Obesity has become a serious health problem during the past decades. According to the definition of WHO, those with a BMI of 25–29·9 and 30 kg/m2 or higher are regarded as individuals with overweight and obesity, respectively(1). Approximately one-third of the world’s population is overweight or obese(Reference Chooi, Ding and Magkos2). As such, the prevalence of overweight and obesity among Iranian adults is, respectively, 27–38·5 % and 12·6–25·9 %(Reference Jafari-Adli, Jouyandeh and Qorbani3).
The American Medical Association has recognised obesity as a complex chronic disease that is highly correlated with the metabolic syndrome (MetSyn)(Reference Pollack4). According to the definition of the International Diabetes Federation in 2009, the MetSyn is characterised by several cardiometabolic abnormalities including hypertension, reduced HDL-cholesterol and elevated TAG, increased fasting plasma glucose (FPG) levels, and a large waist circumference (WC)(Reference Alberti, Eckel and Grundy5). Having one component of the MetSyn can increase the risk of developing MetSyn and CVD later in life. A pooled analysis of thirty-four observational studies in 2017 reported that the global presence of MetSyn in young adults aged 18–30 years was 4·8–7 %(Reference Nolan, Carrick-Ranson and Stinear6). Prevalence of the MetSyn is indicated in approximately 25 % of all adults with raised prevalence in advanced ages(Reference Nolan, Carrick-Ranson and Stinear6). Between 27·46 % and 33·7 % of Iranian adults have been identified with MetSyn, which is much higher than their counterparts in developed countries(Reference Azizi, Salehi and Etemadi7,Reference Ghobadi, Rostami and Marzijarani8) . The high prevalence of obesity and MetSyn in Iran highlights the need for careful investigations to determine potential risk factors associated with obesity and cardiometabolic abnormalities.
The prevalence of vitamin D deficiency (25(OH)D of < 20 ng/ml) is high among Iranian populations(Reference Tabrizi, Moosazadeh and Akbari9). According to evidence, a low level of serum vitamin 25(OH)D concentration (serum 25(OH)D of 21–29 ng/ml) is related to obesity(Reference Golzarand, Hollis and Mirmiran10) and MetSyn(Reference Alsharairi11). Several observational studies have suggested an inverse association between serum 25(OH)D concentration and components of the MetSyn including BMI, FPG, WC, systolic blood pressure (SBP) and diastolic blood pressure, insulin levels, and homoeostasis model assessment of insulin resistance (HOMA-IR) index(Reference Gannagé-Yared, Chedid and Khalife12–Reference Wortsman, Matsuoka and Chen17).
A significant association has also been observed between serum vitamin D and levels of cardiorespiratory fitness (CRF), with higher serum 25(OH)D predicting better CRF(Reference Farrell and Willis18). CRF is used to evaluate a person’s ability to perform physical work and, in fact, is a proxy of the ability to transport inhaled oxygen to the mitochondria(Reference Ross, Blair and Arena19). Current evidence suggests that CRF is an important health indicator(Reference Lee, Artero and Sui20) and is inversely associated with obesity(Reference Ingle, Mellis and Brodie21,Reference Lee, Kim and Kang22) and MetSyn(Reference Farrell and Willis18,Reference Hong, Lee and Park23) .
Due to the high prevalence of obesity and MetSyn and lack of investigation around the interaction of CRF and vitamin D, the purpose of the present investigation was to assess the association of CRF, measured by VO2max, and serum vitamin D and their joint association with obesity and MetSyn. The current study is one of the first studies to explore the combined association of Vitamin D and CRF with MetSyn and obesity in Tehranian adults.
Materials and methods
Study population
The sample size was calculated based on the correlation between CRF and serum vitamin D levels(Reference Mowry, Costello and Heelan24). The correlation observed in the articles was 0·36. Taking into account the correlation coefficient with 95 % confidence and a maximum estimation error of 5 %, the sample size was 135. Since the objectives of the study are interaction, to increase the statistical power of the study, the sample size was doubled and 270 people will be registered for the study. In this cross-sectional study, individuals with ages ranged from 20 to 60 years (n 270) who were referred to the School of Nutritional Sciences and Dietetics at Tehran University of Medical Sciences were entered into the study. Individuals were invited to participate in the study through advertisements in the local media. Participants were generally healthy and free of medications and had no acute or chronic infection or inflammatory disease. Subjects were excluded if they used any medication or supplementation or were lactating or pregnant at the time of the study. The Ethical Committee of the Tehran University of Medical Sciences approved the study protocol (ethical approval ID: IR.TUMS.VCR.REC.1397·472). All subjects received information about the procedure of the study and then provided written informed consent before entering the study.
Demographic factors
Trained interviewers recorded data about age, sex (male and female), education (under diploma and diploma), marriage (single and married) and smoking status (never, quit, light, moderate and heavy smoker) by using pre-specified data extraction forms.
Physical activity
The generally validated International Physical Activity Questionnaire (IPAQ) was applied to evaluate physical activity (PA) levels(Reference Craig, Marshall and Sjöström25). PA levels were expressed as metabolic equivalent minutes per week (MET-min/week)(Reference Ainsworth, Haskell and Whitt26) and accordingly, subjects were classified into three groups as follows: no or low PA (< 3000 MET-min/week) and moderate and high PA (> 3000 MET-min/week).
Dietary assessments
In this study, we assessed the dietary intake of the participants by using a valid and reliable FFQ with 168 food items(Reference Mirmiran, Esfahani and Mehrabi27). Expert dietitians through face-to-face interviews have asked the frequency (daily, weekly, monthly and yearly) and amount of consumption of each food item during the past year. The portion size of each food consumed was converted to grams per d by household measures(Reference Ghaffarpour, Houshiar-Rad and Kianfar28). Dietary intake of energy and nutrients was estimated using Nutritionist IV software based on the US Department of Agriculture food composition database that has been modified for Iranian foods(Reference Haytowitz, Lemar and Pehrsson29).
Anthropometric measurements
The participants’ height was measured using a stadiometer with a sensitivity of 0·1 cm (Seca). Weight was measured by adult’s digital scales (808Seca) to the nearest 0·1 kg, with barefoot and in light clothing. BMI was calculated as weight (kilograms) divided by squared height (metres). WC was measured to the nearest 0·1 cm at the midpoint between the bottom of the rib cage and the top of the lateral border of the iliac crest with participants in the standing position at the end of a normal expiration. Quantities of fat mass and fat-free mass were measured using a standard impedance technique (InBody 270, Biospace). Blood pressure was measured twice, after at least 10–15 min rest period, on the right arm, in a seated position by the use of a digital barometer (BC 08, Beurer).
Biochemical assessments
Blood samples were collected in the morning after participants had been seated for 30 min and had fasted overnight (at least 12 h). The enzymatic method, based on colorimetry, and commercial kits (Pars Azmoun) with the automatic machines (Selecta E, Vitalab) were used for measuring the blood sugar and serum lipid values. FPG was measured by using a commercial kit (Pars Azmoun), based on the enzymatic pigmentation method (glucose oxidase). Phenol aminoanthidine cholesterol oxidase method was used for measuring serum HDL-C. Serum TAG was measured by using the enzymatic glycerol-3 phosphate oxidase enzyme phenytoin antiheroine method. All participants were tested on the same day. All samples were analysed for 25(OH)D and 1,25-(OH)2D3 concentrations by ELISA method using the following kits: 25-(OH) D ELISA kits (Monobind) with inter- and intra-assay CV of 10·4 to 11·5 %, respectively, and 1,25-(OH)2D3 ELISA kits (Crystal Day) with inter- and intra-assay CVs of 9·8 to 10·3 %, respectively. The lower detection limit of 25(OH)D and 1,25-(OH)2D3 were 4 ng/ml and 4·8 pmol/l, respectively.
Definition of obesity and metabolic syndrome
A BMI of 30 kg/m2 or higher was considered general obesity(1). The MetSyn was defined using a modification of the criteria presented by the International Diabetes Federation(Reference Alberti, Eckel and Grundy30). Participants with three or more of the following components were regarded as having the MetSyn: (1) serum TAG ≥ 150 mg/dl (1·7 mmol/l); (2) HDL-C < 40 mg/dl (1·0 mmol/l) in males and < 50 mg/dl (1·3 mmol/l) in females; (3) systolic blood pressure ≥ 130 and/or diastolic blood pressure ≥ 85 mmHg; (4) WC ≥ 102 cm in men and ≥ 88 cm in women and (5) FPG ≥ 100 mg/dl (5·6 mmol/l).
Assessment of diet quality
The traditional dietary approaches to stop hypertension (DASH) diet scoring was used to assess the dietary quality which was first specified by Fung et al. (Reference Fung, Chiuve and McCullough31). DASH score considers eight components including fruits, vegetables, nuts and legumes, whole grains, low-fat dairy products, Na, red and processed meats, and sweetened beverages. Iranian people do not consume whole grains, so this component was not considered to measure DASH. The scoring system is based on quintiles with the lowest consumption receiving one point and the top quintile receiving five points for healthy components. The scoring for Na, red and processed meats and sweetened beverages are reversely coded so that quintile 1 receives five points and quintile 5 receives one point. The overall score ranges from 8 (the lowest adherence)to 40 (the highest adherence).
Assessment of cardiorespiratory fitness
The maximum rate of oxygen consumed (VO2max) was measured using a treadmill and respiratory gas analyzer (Cortex Metabolizer 3B). The subjects warmed up for 5 min on the treadmill at a speed of 5 km/h, next the Bruce test was used to determine the VO2max, following standard procedures(Reference Lobo, Gianotti and Adiamah32). After completing the Bruce test, the subjects walked at a speed of 4 km/h to cool down for 3 min and were encouraged to perform 5 to 10 min of stretching. The conditions for test cessation were the heart rate up to 90 % of the maximum heart rate, a RER over 1·1 and having a plateau in oxygen intake, despite increases in exercise intensity. The CRF was expressed as VO2max and those in the above-median category (> 32 v. < 32) were considered to have CRF.
Statistical analyses
Before analysis, normality distribution was tested by applying Kolmogorov–Smirnov’s test. Values of quantitative and qualitative variables were reported, respectively, as mean and standard deviation and frequency (per cent) across tertiles of the 25(OH)D and 1,25(OH)D. The frequency of qualitative variables among serum vitamin D tertiles was compared using the χ 2 test. The means of quantitative variables were compared using the ANOVA test. The OR and 95 % CI of general obesity and MetSyn across tertiles of serum 25(OH)D, 1,25 (OH)D and CRF were estimated through binary logistic regression analysis in three target models: model 1 adjusted for age and sex, model 2 adjusted for age, sex and smoking status, and model 3 adjusted for age, sex, smoking status, education and PA. To investigate the potential interaction of vitamin D and CRF on obesity and MetSyn, we first divided each of them into two groups based on the median, then we combined each group of vitamin D with another group of CRF. Participants were subdivided according to the median age (under and above 33 years) and DASH score (under and above 24 points). Finally, the OR of obesity and MetSyn were estimated by binary logistic regression and were presented with 95 % CI. The Statistical Package for the Social Sciences (SPSS version 16; SPSS Inc.) was used for performing all statistical analyses. The statistical significance level was defined as P < 0·05.
Results
Baseline features
Subject characteristics within each tertile of serum 25(OH)D and 1,25(OH)D are shown in Table 1. The mean age of participants was 36·7 ± 13·2 years, of whom 33·4 % were men. There was a rising trend in age, weight, BMI and fat mass across tertiles of 25(OH)D, and values of occupation were statistically significant (P < 0·001). In the first tertile of 25(OH)D, the percentage of participants with a moderate level of PA (39·3 %) was higher than those with a low (35·7 %) and high level (25 %) of PA. This result can be also seen in the second tertile; by contrast, in the third tertile of 25(OH) D, the proportion of people with the low level of PA (41·6 %) was higher. Additionally, the percentage of participants with a moderate level of PA (47·7 %) in the first and third tertile of 1,25(OH) D were higher than the other two levels of PA. While in the second tertile of 1,25(OH) D, subjects with a low level of PA (40·7 %) showed a higher proportion. Values of biochemical variables, blood pressure, Vo2max, WC and fat-free mass did not differ within tertiles of 25(OH)D. There were no differences in terms of demographic characteristics, anthropometric measures, biochemical variables, Vo2max and blood pressure across tertiles of 1,25(OH)D. Also, there was an increasing trend in age and BMI across tertiles of 25(OH)D among people who had a low DASH score. There were no differences in terms of demographic characteristics, anthropometric measures, biochemical variables, Vo2max and blood pressure across tertiles of 1,25(OH)D (online Supplementary Table 1).
Table 1. Characteristics of the study participants across tertiles of 25(OH)D (ng/ml) and 1·25(OH)D(ng/ml)
(Number and percentages; mean values and standard deviations)
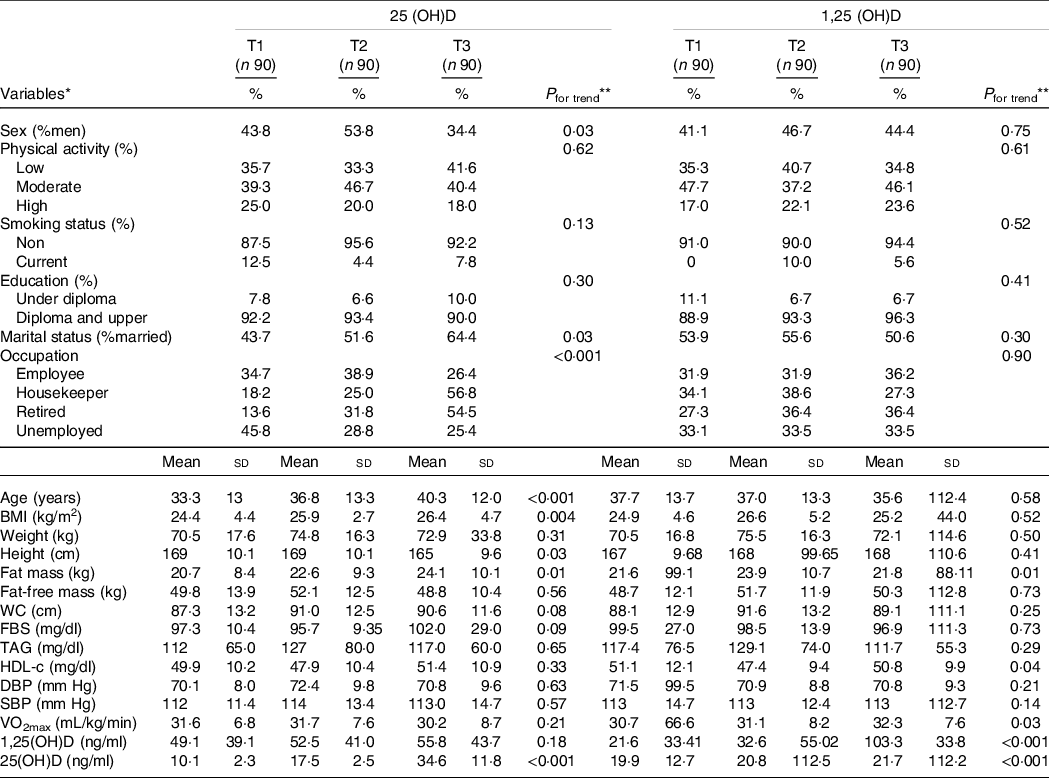
WC, waist circumference; FBS, fasting blood sugar; DBP, diastolic blood pressure; SBP, systolic blood pressure; VO2max, maximal oxygen uptake; T, tertile.
* Data are presented as n (%) for categorical variables and or mean ± standard deviation for continuous variables.
** The one-way ANOVA and the χ 2 test were used for comparison of continuous and categorical variables among tertiles of 25(OH)D (ng/ml) and 1,25(OH)D(ng/ml), respectively. P < 0·05 was considered significant.
Association of serum 25(OH)D and 1,25(OH)D with obesity and metabolic syndrome
Table 2 presents the results of the logistic regression analysis (OR and 95 % CI) for the association of serum 25(OH)D and 1,25(OH)D with obesity and MetSyn and its components in the crude and adjusted models. The results showed that there was no association between serum 25(OH)D and 1,25(OH)D and MetSyn and its components. There was also no association between serum 25(OH)D and general adiposity. However, those in the second tertile of 1,25(OH)D had a higher likelihood of having general obesity as compared with the first tertile either in the crude (OR: 3·01, 95 % CI 1·29, 7·00; P = 0·01) or in the fully adjusted model (OR: 3·37, 95 % CI 1·30, 8·94; P = 0·01). Those in the second tertile of 1,25(OH)D with a low DASH score had a higher likelihood of having general obesity as compared with the first tertile either in the crude (OR: 3·30, 95 % CI 1·07, 10·1; P = 0·03) or in the fully adjusted model (OR: 3·60, 95 % CI 1·01, 14·68; P = 0·05). Besides, those who were in the third tertile of 1,25(OH)D with a low DASH score were at more risk of having a low HDL serum concentration compared with the first and second tertile both in the crude (OR: 2·32, 95 % CI 1·06, 5·08; P = 0·03) and fully adjusted model (OR: 2·13, 95 % CI 1·01, 4·98; P = 0·05) (online Supplementary Table 2).
Table 2. Obesity and metabolic syndrome and its components in the tertile of vitamin D
(Odd ratio and 95 % confidence intervals)
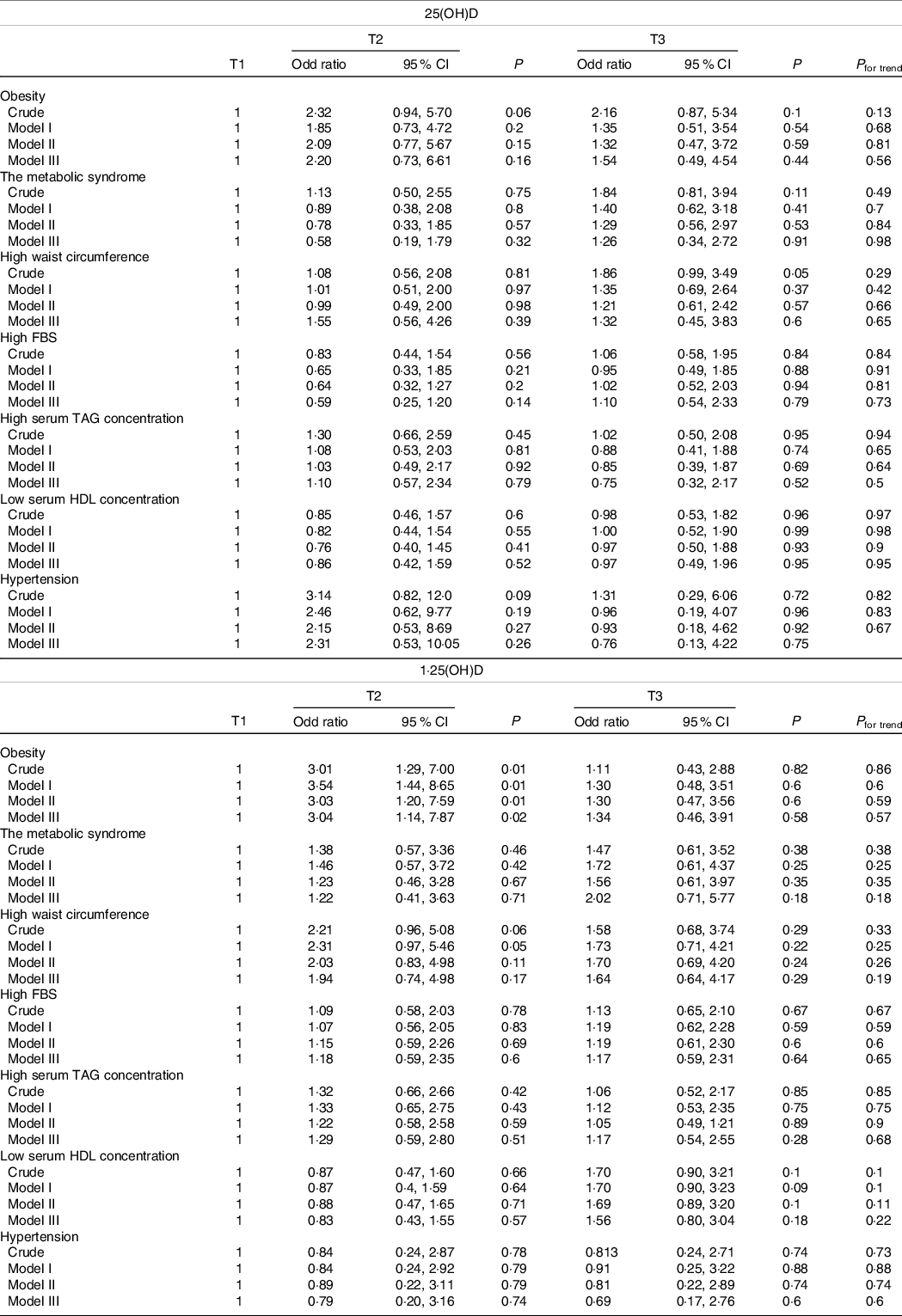
FBS, fasting blood sugar; T, tertile.
P for trend is obtained by logistic regression analysis.
Model I: adjusted for the effect of sex and age.
Model II: adjusted for the effect of sex, age and smoking.
Model III: adjusted for the effect of sex, age, smoking, physical activity, occupation and education. P < 0·05 was considered significant.
Association of cardiorespiratory fitness with obesity and metabolic syndrome
Table 3 illustrates the OR of obesity and MetSyn and its components in CRF tertiles. There was no association between CRF and MetSyn and its components. The exception was central obesity. The OR of central obesity for the second and third tertiles of CRF were, respectively, 0·44 (95 % CI 0·22, 0·87; P = 0·02) and 0·12 (95 % CI 0·05, 0·32; P < 0·001) in the maximally adjusted model that controlled for age, sex, smoking, education and PA (P for trend = 0·001). This was also the case for general adiposity, where being the second and third tertile of CRF was associated with a 75 % and a 93 % lower likelihood of general obesity by BMI, respectively (P for trend < 0·001). There was also a significant positive association between CRF and increased FPG.
Table 3. Association between cardiorespiratory fitness and obesity and components of the metabolic syndrome
(Odds ratio and 95 % confidence intervals)
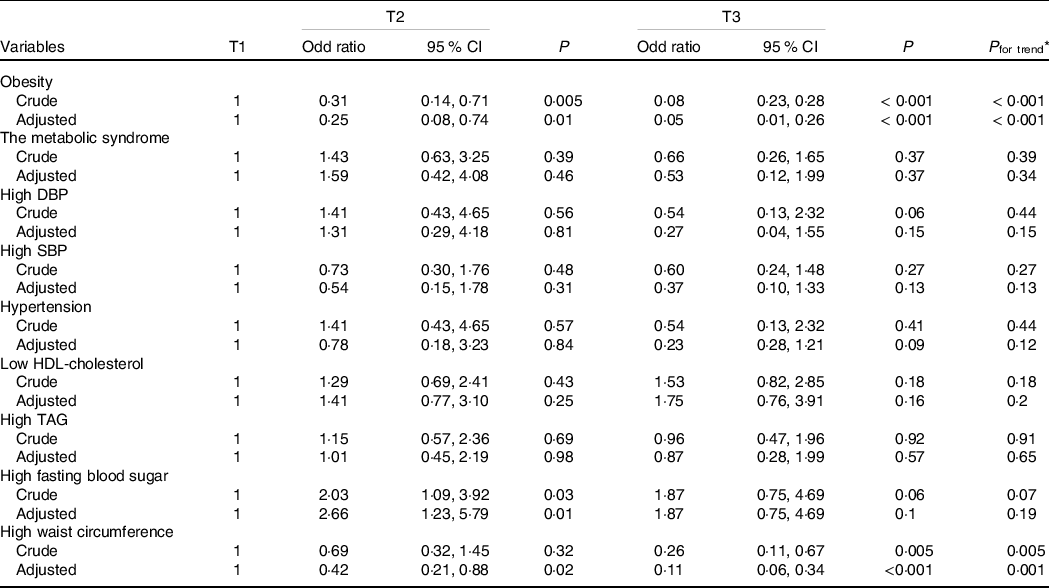
DBP, diastolic blood pressure; SBP, systolic blood pressure.
* Obtained by logistic regression analysis, adjusted for age, sex, smoking, physical activity, occupation and education.
Joint association of vitamin D and cardiorespiratory fitness with obesity and metabolic syndrome
Table 4 presents the joint association of serum vitamin D levels and CRF with obesity. The results showed that those with high CRF, either with low or high serum 1,25(OH)D, had a lower chance of having general obesity by BMI. The OR of general obesity was 0·23 (95 % CI 0·06, 0·91; P = 0·04) for those with high CRF and low serum 1,25(OH)D, and 0·12 (95 % CI 0·02, 0·68; P = 0·02) for those with high CRF and high serum 1,25(OH)D, as compared with those with low CRF and low serum 1,25(OH)D. There was no association between high serum 1,25(OH)D and low CRF with general obesity. Table 4 presents the joint association of serum vitamin D levels and CRF with obesity. Also, those who had a low DASH score with high CRF, with low serum 1,25(OH)D, had a lower chance of having general obesity by BMI. The OR of general obesity was 0·09 (95 % CI 0·04, 0·58; P = 0·04) for those with a high CRF and low serum 1,25(OH)D, as compared with those with low CRF and low serum 1,25(OH)D. There was no association between high serum 1,25(OH)D and low CRF with general obesity (online Supplementary Table 3).
Table 4. Combined association of serum vitamin D and CRF with obesity and metabolic syndrome
(Odds ratio and 95 % confidence intervals)
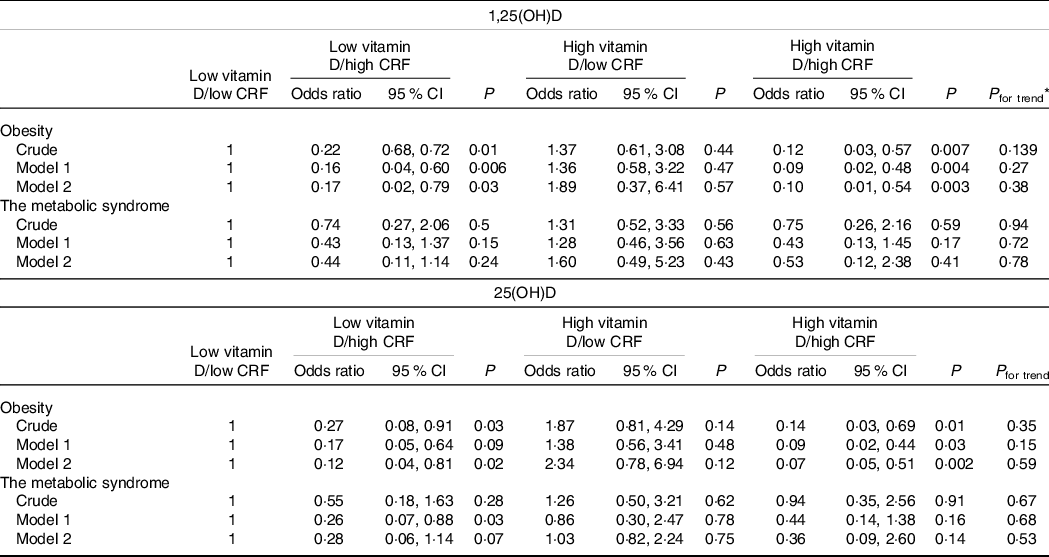
CRF, cardiorespiratory fitness.
* Obtained by logistic regression analyses. Model 1 adjusted for age and sex. Model 2 additionally adjusted for physical activity, smoking, occupation and education.
Similar findings were observed for the joint association of serum 25(OH)D and CRF with general obesity. There was a significant inverse association for those with low serum 25(OH)D and high CRF (OR: 0·17, 95 % CI 0·05, 0·64; P = 0·009) compared with those with low serum 25(OH)D and low CRF in the model that controlled for age and sex. However, the association became non-significant when we additionally controlled for education, smoking and PA. There was also a strong inverse association between high serum 25(OH)D and high CRF with obesity (OR: 0·13, 95 % CI 0·02, 0·75; P 0·02) compared with those with low serum 25(OH)D and low CRF in the fully adjusted model. There was no association between high serum 25(OH)D and low CRF with general obesity. There was a significant inverse association among those who had a low DASH score with low serum 25(OH)D and high CRF (OR: 0·11, 95 % CI 0·01, 0·98; P = 0·04) compared with those with low serum 25(OH)D and low CRF in the crude model. This inverse association remained in adjusted models. There was no association between high serum 25(OH)D and low/high CRF with general obesity (online Supplementary Table 3).
Table 4 also indicates the joint association of serum 25(OH)D and 1,25(OH)D and CRF with MetSyn according to DASH score. The analyses indicated that there was no significant association between high CRF, neither with high nor low serum 25(OH)D and 1,25(OH)D, with MetSyn compared with those with low CRF, either with low 25(OH)D or low 1,25(OH)D. Besides, there was no association between low CRF, neither with high 25(OH)D nor high 1,25(OH)D, with MetSyn compared with those with low CRF and low serum 25(OH)D and 1,25(OH)D.
Supplementary Table 4 presents the joint association of serum 25(OH)D and 1,25(OH)D and CRF with obesity according to age. The OR of general obesity for whom were under 33 years was 8·52 (95 % CI 1·02, 70·93; P = 0·04) with low CRF and high serum 25(OH)D in the fully adjusted model, as compared with those with low CRF and low serum 25(OH)D. There was no association between other groups with general obesity. Also, the OR of general obesity for those were above 33 years was 0·19 (95 % CI 0·03, 0·96; P = 0·04) with high CRF and low serum 25(OH)D after adjusting for sex and age. The analyses showed that there was no significant association between high CRF, neither with high nor low serum 25(OH)D and 1,25(OH)D, with MetSyn compared with those with low CRF, either with low 25(OH)D or low 1,25(OH)D among both groups under and above 33 years.
Discussion
To the best of our knowledge, the current study is the first study of its kind assessing the individual and joint association of serum vitamin D and CRF with general and abdominal obesity and MetSyn in adults. The present study indicated that there was no association between serum 25(OH)D and 1,25(OH)D and the likelihood of general adiposity and MetSyn and its components in adults. The exception was central adiposity for which the results showed that those in the second tertile of serum 1,25(OH)D had a higher odd of having central adiposity by WC. There was no association between serum 25(OH)D and central adiposity. We also investigated the association of CRF with obesity and MetSyn. The results showed that there was a strong inverse association between CRF and general and abdominal adiposity. However, our results failed to show any significant association between CRF and MetSyn. The joint association of vitamin D and CRF suggested a potential effect modification by vitamin D status, in a way that the inverse association of CRF with obesity was stronger in those with high serum vitamin D than those with low serum vitamin D.
The results of the present study showed that serum levels of 25(OH)D were not related to obesity; however, we observed that 1,25(OH)D was positively strongly associated with obesity. Previous studies suggested an inverse association between serum vitamin D and obesity(Reference Pereira-Santos, Costa and Assis33). This can be partially explained by the dilution of intracutaneously synthesised or ingested vitamin D in adipose tissue and less sunlight exposure because of less participation in outdoor activities(Reference Pourshahidi34). Baradaran et al. reported that there was no significant association between serum vitamin D levels and BMI in Iranian adolescents(Reference Baradaran, Behradmanesh and Nasri35). Besides, it is not exactly determined whether vitamin D deficiency is a cause or consequence of adiposity(Reference Vranić, Mikolašević and Milić36). The inconsistent results found for 25(OH)D and 1,25 (OH)D may be due to the effects of inflammatory status on levels of 25(OH)D and 1,25(OH)D. Adiposity is generally associated with low-grade systemic inflammation(Reference Irandoost, Ebrahimi-Mameghani and Pirouzpanah37). There is evidence that levels of 25(OH)D in some inflammation-related conditions decreases and by contrast, levels of 1,25(OH)D increase due to its production in the mitochondria of active macrophages(Reference Waterhouse, Marshall and Fenter38). This may potentially explain the inconsistent associations of 25(OH)D and 1,25(OH)D with adiposity.
In this study, most subjects among vitamin D tertiles had moderate to low levels of PA. Obese people usually have a low level of PA that leads to less sunlight exposure due to less outdoor activities and higher chance of vitamin D deficiency(Reference Chooi, Ding and Magkos2). On the other hand, there is a hypothesis that vitamin D deficiency resulting from having less sunlight exposure and outdoor activities leading to obesity. So, it cannot conclude that whether a low level of outdoor activities leads to vitamin D deficiency and obesity or obesity leads to a sedentary lifestyle and vitamin D deficiency.
In this cross-sectional study, we did not found any significant association between serum 25(OH)D and 1,25(OH)D with MetSyn and its components in adults. Similar to our study, In a Korean cross-sectional study, no association was observed between low levels of serum vitamin D and MetSyn(Reference Kim, Lim and Kye39). Another study presented that serum 25(OH)D was not a significant predictor of MetSyn in Africans and Asians who lived in India(Reference George, Norris and van Deventer40). Ghobadi et al. found no relationship between serum 25(OH)D and odds of MetSyn in Iran(Reference Ghobadi, Rostami and Marzijarani8). Also, no statistically significant relationships were reported between circulating vitamin D3 levels and components of MetS in premenopausal women in Poland(Reference Wieder-Huszla, Jurczak and Szkup13). By contrast, a Korean study among postmenopausal women demonstrated a significant positive relationship between low levels of serum vitamin D3 and MetSyn and some of its components such as hypertriacylglycerolaemia and hypertension(Reference Song and Park41). Another population-based cohort study of 3240 middle-aged and elderly adults in the Netherlands showed a positive association between vitamin D deficiency and odds of MetSyn(Reference Vitezova, Zillikens and Van Herpt42). Vitamin D deficiency is associated with a series of cardiometabolic abnormalities that may link it with MetSyn(Reference Miñambres, Sanchez-Quesada and Pérez43). Inconsistent findings across studies might be due to different clinical features and demographics of the study populations and the small number of study participants in the present study.
In this cross-sectional study, we observed a significant inverse association between high CRF with general and abdominal obesity. In a Finnish cross-sectional study, CRF was inversely associated with general and abdominal obesity, with the level of CRF was more closely related to WC than with BMI(Reference Fogelholm, Malmberg and Suni44). Our observation was similar to that of a cross-sectional study in South Asia among postmenopausal women, in which VO2 peak was negatively associated with BMI and WC(Reference Lesser, Dick and Guenette45). Low CRF can be a consequence of a sedentary lifestyle. Low PA levels can lead to a positive energy balance and thereby can increase fat stores in the body. This may partly explain the inverse association between CRF and obesity observed in the present study.
The present study also suggested that there was no association between CRF and MetSyn. In agreement with our study, it has been reported that CRF was not correlated with any individual components of the MetSyn after adjusting for sex, age and body composition in overweight Latino youths(Reference Shaibi, Cruz and Ball46). However, in contrast to our study, Hassinen et al. found that CRF was strongly negatively related to the risk of MetSyn in a population-based sample of the elderly(Reference Hassinen, Lakka and Savonen47). A cohort study between the US firefighters demonstrated strong inverse associations between MetSyn and CRF(Reference Baur, Christophi and Kales48). The null association between CRF and MetSyn observed in our study may be due to the small sample size that might decrease the power of the statistical analyses.
There is evidence that there might be an association between CRF and serum vitamin D status(Reference Ardestani, Parker and Mathur49). This association strengthens with diet quality in a way that the risk of obesity was more in subjects who intake components that had low quality. According to several studies, overall diet quality is most likely to be a significant component of the diet–obesity relationship(Reference Wolongevicz, Zhu and Pencina50–Reference Quatromoni, Pencina and Cobain52). A probable clarification is that subjects with the poorest diet quality intake diets were lower in energy, carbohydrate and micronutrients, and other healthy components and higher in total fat, particularly saturated fat and processed meat(Reference Wolongevicz, Zhu and Pencina50). A possible explanation is that CRF relates to daily PA, which could be connected to sunlight exposure and hence to the synthesis of vitamin D. On the other hand, low vitamin D levels can decline cardiac output and raise peripheral vessel resistance, leading to reduced VO2max(Reference Ardestani, Parker and Mathur49). Besides, vitamin D deficiency can lead to myocardial hypertrophy, increased blood pressure and endothelial dysfunction, all of which can diminish CRF(Reference Zittermann, Schleithoff and Tenderich53). On the other hand, this interaction can be influenced by ageing. It may be because of different factors including hormonal changes during ageing, decreasing RMR by 2–3 % per decade after the age of 20 years and changing in body composition(Reference Michalakis, Goulis and Vazaiou54). Our results also indicated that values of Vo2max increased proportionally across tertiles of serum 1,25(OH)D. The joint association of vitamin D and CRF suggested a potential effect modification by vitamin D status, in a way that the inverse association of CRF with obesity was stronger in those with high serum vitamin D than those with low serum vitamin D among older people who intake low diet quality.
To our knowledge, this is the first study to examine the individual and joint association of vitamin D status and CRF with obesity, MetSyn and its components in a sample of young adults. The strengths of this study can be pointed to the assessment of biochemical factors, anthropometric and metabolic parameters together. We also used valid tools and procedures to measure Vo2max. In addition, we measured both 25(OH)D and 1,25(OH)D to present a more precise estimation of serum vitamin D status. We used ELISA method to measure vitamin D status. Among the existing methods, LC-MS/MS or a ligand-binding assay (such as an immunoassay platform like a competitive ELISA or a competitive chemiluminescent immunoassay, or competitive receptor-binding assays, the LC-MS/MS method is generally considered to be the most accurate one for the measurement of serum 25(OH)D levels(Reference Wallace, Gibson and De La Hunty55). Nevertheless, the LC-MS/MS techniques need expensive equipment, large plasma sample volume and specialized staff. These difficulties make the commercial ELISA the most popular method for the measurement of plasma 25(OH)D concentration(Reference Zerwekh56).
This study would also benefit from extensive collected data such as behavioural variables and social background characteristics, which could affect the outcomes. However, this study had limitations. Due to cross-sectional design, longitudinal analyses are necessary to draw more definitive conclusions about the joint association of vitamin D status and CRF with obesity and MetSyn. Also, this is a cross-sectional study and as with all observational studies, true causality is impossible to capture with observational studies. Besides, the relatively small sample size of the present study diminished the power of the study. The target population in this study was apparently healthy adults. Participants were volunteers and invited using advertisements in the local media. Thus, our participants may not be a true representative of the whole population. Moreover, we mentioned our recruitment method as a limitation in the text. Also, our results may have been affected by selection bias. However, to minimise the bias, we tried to adjust for factors that may affect outcomes. In this study, most participants among vitamin D tertiles had moderate to low levels of PA, and these levels of PA were calculated according to IPAQ so outdoor activity was not measured.
Conclusion
This cross-sectional study provided evidence for the individual and joint association of vitamin D status and CRF with obesity and MetSyn. This study showed that there was no association between serum vitamin D levels, neither 25(OH)D nor 1,25(OH)D, with general obesity and MetSyn. We found a strong inverse association between CRF and the likelihood of general and abdominal obesity. There was also no association between CRF and MetSyn. More research with larger sample size and with prospective design is needed to fully investigate the joint association of vitamin D status and CRF with obesity and MetSyn.
Acknowledgements
The authors thanks all those who participated in this study.
This manuscript has been granted by the Tehran University of Medical Sciences (Grant No: 33887). The funder had no role in the design, analysis or writing of this article.
S. S. B. and K. D. J. contributed to the conception anddesign of the research; Z. N. and H. M. contributed to the acquisition of data; M. F. and F. D. F. participated in the analysis and interpretation of the data; M. F. drafted the manuscript; and A. J. critically revised the manuscript; S. S. B. agrees to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
The authors declare that they do not have any conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521003196









