical bars. A,B,CMean values with unlike letters were significantly different (P< 0·05). a,b,cMean values with unlike letters were significantly different (P< 0·05). *Mean values were significantly different between the NBW and IUGR groups at a given age (P< 0·05).
Discussion
The main finding of the present study is that the intestinal expression of b0,+AT in mRNA and protein is much higher at birth than in the suckling periods. Furthermore, piglets with IUGR were characterised by a markedly decreased expression of the AA transporter at birth, and a continued decrease albeit less marked in both protein and mRNA expressions in later days of the suckling period. In addition, plasma concentrations of arginine and lysine were lower at some time points in IUGR piglets, whereas the cyst(e)ine level was not different from that in NBW piglets.
It has been demonstrated that body weight at weaning is closely related to the body weight at birth, and body weight at weaning depends on the amount of the sow's milk consumption during the suckling period(Reference Yu, Hua and Xu28, Reference Zhang, Liu and Luo29, Reference Wang, Shi and Zhang40). Using liquid milk replacer for piglets during lactation can successfully increase weaning weights; however, it does not represent a very effective approach for piglets with small birth weights(Reference Reeds, Burrin and Stoll30–Reference He, Ren and Kong32, Reference Wolter and Ellis41–Reference King, Boyce and Dunshea43). In the present experiment, the IUGR and NBW piglets were under natural breeding conditions. Therefore, absolute milk intakes were presumably different between these two groups. However, for both NBW and IUGR piglets, the relative milk consumption is known to be similar, and this parameter is not affected by the body weight of piglets, or by the pre-weaning growth rate(Reference He, Ren and Kong32, Reference Liu, Geng and Shu33, Reference Wolter, Ellis and Corrigan44, Reference Campbell and Dunkin45).
A multi-comparison between the two groups illustrated that b 0,+AT mRNA expression of NBW piglets was higher than that of IUGR piglets at birth, while this difference was less significant from days 7 to 14. Although, at birth, it is clear that the different expression of b0,+AT is not related to milk suckling (since the animals were not allowed to suckle milk from sows), we cannot exclude that afterwards in suckling piglets, the expression of b0,+AT was dependent on both body weight and milk consumption.
The fact that protein expression of b0,+AT in IUGR piglets was generally lower than that in NBW piglets may partly explain why IUGR piglets are not able to overcome the loss of growth at birth in comparison with NBW piglets(Reference Wu, Bazer and Wallace1, Reference Tang, Yin and Nyachoti34). From days 0 to 7, the expression profile of the AA transporter in mRNA and protein was not very different. However, the expression of the protein was not decreased as sharply as the corresponding mRNA expression, suggesting post-transcriptional regulation of this transporter in the intestine(Reference Yao, Yin and Chu35, Reference Xu, Phillips and Schulten46). Plasma concentrations of AA markedly increase in the bloodstream in pigs 1 h after meals(Reference Yin, Baidoo and Schulze36, Reference Blachier, Guihot-Joubrel and Vaugelade47). AA concentrations in the bloodstream result from numerous parameters including protein consumption, protein digestion in the small intestine, intestinal absorption and metabolism of AA, and AA metabolism in piglet tissues. Then, measurement of AA concentrations in circulating blood plasma represents the net result of these different complex events. With these reservations in mind, AA concentrations in blood plasma have been considered as an indicator of AA utilisation(Reference Kong, Yin and He37, Reference Wang, Gu and Tang38, Reference Bertolo, Pencharz and Ball48, Reference Wang, Chen and Li49). In the present study, a significant decrease of plasma arginine concentration in IUGR piglets occurred during early lactation when compared with NBW piglets. A similar result was found with lysine, but not with cyst(e)ine.
IUGR is considered by pig breeders as a serious concern since it may lead to increased mortality of newborn piglets(Reference Wu, Bazer and Wallace1, Reference Tang, Yin and Nyachoti34). IUGR pigs are often eliminated for their increased susceptibility to the onset of pathogenic diseases, permanently impaired growth and suboptimal carcass quality(Reference Muller, Janoviak and Miserez39–Reference Wolter and Ellis41, Reference Bröer50–Reference Thornbury, Sibbons and van Velzen52). Interestingly, a shift in AA transport capacity within the fetoplacental unit in ovine IUGR has been reported(Reference Azain, Tomkins and Sowinski42, Reference King, Boyce and Dunshea43, Reference Regnault, Friedman and Wilkening53, Reference Wang, Wu and Lin54).
The SLC7A9 gene is the one encoding the AA transporter b0,+AT. Mutations in the SLC7A9 gene result in the impaired transport of cationic AA by the gastrointestinal tract(Reference Wolter, Ellis and Corrigan44, Reference Campbell and Dunkin45, Reference Bisceglia, Fischetti and Bonis55, Reference Ercolani, Sahota and Schuler56). Changes in b0,+AT during early developmental stages and how they relate to intestinal functions have previously been studied in several other mammalian species(Reference Wu, Bazer and Wallace1, Reference Bröer57). However, no information is available regarding b0,+AT expression in IUGR piglets, especially during the suckling period. In the present experiment, b0,+AT expression was markedly decreased from days 0 to 7 in NBW piglets, while from days 7 to 21, it showed a relatively steady expression profile associated with the gradual maturation of the intestine. This time coarse of b0,+AT expression is in accordance with our previous study performed in the duodenum and jejunum of another miniature breed, i.e. the Tibetan pig(Reference Wang, Gu and Tang38, Reference Xu, Phillips and Schulten46). In accordance with the results of the present study, Sperandeo et al. (Reference Sperandeo, Annunziata and Bozzato58) found that the b0,+AT protein was down-regulated in the intestine of an IUGR mouse model.
The present results showing the relationship between IUGR and intestinal b0,+AT AA transporter expression suggest that IUGR may limit the absorption of several essential and semi-essential AA in piglets, therefore possibly contributing to the severe reduction of piglet body-weight gain in the suckling period. Additional work is required in order to test new nutritional strategies in order to counteract the decreased expression of the b0,+ transport system in the small intestine of IUGR piglets.
Acknowledgements
The present study was funded by the National Natural Science Foundation of China (31110103909, 30901040 and 31101729) and the National Basic Research Project (2012CB124704 and 2013CB127301). The authors' contributions were as follows: X. K. and Y. Y. conceived and designed the study. W. W. analysed and interpreted the data, and wrote the manuscript. F. B. was involved in the data analysis and critically revised the manuscript for important intellectual content. D. F., J. P., H. Y. and J. G. performed the experiments for the data acquisition. W. C. revised the manuscript. All authors read and approved the final version of the manuscript. All the authors declare that they do not have any competing interests.










