The maintenance of energy balance is an important principle of all life. Animals have to ensure that long-term energy intake matches energy expenditure to avoid either starvation or overfeeding. In our modern society, continuous malnutrition, especially the consumption of a Western-style diet enriched with saturated fat and sugars, has drastically increased the prevalence of diet-induced obesity (DIO) and the development of the metabolic syndrome(Reference Zhang, Zhang and Zhang1), thus causing an enormous financial burden on modern healthcare systems. To mimic DIO in rodent models, diets high in fat and/or carbohydrates are commonly used. Most experiments to investigate the adverse effects of a high-fat diet (HFD) on energy metabolism are performed using diets enriched with animal fats, such as lard, as a predominant fat source. Lard serves as a dietary source of SFA, especially of palmitic acid, the most abundant fatty acid (FA) in lard (about 40 % of total FA), and MUFA such as oleic acid (about 50 % of total FA)(2). The consumption of such an animal fat-enriched HFD is well known to induce obesity and the metabolic syndrome in various animal models and humans by increasing overall food intake and by disrupting normal feeding patterns(Reference Hariri and Thibault3–Reference Hatori, Vollmers and Zarrinpar5).
In contrast to the Western-style diet, diets considered beneficial for metabolic health, such as the Mediterranean diet, contain high amounts of MUFA (up to 55 % of total fat) and PUFA (up to 15 % of total fat) such as n-3 PUFA DHA and EPA(Reference Davis, Bryan and Hodgson6). Some studies using diets enriched with PUFA instead of lard could demonstrate that not fat per se is harmful to metabolic health; rather the ratio of SFA:PUFA appears to be important(Reference Hariri and Thibault3,Reference Buettner, Parhofer and Woenckhaus7) . However, it is still controversial if dietary PUFA supplementation can positively affect body weight and metabolic health, especially in obese individuals. Whereas several studies reported reduced body weight gain, with or without decreased energy intake, in rodents exposed to diets supplemented with n-3 PUFA(Reference Sato, Kawano and Notsu8–Reference Ruzickova, Rossmeisl and Prazak12), others could not find a beneficial effect of the diet on body mass(Reference Belzung, Raclot and Groscolas13,Reference Rokling-Andersen, Rustan and Wensaas14) . Similarly, most studies in humans did not reveal any beneficial effects of a diet supplemented with n-3 PUFA on body weight even though energy intake was reduced(Reference Summers, Fielding and Bradshaw15–Reference Noreen, Sass and Crowe18). However, some studies suggest a beneficial effect on body fat mass in both human and rodents(Reference Baillie, Takada and Nakamura9,Reference Summers, Fielding and Bradshaw15,Reference Du, Jin and Fang17,Reference Noreen, Sass and Crowe18) . The main reasons for these conflicting results are probable differences in diet composition, the type and amount of fat used, which makes it difficult to compare between individual studies. Hence, further research is urgently required to elucidate the effect of different macronutrient compositions and ratios on whole-body energy metabolism.
In line with this, the present study aimed to investigate the role of FA composition of an HFD, enriched with either New Zealand green-lipped mussel (Perna canaliculus, endemic to New Zealand) oil or MSC Hoki (Macruronus novaezelandiae, blue grenadier) liver oil, on energy homeostasis and metabolic health in mice. We identified interesting, novel beneficial effects of HFD enriched with New Zealand green-lipped mussel oil, which led to a marked reduction in body weight in DIO mice or prevented HFD-induced body weight gain, which was associated with a reduction in visceral fat mass.
Experimental methods
Ethical approval
All animal experiments were performed in accordance with the New Zealand Animal Welfare Act and associated guidelines, and received approval from the University of Otago Animal Ethics Committee.
Animals and diet composition
Adult C57BL/6J male mice were obtained from the University of Otago Hercus Taieri Resource Unit and housed individually under controlled conditions, such as an ambient temperature of 22 ± 1°C and a 12 h light–12 h dark cycle. The age of animals at the beginning of the experiment is indicated in Table 1. Animals had ad libitum access to water and food.
Table 1. Age of animals at the beginning of feeding paradigm

For metabolic studies, ten cohorts of C57BL/6J mice aged 10–12 weeks (n 8) were fed various isoenergetic HFD with 60 % energy derived from fat, or a low-fat diet (LFD) with 10 % energy from fat (D12450J; Research Diets) for 1 week (Table 2). Across the nine different HFD, only the source of fat varied. Whereas the standard HFD was enriched with lard, four HFD contained different ratios of lard and New Zealand green-lipped mussel oil (kindly donated by Aroma), with M1 containing the lowest mussel oil concentration and M4 the highest. Similarly, four HFD contained different ratios of lard and MSC Hoki liver oil (kindly donated by Aroma), with F1 containing the lowest fish oil concentration and F4 the highest. All HFD were customised by Research Diets, and the exact composition can be found in Table 3. The FA composition of New Zealand green-lipped mussel (analysed by Eurofins ELS Limited) and MSC Hoki liver oil (analysed by Cawthron Institute) is summarised in Table 4. Body weight and food intake were manually monitored daily throughout the experiment.
Table 2. Ratio of fat sources in different diets

LFD, low-fat diet; HFD, high-fat diet; M1–M4, mussel oil-enriched diets 1–4; F1– F4, fish oil-enriched diets 1–4.
Table 3. Diet compositions

LFD, low-fat diet; HFD, high-fat diet; M1–M4, mussel oil-enriched diets 1–4; F1–F4, fish oil-enriched diets 1–4.
* To convert kcal to kJ, multiply by 4·184.
Table 4. Fatty acid (FA) composition of Hoki liver oil and New Zealand (NZ) green-lipped mussel oil

To elicit age-related changes in body weight and food intake, approximately 1-year-old male C57BL/6J mice, either pre-fed with an LFD or a standard HFD for 10 months, were fed an LFD (n 6 and 4, respectively), an HFD (n 4 and 6, respectively) or the mussel oil-enriched M4 diet (n 7 and 7, respectively) ad libitum for 4 weeks. One additional cohort of mice pre-fed with HFD only received restricted quantities of a standard HFD, energetically matched to the M4 cohort, to exclude that bodyweight differences were only due to a reduction in energetic intake (n 3, pair-fed). Body weight and food intake were measured daily. To further investigate the body composition of these mice, liver and different white adipose tissue (WAT) depots such as epididymal, retroperitoneal and perirenal fat pads were collected and weighed.
Indirect calorimetry
Indirect calorimetry experiments were based on an open-flow multichannel respirometer system (Promethion; Sable Systems International), allowing simultaneous monitoring of energy metabolism and animal behaviour. Three cohorts of male C57BL/6J mice (n 8) aged 10–12 weeks were placed into metabolic cages 2 d prior to the diet change to either an LFD, an HFD or the M4 diet to adapt to the new environment. Animals were kept in metabolic cages for 1 week and had ad libitum access to food and water. For the analysis of oxygen consumption and carbon dioxide production to determine energy expenditure, RMR and respiratory quotient (RQ), the air flow rate in metabolic cages was adjusted to 2000 ml/min, and the extracted air was dried in a cooling trap and analysed for O2, CO2 and H2O content. RMR was defined as the mean value of energy expenditure during the 30 min when the activity score was lowest. Body weight and water intake were monitored simultaneously, and animal activity was determined as pedestrian locomotion and time spent in locomotion or motionless using an integrated laser beam break-based system. The BXYZ Beambreak Activity Monitor (Promethion; Sable Systems International) consists of a grid of laser beams throughout the cage. If a mouse moves around, it passes some of these beams and breaks the light for a period of time. In this way, the exact position of the mouse in the cage can be tracked. Depending on the frequency and distance of broken beams, the activity level and type of activity can be concluded. There were no running wheels in the cages during the experiment. The recorded data were subsequently analysed with ExpeData (Sable Systems International) and the automated analysis scripts provided. Data from 24 or 12 h (day v. night) periods were combined, and the average or sum over 7 consecutive days was calculated as indicated in figure legends.
Statistics
One-way or two-way ANOVA, if appropriate for repeated measurements, with Tukey’s post hoc analyses were used to find differences between means. P values <0·05 were considered statistically significant. For all statistical analyses, GraphPad Prism 7 was used.
Justification of sample size
Power calculation was performed in previous studies to assess the sample size required to detect significant differences in body weight, food intake and energy expenditure after dietary manipulations. As an example for assessing changes in body weight, we calculated a sample size of eight based on a known average body weight of 40 (se 2·5) g and an anticipated reduction of 5 g, a power of 80 % and α of 0·05. This sample size, however, slightly varied due to unknown effect sizes and natural variability working with animals.
Results
Mussel oil-enriched high-fat diet reduces diet-induced body weight gain without affecting food intake
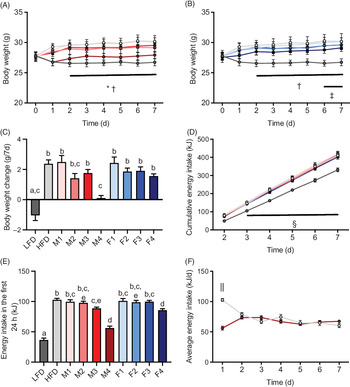
To determine the immediate effects of FA composition on food intake, body weight and energy expenditure, male C57BL/6J mice were fed ad libitum with either a LFD or one of several HFD with different FA compositions for 1 week. Whereas the body weight of mice fed an LFD or M4 diet remained constant over the duration of the experiment, mice fed HFD, M1, M3, F1, F2, F3 or F4 diet significantly gained weight compared with animals fed an LFD (P < 0·0001) (Fig. 1(A)–(C)). The final body weight of M2-fed mice did not significantly differ from any other group; however, there was a trend towards increased body weight. The absence of body weight gain in mice fed M4 diet was not due to decreased food consumption as the cumulative energy intake was identical between different HFD groups over the duration of the experiment (Fig. 1(D)). The only exception was that M3-, M4- and F4-fed cohorts exhibited a brief reduction in food intake compared with HFD-fed animals only within the first 24 h after the diet was changed (Fig. 1(E)). Interestingly, the reduced energetic intake in M4-fed mice within the first 24 h matched the average energy intake in HFD-fed animals at days 2−7, whereas HFD-fed animals increased their energy intake drastically in the first 24 h after exposure to the diet (Fig. 1(F)).

Fig. 1. A green-lipped mussel oil-enriched high-fat diet (HFD) can prevent HFD-induced weight gain in young adult male mice. Male wild-type mice (C57BL/6J), 10–12 weeks old, were fed a low-fat diet (LFD) (10 % energy from fat), a standard HFD enriched with lard or one of various customised HFD (all 60% energy from fat) with different concentrations of New Zealand green-lipped mussel oil (M1, lowest concentration–M4, highest concentration) or Hoki fish oil (F1, lowest concentration–F4, highest concentration) for 1 week. Body weight trajectory of mice fed mussel oil (A) or fish oil diet (B). (C) Body weight change over 7 d. (D) Cumulative energy intake from day 2 to day 7. (E) Energy intake in the first 24 h after the diet was changed. (F) Average energy intake of HFD- and M4-fed mice. Data are means with standard errors. a,b,c,d,e Mean values with unlike letters are significantly different (P < 0·05, one-way ANOVA followed by Tukey’s post hoc analysis, n 8). * Significant differences between LFD and M3. † Significant difference between LFD and HFD. ‡ Significant difference between LFD and F3. § Significant differences between LFD and all other HFD groups. || Significant differences between HFD- and M4-fed mice (P < 0·05, two-way ANOVA, n 8). (A) ![]() , LFD;
, LFD; ![]() , HFD;
, HFD; ![]() , M1;
, M1; ![]() , M2;
, M2; ![]() , M3;
, M3; ![]() , M4. (B)
, M4. (B) ![]() , LFD;
, LFD; ![]() , HFD;
, HFD; ![]() , F1;
, F1; ![]() , F2;
, F2; ![]() , F3;
, F3; ![]() , F4. (D)
, F4. (D) ![]() , LFD;
, LFD; ![]() , HFD;
, HFD; ![]() , M1;
, M1; ![]() , M2;
, M2; ![]() , M3;
, M3; ![]() , M4;
, M4; ![]() , F1;
, F1; ![]() , F2;
, F2; ![]() , F3;
, F3; ![]() , F4. (F)
, F4. (F) ![]() , HFD;
, HFD; ![]() , M4.
, M4.
Dietary n-3 PUFA modulates energy expenditure and water homeostasis
Since the M4 diet did not lead to body weight gain observed in HFD-fed mice, with a decrease in food intake only on the first day, we next investigated whether the beneficial effects of M4 diet on body weight are due to metabolic alterations. Therefore, general metabolic parameters such as total energy expenditure, RMR, oxygen consumption (VO2), carbon dioxide production (VCO2) and RQ were measured by indirect calorimetry. Water intake was monitored in parallel with a scale-based system. Mice were transferred to metabolic cages; after 2 d of habituation, their diet was changed to either LFD, standard HFD or the M4 diet for 1 week as in experiment 1. Similar to the results obtained in experiment 1, mice fed M4 diet did not gain weight in comparison with the group fed LFD (Fig. 2(A) and (B)), whereas mice fed a standard HFD gained an average of 1·6 (se 0·3) g (P = 0·02). Interestingly, from day 4 onwards, the cumulative water intake was significantly higher in mice fed HFD in comparison with both LFD (P < 0·04) and M4 diet cohorts (P < 0·05) (Fig. 2(C)). Surprisingly, mice fed M4 diet exhibited reduced energy expenditure in comparison with mice fed LFD (P = 0·01) or HFD (P = 0·04) (Fig. 2(D) and (E)). There was no difference in RMR between groups (Fig. 2(F)). Oxygen consumption was significantly reduced in M4 animals in the active phase (P = 0·04) (Fig. 2(G) and (H)). In comparison with mice fed HFD or M4 diet, LFD animals displayed increased CO2 production in their active phase (P < 0·0001), which is reflected by a higher RQ value (1·0), suggesting that mainly carbohydrates were metabolised (Fig. 2(I)). The RQ value of HFD- and M4-fed mice was 0·89 and 0·88, respectively, suggesting the utilisation of mixed energy sources.

Fig. 2. A green-lipped mussel oil-enriched high-fat diet (HFD) decreases total energy expenditure. Male wild-type mice (C57BL/6J), 10–12 weeks old, were fed a low-fat diet (LFD) (10 % energy from fat), a standard HFD enriched with lard or a customised HFD enriched with New Zealand green-lipped mussel oil (M4, both 60 % energy from fat) for 1 week. Average body weight (A), body weight change over 7 d (B), water intake (C), energy expenditure (D, E), RMR (F), oxygen consumption (G), carbon dioxide production (H) and the resulting respiratory quotient values (I) as determined by metabolic cages. Data are means with standard errors. a,b Mean values with unlike letters are significantly different (P < 0·05, one-way ANOVA followed by Tukey’s post hoc analysis, n 8). * Significant difference between LFD and HFD. † Significant differences between LFD and both HFD and M4. ‡ Significant differences between M4- and both HFD- and LFD-fed mice. § Significant difference between HFD- and M4-fed mice (P < 0·05, two-way ANOVA, n 8). (A, C, D) ![]() , LFD;
, LFD; ![]() , HFD;
, HFD; ![]() , M4. (G, H)
, M4. (G, H) ![]() , LFD;
, LFD; ![]() , HFD;
, HFD; ![]() , M4.
, M4.
Feeding a high-fat diet reduces fine movements independent of dietary fatty acid composition
The total energy expenditure is composed of basal energy expenditure, which includes all the energy required to maintain fundamental metabolic functions in all cells of the body, thermal effect of feeding and energy expenditure during activity. Since the total energy expenditure was decreased in mice fed M4 diet, but there was no modulation of RMR, the activity of the animals was monitored in metabolic cages with a laser beam break-based system. There was no difference in the distance travelled or time spent in pedestrian locomotion between the groups (Fig. 3(A) and (B)). However, mice fed LFD spent less time being inactive than mice fed HFD or M4 diet (P < 0·0001), suggesting a higher frequency of fine movements (due to the absence of time spent in locomotion or being inactive), such as grooming (Fig. 3(C)).

Fig. 3. A high-fat diet (HFD) has no effect on locomotor activity in young adult mice independent of dietary fat composition. Male wild-type mice (C57BL/6J), 10–12 weeks old, were fed a low-fat diet (LFD) (10 % energy from fat), a standard HFD enriched with lard or a customised HFD enriched with New Zealand green-lipped mussel oil (M4, both 60 % energy from fat) for 1 week. (A) Total distance covered by pedestrian locomotion during active dark phases and inactive light phases. (B, C) Time spent in pedestrian locomotion (B) or being inactive (C). Data are means with standard errors. a,b Mean values with unlike letters are significantly different between treatments during light or dark phase (P < 0·05, one-way ANOVA, n 8). (C) ![]() , LFD;
, LFD; ![]() , HFD;
, HFD; ![]() , M4.
, M4.
Consumption of a mussel oil-enriched high-fat diet has beneficial effects on body weight and fat mass in middle-aged lean and diet-induced obese mice
As previously seen in young adult mice, middle-aged mice transferred from LFD to HFD for 4 weeks gained 13·2 (se 1·2) g, but only 5·4 (se 1·2) g when transferred to M4 diet (Fig. 4(A) and (C)). In alignment with this observation, mice pre-fed HFD lost 2·8 (se 0·4) g after they were transferred to M4 diet, but not mice that remained on HFD (1·5 (se 0·6) g) (Fig. 4(B) and (C)). Energetic intake was significantly lower in mice fed LFD (P < 0·04), but consistent with the first experiment in young mice, there was no difference in food intake between HFD-fed, M4-fed or energetically pair-fed groups, independent of pre-feeding with either LFD or HFD for 10 months (Fig. 4(D)–(F)).

Fig. 4. Prolonged feeding of green-lipped mussel oil-enriched high-fat diet (HFD) can not only prevent HFD-induced weight gain but also decrease body weight in mice with diet-induced obesity. Male wild-type mice (C57BL/6J) were fed with either a standard HFD (grey background) or a low-fat diet (LFD) (white background) for 10 months before their diet was switched to either LFD, a standard HFD or New Zealand green-lipped mussel oil-enriched HFD (M4) ad libitum for 4 weeks. One additional cohort of mice pre-fed with HFD only received a restricted amount of HFD energetically pair-fed to the M4 cohort (pair-fed). (A, B) Body weight of mice pre-fed an LFD (A) or an HFD (B) after the diet was changed. (C) Body weight change over 28 d. (D–F) Energy intake in LFD (D) and HFD pre-fed mice (E) after the diet was changed. Data are means with standard errors. a,b,c,d,e Mean values with unlike letters are significantly different (P < 0·05, one-way ANOVA followed by Tukey’s post hoc analysis, n 3–7). * Significant differences between LFD and HFD. † Significant differences between LFD and both HFD and M4 (P < 0·05, two-way ANOVA, n 3–7). (A, D) ![]() , LFD;
, LFD; ![]() , HFD;
, HFD; ![]() , M4. (B, C)
, M4. (B, C) ![]() , LFD;
, LFD; ![]() , HFD;
, HFD; ![]() , M4;
, M4; ![]() , pair-fed.
, pair-fed.
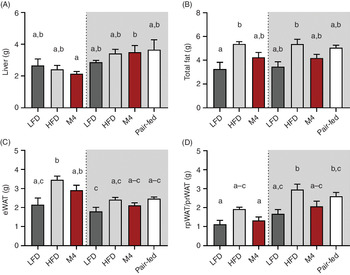
Tissue weight analysis revealed that liver weight was unaffected by diet consumption (Fig. 5(A)). Furthermore, no significant difference in total fat mass was observed between animals fed HFD for 4 weeks compared with mice fed HFD for a longer duration (>10 months) (Fig. 5(B)). Consistently, a complete reduction of total fat mass was observed in mice that were fed HFD for a longer duration (10 months) followed by an exposure to LFD for 4 weeks. Mice fed HFD had a total fat mass of 5·4 (se 0·2) g (LFD pre-fed, P = 0·03) and 5·4 (se 0·4) g (HFD pre-fed, P = 0·01) compared with 3·3 (se 0·6) g for mice fed LFD, whereas the total fat mass of two mussel oil-fed cohorts did not differ from the latter group (Fig. 5(B)). Comparing different fat depots, it appeared that mice that were fed an LFD for 10 months prior to a change to either LFD, HFD or M4 diet stored fat in the epididymal WAT (Fig. 5(C)). Whereas, mice that were fed an HFD for 10 months before the diet was changed stored fat as retroperitoneal or perirenal WAT (Fig. 5(D)).

Fig. 5. Prolonged feeding of green-lipped mussel oil-enriched high-fat diet (HFD) reduces body weight in mice with diet-induced obesity by reducing visceral fat depots. Male wild-type mice (C57BL/6J) pre-fed either a standard 60 % HFD (grey background) or a low-fat diet (LFD) (white background) for 10 months were switched to either ad libitum LFD, HFD or M4 diet for 4 weeks. One additional HFD pre-fed cohort was energetically pair-fed with the M4 group. Tissue weight of liver (A), total fat collected (B), epididymal white adipose tissue (eWAT) (C) and retroperitoneal WAT (rpWAT) and perirenal WAT (prWAT) (D). Data are means with standard errors. a,b,c Mean values with unlike letters are significantly different (P < 0·05, one-way ANOVA, n 3–7).
Discussion
To determine the effects of dietary FA composition on body weight and food intake, 12-week-old mice were fed an HFD enriched with lard, New Zealand green-lipped mussel oil or Hoki liver oil in different concentrations for 1 week. Intriguingly, high dietary concentrations of mussel oil (M4 diet) were able to completely prevent HFD-induced weight gain. The weight-reducing effects of M4 diet were superior to a diet enriched with Hoki liver oil, suggesting that mussel oil has a greater potential in regulating energy homeostasis and improving metabolic health compared with Hoki liver oil. This weight-reducing effect of mussel oil-enriched HFD compared with a standard HFD and fish oil-enriched HFD is remarkable since all diets have the same energy density and fat content and only differ in FA composition. Since both fish and mussel oil are a complex mixture of a variety of different FA, it is difficult to elucidate whether a specific FA or a combination of FA is responsible for the beneficial effects on metabolic health. Until recently, research has mainly focused on EPA and DHA as two important n-3 PUFA. Thus far, a multitude of advantageous effects of these PUFA on metabolic and cardiovascular health have been demonstrated(Reference Kalupahana, Claycombe and Newman19–Reference Calder22). In line with this, New Zealand green-lipped mussel oil contains 40·9 g/100 g n-3 PUFA, which is approximately twice that of Hoki liver oil, with only 20·7 g/100 g. Especially the concentrations of DHA and EPA are 18·3 and 20 g/100 g, respectively, in mussel oil, which is significantly higher compared with Hoki fish oil (11·3 and 5·1 g/100 g, respectively). However, it needs to be considered that variations in other FA between diets might contribute to the observed effects as well (Table 4). Palmitoleic acid and docosapentaenoic acid, for example, are two emerging FA found in fish and other marine animals that only recently got research attention(Reference Bolsoni-Lopes, Festuccia and Chimin23–Reference Weylandt26).
Whereas mice fed an LFD had a significantly lower energy intake compared with all cohorts fed an HFD, the energy intake of different groups fed an HFD was equal from day 2 onwards and independent of the diet’s FA composition. Differences in the diet’s odour or texture did not seem to affect food intake. Therefore, we anticipated that the total energy expenditure of mice fed a mussel oil-enriched diet would be increased to account for weight differences. Surprisingly, this was not the case, but a significant reduction in total energy expenditure was observed by indirect calorimetry. Also, a behavioural analysis revealed that a reduction in total energy expenditure was not caused by a decreased physical activity. The total energy expenditure is composed of RMR, which is the basal energy required for cellular processes; energy spent during a physical activity; and thermal effect of feeding, which includes all the energy spent for consuming, digesting and assimilating food. Since RMR and physical activity were unchanged, it was likely that the thermal effect of feeding declined in M4-fed mice. Unfortunately, it is only possible to measure total energy expenditure by indirect calorimetry, but not heat production or loss. Therefore, no conclusion about any thermal effects can be made. Direct calorimetry would be required to determine how much of total energy expended was used for ATP and how much for heat production. This is important to consider since other studies reported an effect of PUFA on the expression of uncoupling proteins and, thus, on oxidative phosphorylation(Reference Baillie, Takada and Nakamura9).
Only a few studies have investigated the effects of FA composition of HFD on body weight and food intake regulation. While in alignment with our findings, they also reported the beneficial effects of PUFA-enriched diets on body weight, insulin resistance and overall metabolism. Vaidya et al. (Reference Vaidya, Gangadaran and Cheema27) used a high-fat/high-sucrose diet supplemented with different concentrations of freeze-dried blue mussel powder in C57BL/6 mice for 12 weeks. Similar to the present study, they observed suppressed weight gain in blue mussel-supplemented animals accompanied by a decrease in systemic inflammation and improved metabolic health. In line with this, Sato et al. (Reference Sato, Kawano and Notsu8) reported reduced weight gain, hyperglycaemia and hyperinsulinaemia in DIO mice fed a high-fat/high-sucrose diet in which only 5 % of total fat was substituted with EPA for 4–20 weeks. Furthermore, studies using EPA (ethyl ester) or EPA-enriched fish oil in various rodent models have shown that PUFA-enriched diets can protect from weight and fat mass gain and associated metabolic complications, lower insulin plasma concentrations, attenuate inflammation and improve insulin sensitivity and glucose tolerance(Reference Kalupahana, Claycombe and Newman19,Reference LeMieux, Kalupahana and Scoggin20,Reference Oliveira, Castro and Belchior28–Reference Chacinska, Zabielski and Ksiazek30) . However, in contrast to the present study, Oliveira et al. (Reference Oliveira, Castro and Belchior28) and Pahlavani et al. (Reference Pahlavani, Ramalingam and Miller29) reported an increase in O2 consumption and whole-body energy expenditure after fish oil consumption to account for a reduced body weight gain.
In the previously mentioned studies, the observed effects of FA composition on energy intake varied drastically, with some studies showing no difference in HFD enriched with either saturated and unsaturated FA(Reference Sato, Kawano and Notsu8,Reference Hainault, Carolotti and Hajduch11,Reference Rokling-Andersen, Rustan and Wensaas14,Reference Kalupahana, Claycombe and Newman19,Reference LeMieux, Kalupahana and Scoggin20,Reference Oliveira, Castro and Belchior28) , whereas others reported a clear decrease in energy intake with n-3 PUFA-enriched diets(Reference Vaidya, Gangadaran and Cheema27,Reference Dziedzic, Szemraj and Bartkowiak31) . The variation in food intake is probably due to differences in diet composition, type of fat used and different food perceptions (e.g. different texture and odour), emphasising an urgent need for more research and better comparability between studies. The accumulation of different PUFA in the adipose tissue, brain or their concentrations in blood after food consumption is another important factor. Neither in the current or previous studies, the PUFA status of these tissues was examined and considered for the interpretation of the observed data.
A possible explanation for the observed difference in body weight between mice fed M4 or HFD is the alteration in water intake. Mice fed an HFD had a significantly elevated water intake in comparison with LFD- and M4 diet-fed animals, suggesting a modulation of their water homeostasis. Another explanation for the observed reduction in weight gain of the M4-fed group could be reduced intestinal fat uptake and, thus, enhanced excretion of FA. To test this, bomb calorimetry experiments should be performed to determine the energy content in the animals’ excreta. FA that is not absorbable by the small intestine gets excreted and should be detectable as excess energy. In line with this, n-3 PUFA-enriched diets demonstrated accelerated gastric emptying in humans(Reference Robertson, Jackson and Fielding32) and decreased postprandial plasma TAG levels(Reference Harris, Connor and Alam33,Reference Roche and Gibney34) . Furthermore, it was demonstrated in DIO mice that both menhaden fish (Brevoortia tyrannus) oil and hard-shelled mussel (Mytilus coruscus) oil can alter the gut microbiome in favour of anti-obesiogenic strains and thus improve intestinal morphology, epithelial barrier function and attenuate toll-like receptor-4-induced inflammatory signalling(Reference Monk, Liddle and Hutchinson35–Reference Rogero and Calder37).
Intriguingly, none of the other studies reported transient abnormalities in food intake restricted to the first day as observed in the present study in M4-fed mice. The latter might be due to less frequent energy intake measurements in those studies. However, it is well described in literature that the intake of saturated fat induces hyperphagia in humans and rodents(Reference Tremblay, Plourde and Despres38–Reference Thaler, Yi and Schur40). Thereby, food intake drastically increases immediately after the diet is changed, but this subsides again over time to a level that is still higher than the basal energy intake observed in animals fed an LFD(Reference Thaler, Yi and Schur40). The M4 diet in the present study seemed to prevent the initial, severe HFD-induced hyperphagia in mice, but not long-term overfeeding. A similar trend can be seen in mice fed M3 or F4 diet. Supporting this idea, Barson et al. (Reference Barson, Karatayev and Gaysinskaya39) demonstrated that the FA composition of a diet can determine subsequent energy intake in male and female rats. Short-term feeding with a diet (50 % energy from fat) containing 82 % lard and 18 % soyabean oil significantly increased the size of a subsequent chow test meal in comparison with a diet composed of 20 % lard and 80 % fish oil. The mechanisms underlying severe, short-term, HFD-induced hyperphagia are not well characterised and require further research.
It is known that metabolism, energy requirements and food perception drastically change with age in humans and rodents. Ageing is associated with a decrease in body mass, energy intake, RMR, physical activity and fat-free mass, whereas the prevalence of some metabolic diseases such as type 2 diabetes might increase(Reference Ogurtsova, da Rocha Fernandes and Huang41,Reference Manini42) . Intriguingly, a diet enriched with New Zealand green-lipped mussel oil was demonstrated to not only counteract an increase in body weight in young adult and middle-aged lean mice but also decreased body weight in middle-aged DIO mice, emphasising the enormous weight-reducing potential of mussel oil. The total energy intake was not altered, independent of pre-feeding or FA composition of HFD. Interestingly, prolonged feeding of M4 diet was associated with reduced visceral fat mass in both HFD and LFD pre-fed mice. The amount of visceral fat, in contrast to subcutaneous fat, is strongly correlated with the risk of development of CVD(Reference Chiba, Saitoh and Takagi43). In line with this, marine n-3 PUFA were shown to promote a reduction in the size of visceral fat depots in DIO rodents and humans, without altering body weight and composition, suggesting a shift in adipose tissue(Reference Rokling-Andersen, Rustan and Wensaas14,Reference Summers, Fielding and Bradshaw15,Reference Oliveira, Castro and Belchior28,Reference de Mello, Schraiber and Goldim44) . This shift in adipose tissue might be due to alterations in lipogenesis and lipolysis mediated by PUFA. In various rodent studies, PUFA were shown to increase lipolysis and FA β-oxidation, and to inhibit lipogenesis in a tissue-specific manner(Reference Saponaro, Gaggini and Carli45–Reference Crescenzo, Mazzoli and Cancelliere49).
In conclusion, the present study provided the first evidence for the advantageous effects of a New Zealand green-lipped mussel oil-enriched diet on body weight and fat deposition not only in young adult mice but also in middle-aged mice. These beneficial properties were not only restricted to lean animals but were also observed in middle-aged DIO mice pre-fed an HFD for 10 months. Intriguingly, the weight-reducing effect was independent of energy intake and physical activity. The consumption of New Zealand green-lipped mussel oil and other marine n-3 PUFA sources could be beneficial for metabolic health in humans as well. However, further research is needed to translate the findings from rodent studies directly to humans. A meta-analysis investigating the effects of fish oil supplements on body weight in humans reported contradicting effects, but doses might have been too low in some studies(Reference Du, Jin and Fang17). One limitation may be the quantity of FA that can be delivered in one meal as mostly n-3 PUFA are administered as supplements in humans, whereas in our study mussel oil was incorporated in rodent diet. Whereas rodents can be fed with a PUFA-enriched diet high not only in fat (up to 60 % of total energy) but also n-3 PUFA, this will be very difficult to achieve in humans. Therefore, further research to determine the biologically effective FA as well as the required doses is urgently required. It may be important to incorporate these FA directly into the diet or to administer them as a certain food product (e.g. functional food), and it remains to be determined whether lower equivalent doses are effective in humans. Many studies using dietary n-3 PUFA supplements have shown that fish oil intake is associated with elevated EPA and DHA concentrations in circulating lipids(Reference McGlory, Galloway and Hamilton50), various body tissues(Reference McGlory, Galloway and Hamilton50–Reference Katan, Deslypere and van Birgelen53) and blood cells(Reference Browning, Walker and Mander51,Reference Katan, Deslypere and van Birgelen53,Reference Popp-Snijders, Schouten and van Blitterswijk54) in a dose- and time-dependent manner(Reference Browning, Walker and Mander51,Reference Katan, Deslypere and van Birgelen53,Reference von Schacky, Fischer and Weber55–Reference Blonk, Bilo and Nauta57) . Epidemiological evidence further suggests that n-3 PUFA supplementation with or without energetic restriction paradigms and/or exercise can not only positively affect body weight, fat mass and energy expenditure and thus reduce the risk of metabolic complications(Reference Buckley and Howe58,Reference Howe and Buckley59) , but can also reduce the risk of CVD by reducing systemic blood pressure, lowering TAG concentrations and improving the vascular function(Reference Innes and Calder21,Reference Shabrina, Tung and Nguyen60–Reference Marik and Varon65) .
Acknowledgements
The present study was funded by a grant from the Royal Society of New Zealand Marsden Fund to A. T.; A. L. received a University of Otago PhD scholarship. Mussel and Hoki oils were kindly provided by Aroma, Christchurch, NZ. The Marsden Fund or Aroma had no role in the design, analysis or writing of the present article.
A. T. and A. L. designed the experiments and analysed the data; A. L. and M. Z. R. performed the experiments; A. T. and A. L. wrote the paper; A. T. provided funding for the present study.
The authors declare no conflicts of interest.