The physical, chemical, sensory and nutritional properties of goat meat at the point of human consumption are influenced by producers, marketers and processors(Reference Casey and Webb1). In many countries, fat is an unpopular constituent of meat for consumers, being considered unhealthy(Reference Wood, Enser and Fisher2). On the other hand, the costly deposition of fat besides the effect on product market value represents a waste of dietary energy(Reference Banskalieva, Sahlu and Goetsch3). The fatty acid (FA) composition of meat usually has little influence on market value of a carcass, whereas the quantity of fat is of greater importance. However, the physical and chemical properties of lipids affect eating, flavor and storing qualities of meat(Reference Wood, Enser and Fisher2), although the composition of meat FA has little influence on carcass market value relative to fat quantity. The manipulation of meat FA profile is becoming a more important consideration because it is related to differences in the nutritional value for human consumption(Reference Shingfield, Bonnet and Scollan4). SFA increase hardness of fat because they are easily solidified upon cooling, which negatively influences meat palatability(Reference Wood, Enser and Fisher2, Reference Banskalieva, Sahlu and Goetsch3). From the human nutrition point of view, enhancing the unsaturated FA (UFA) content of meat, at the cost of SFA, translates into a healthier product. Dietary intake of SFA has been associated with elevated serum cholesterol levels and increased risk of CVD in humans(Reference Shingfield, Bonnet and Scollan4).
Ruminant meat products are, however, important natural dietary sources of conjugated linoleic acids (CLA), which is of interest for human health. In particular, the cis-9, trans-11-CLA isomer has been shown to possess anticarcinogenic properties(Reference Shingfield, Bonnet and Scollan4). CLA isomers are produced by incomplete biohydrogenation of linoleic acid in the rumen. The cis-9, trans-11-CLA may also be made endogenously in ruminant tissues from vaccenic acid (trans-11-18 : 1) by stearoyl-CoA desaturase(Reference Bauman, Baumgard and Corl5). Furthermore, the CLA content of meat can be increased by several dietary strategies, for example, adding CLA to the diet of the animal(Reference Gillis, Duckett and Sackmann6–Reference Schlegel, Ringseis and Shibani10). To prevent biohydrogenation of these biologically active molecules, CLA must be supplied in rumen-protected forms.
There is considerable interest in adding rumen-protected CLA (rpCLA) in animal feeds in the expectation that they may improve production efficiency and meat quality for its distributive effect between fat and lean(Reference Mersmann11). Furthermore, they are incorporated into meat for providing value-added ‘healthful’ meat products for human consumption(Reference Bauman, Baumgard and Corl5). Many studies have focused on manipulating the FA composition and fat content of goat’s meat(Reference Najafi, Zeinoaldini and Ganjkhanlou12–Reference Turner, Cassid and Zerby15) The trans-10, cis-12 is another isomer of CLA that affects lipid metabolism(Reference Pariza16). Reduced body fat, increased lean tissue growth and meat FA alteration were reported in several species, including growing lambs(Reference Wynn, Daniel and Flux9), beef heifers(Reference Gillis, Duckett and Sackmann6, Reference Gillis, Duckett and Sackmann8, Reference Schlegel, Ringseis and Shibani10), beef bulls(Reference Schiavon, Marchi and Tagliapietra17) and dairy bulls(Reference Gómez, Mendizabal and Sarriés18) after dietary supplementation with a mixture of the cis-9, trans-11-CLA and trans-10, cis-12-CLA isomers. Fast growing ruminants have protein requirements that exceed the amount provided by microbial protein, and they might, therefore, benefit from supplemental dietary protein with low ruminal degradability(19). For example, growing lambs fed a diet containing high rumen undegradable protein (RUP) content (289 g/kg of dietary crude protein (CP)) had higher growth performance compared with lambs fed a diet with medium (229 g/kg of dietary CP) or low (161 g/kg of dietary CP) RUP content(Reference Haddad, Mahmoud and Talfaha20). Rumen-protected CLA (rpCLA) has been proposed to exert some protein-sparing effects(Reference Pariza, Park and Cook21). These effects might be more evident with ruminants having high protein requirements(Reference Schiavon, Tagliapietra and Dalla Montà22). Furthermore, von Soosten et al.(Reference von Soosten, Meyer and Piechotta23) found that rpCLA exerted a protective effect against excessive use of body reserves in early lactating primiparous Holstein cows, and increased protein accretion, and they suggested a role of CLA in metabolic mechanisms of nitrogen partition in different body functions. In addition, increased protein accretion was reported in growing bulls fed dietary rpCLA(Reference Schiavon, Tagliapietra and Dalla Montà22). The possible CLA effects on protein anabolism might be mediated by anabolic hormones like insulin-like growth factor-1 (IGF-I)(Reference Lucy, Jiang and Kobayashi24). The high lean tissue deposition and the low body fat deposition in CLA-treated growing animals may require an increase in dietary protein quality to maintain protein synthesis. On other hand, increasing dietary RUP level probabaly increased duodenal flow of essential amino acids for supporting higher growth requirements. To our knowledge, no information is available on the effects of dietary RUP level, rpCLA supplementation and their interaction on meat quality in growing goat kids in terms of fatness, FA profile and CLA content. We hypothesised that supplementing rpCLA in diets of goat kids containing higher level of dietary RUP not only would improve meat FA profile but would also reduce its fat content. Therefore, the objective of the present study was to determine the effect of dietary RUP and rpCLA level and their interaction on meat quality of growing goat kids.
Methods
Animal welfare
All procedures including animal welfare, husbandry and experimental procedures were evaluated and approved by the Institutional Animal Care and Ethics Committee of the Iranian Council of Animal Care(25).
Experimental design and animal management
Thirty-two (4 months old; sixteen males and sixteen females) Iranian native Kurdish goat kids (13·06 ± 1·08 kg body weight (BW)) were balanced by sex and BW, and then randomly allocated to one of four experimental diets (eight animals per group) in a factorial design. Diets were formulated differing in RUP density (low RUP (LR): RUP = 250 g/kg of dietary CP or high RUP (HR): RUP = 350 g/kg of dietary CP) and either supplemented (on DM basis) with 15 g/kg rpCLA or 12 g/kg hydrogenated soyabean oil (HSO) resulting in four experimental diets: LR-HSO, LR-rpCLA, HR-HSO and HR-rpCLA (Table 1).
Table 1. Ingredients and chemical composition of experimental diets fed to growing goat kids
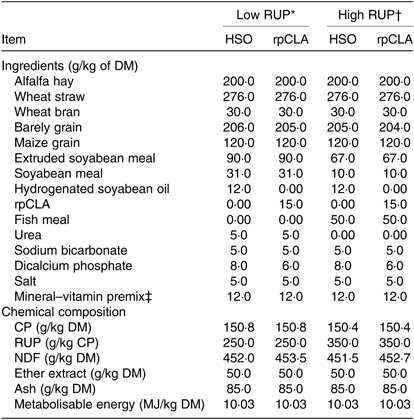
RUP, rumen undegradable protein; HSO, hydrogenated soyabean oil; rpCLA, rumen-protected conjugated linoleic acid; CLA, conjugated linoleic acid; CP, crude protein; NDF, neutral-detergent fibre.
* Diets containing low RUP level (250 g/kg CP).
† Diets containing high RUP level (350 g/kg CP).
‡ Each kg of the premix contains: vitamin A, 500000 IU; vitamin D3, 100000 IU; vitamin E, 100 mg; Ca, P, Mg, Na, Mn, Fe, Cu, Zn, Co, iodine and Se, 180 000, 90 000, 19 000, 60 000, 2000, 3000, 300, 3000, 100, 100 and 1 mg, respectively.
Animals were individually housed in pens (1 × 2 m). The total period of the experiment was 100 d with 20 d for animals’ adaptation to pens and 80 d for data collection. On days 1 and 15 of the adaptation period, all kids received anthelmintic drugs (Ivermectin drench, 0·2 mg/kg BW; Albendazole boluses, 5 mg/kg BW; Damloran Pharmaceutical Co.) and were vaccinated against enterotoxaemia (Razi Institute).
The experimental diets were formulated to be isonitrogenous and isoenergetic(19). Dietary RUP content was increased from 250 to 350 g/kg of dietary CP by replacing extruded soyabean seed, soyabean meal and urea with fish meal. CP degradability of feedstuffs was measured by the nylon bags technique(26). Diet metabolisable energy content was calculated based on standard tables(19). The rpCLA supplement (Lutrell, BASF) consisted of methyl esters of CLA bound to a silica matrix and was composed of 785 g/kg of lipid, 200 g/kg of ash, and 15 g/kg of moisture. The lipid portion contained 100 g/kg of cis-9, trans-11-CLA; 100 g/kg of trans-10, cis-12-CLA; 140 g/kg of sunflower oil and 445 g/kg of stearic acid and palmitic acid. The HSO was composed of 990 g/kg lipid and 10 g/kg moisture. The lipid portion contained 878 g/kg of FA, almost exclusively represented by stearic and palmitic acids. The FA profile of experimental diets is shown in Table 2. The diets were offered ad libitum three times daily, and animals had free access to water. Experimental diets were sampled weekly, stored at –18°C and composited at the end of study pending further analyses. Samples of diets were dried in a forced air oven at 55°C for 72 h, ground to pass through 1-mm screen using a Wiley mill (Thomas Scientific) and analysed in duplicate according to Association of Official Analytical Chemists(27) for DM (method 934.01), CP (method 976.05), ash (method 942.05) and ether extract (method 973.18). Neutral-detergent fibre was determined using sodium sulphite, but without amylase, and expressed inclusive of residual ash(Reference Van Soest, Robertson and Lewis28).
Table 2. Fatty acid (FA) composition of experimental diets fed to growing goat kids
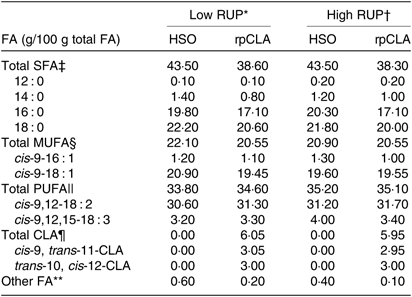
RUP, rumen undegradable protein; HSO, hydrogenated soyabean oil; rpCLA, rumen-protected conjugated linoleic acid; CLA, conjugated linoleic acid; CP, crude protein.
* Diets containing low RUP level (250 g/kg CP).
† Diets containing high RUP level (350 g/kg CP).
‡ Total SFA = 12 : 0 + 14 : 0 + 16 : 0 + 18 : 0.
§ Total MUFA = cis-9-16 : 1 + cis-9-18 : 1.
|| Total PUFA = cis-9,12-18 : 2 + cis-9,12,15-18 : 3.
¶ Total CLA = cis-9, trans-11-CLA + trans-10, cis-12-CLA.
** Unidentified FA.
DM intake, average daily gain and feed efficiency
The amount of both offered and refusal feed was recorded daily before the morning feeding. During the experiment, the kids were weighed individually, and average daily gain (ADG), DM intake (DMI) and feed efficiency (FE) were determined.
Slaughter and carcass measurements
At the end of feeding period, all animals were weighed after an overnight fast (12 h). After slaughter, the non-carcass parts of the body were removed, and hot carcass and internal fat including peritoneal, omental and mesenteric fats were weighed. Then, the carcass was split into two equal halves (left and right). The right half of carcass was cut into five primal cuts including neck, shoulder, breast-flank, loin and leg(27, Reference Colomer-Rocher, Morand-Fehr and Kirton29). The cuts were weighed and expressed as a percentage of the total weight of the right half of the carcass. Dressing percentage was calculated by dividing hot carcass weight by final BW for each animal.
Meat quality measurements
All meat quality measurements were made on the left half of the carcass. Colour parameters of the Longissimus dorsi (LD) muscle was measured using a Hunter Lab colorimeter (Hunter Laboratories, model DP-9000) using a 2° observation angle, D25 illuminant and 4·5-cm aperture on five anatomical positions, and the mean was taken as final value(Reference Gómez, Mendizabal and Sarriés18). Meat colour was expressed according to the CIE-Lab colour space by reporting L* (lightness), a* (redness) and b* (yellowness) values(30). Samples (100 g) of LD of each animal were obtained and stored at –40°C until analysis. Samples were thawed at 4°C and ground with a hand grinder until they were completely blended. Subsamples were analysed in duplicate for DM, CP and total lipid content(27). The ash content was calculated by subtracting the percentages of CP, fat and moisture from 100(Reference Schiavon, Marchi and Tagliapietra17).
Fatty acid analysis
The FA composition of LD muscle was determined after extraction of total lipids in accordance with Folch et al.(Reference Folch, Lees and Sloane Stanley31). Briefly, a 0·5-g of LD sample (duplicate) was placed in a 5-ml screw-top test tube, 2 ml of methanolic potassium hydroxide (2 M) were added, and then, the tube cap was tightened and the tube was vigorously shaken for 2 s. Then, 2 ml of hexane were added and the tube was shaken for 5 s. Then, the tube was placed in an ultrasonic bath for 15 min at 35°C. The upper layer was separated and passed through a filter (0·45 m) containing sodium sulfate anhydrous, and then, 1 µl of filtrate was injected into a GC (Youngling 6100). The GC was equipped with a J&W CP-Sil 88 fused silica capillary column (100 m × 0·25 mm, 0·20 m film thickness, Agilent Technologies). The temperatures of injector and detector ports were set at 270°C and 300°C, respectively. The FA composition was analysed by an isotherm program. The column temperature was held at 175°C for 60 min. Nonadecanoic acid (C19 : 0) was used as an internal standard to quantify the individual and total fatty acid methyl esters (FAME). The identification of individual FAME was based on a standard mixture of 37 Component FAME Mix (Sigma–Aldrich, Supelco-18919-1AMP, F.A.M.E. Mix, C4-C24) and sixty individual FAME standards (Sigma–Aldrich). The identification of CLA isomers was based on co-injection with commercial standard mixtures (Sigma–Aldrich). FA were expressed as g/100 g of total FAME.
Calculations
Total SFA was calculated as the sum of 10 : 0, 12 : 0, 14 : 0, 16 : 0, 17 : 0, 18 : 0, 20 : 0 and 22 : 0. Total MUFA was considered as the sum of cis-9-14 : 1, cis-9-16 : 1, cis-9-17 : 1, cis-9-18 : 1 and trans-11-18 : 1. Total PUFA was the sum of cis-9,12-18 : 2 and cis-9,12,15-18 : 3. Total CLA was calculated as the sum of cis-9, trans-11-CLA and trans-10, cis-12-CLA. Desaturase indexes based on 14 carbon, 16 carbon, 18 carbon and CLA were computed as (cis-9-14 : 1/(14 : 0 + cis-9-14 : 1)), (cis-9-16 : 1/(16 : 0 + cis-9-16 : 1)), ((cis-9-18 : 1/(18 : 0 + cis-9-18 : 1)) and (cis-9, trans-11-CLA/(trans-11-18 : 1 + cis-9, trans-11-CLA)), respectively(Reference Schiavon, Marchi and Tagliapietra17).
Statistical analysis
Sample size calculations of eight animals per group were determined based on previous studies(Reference Abijaoudé, Morand-Fehr and Tessier32–Reference Koluman, Boga and Silanikove34), with a statistical power of 0·8. Data (performance, carcass parameters, meat proximate analyses, colour measurement and meat FA profile) were statistically analysed as a 2 × 2 factorial arrangement based on a randomised complete block design using PROC MIXED of SAS 9.4(35) with the following model:
where Y ijkl is dependent variable; μ is the overall mean; RUPi is effect of dietary RUP level (HR v. LR); rpCLAj is the effect of dietary rpCLA level (HSO v. rpCLA); (RUP × rpCLA)ij is the interaction of RUP and rpCLA; B k is the block effect of kid sex; A l (B k) is the random effect of kid within the block; and ε ijkl is the random residual error. Significant differences among treatments were tested using least-squares means with the probability difference option with significance declared at P ≤ 0·05, and trends at 0·05 < P ≤ 0·10.
Results
Performance
Effect of experimental diets on DMI, ADG and FE are shown in Table 3. Goat kids fed the HR-rpCLA diet had the lowest DMI and the greatest ADG and FE compared with the other groups (P<0·05).
Table 3. Effect of dietary rumen undegradable protein (RUP) and rumen-protected conjugated linoleic acid (rpCLA) level on performance and carcass parameters of growing kids
(Mean values with pooled standard errors)
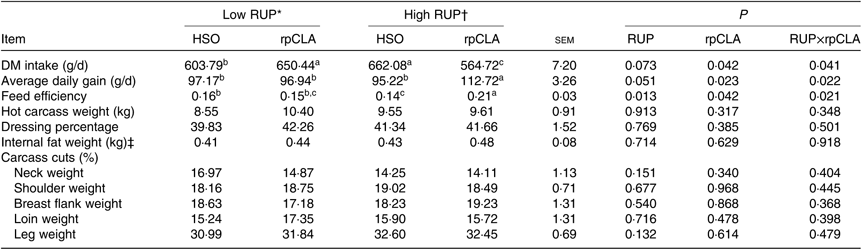
HSO, hydrogenated soyabean oil; CP, crude protein.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Diets containing low RUP level (250 g/kg CP).
† Diets containing high RUP level (350 g/kg CP).
‡ Internal fat included the sum of peritoneal, omental and mesenteric fats.
Carcass components
Dietary RUP level, rpCLA supplementation and their interaction had no effect on hot carcass weight, dressing percentage, internal fat weight and carcass cut percentage (P > 0·05; Table 3).
Meat chemical composition and colour parameters
Table 4 presents the effect of dietary RUP level, rpCLA supplementation and their interaction on meat chemical composition and colour parameters of growing kids. Meat moisture, protein and ash contents were not affected by dietary RUP and rpCLA interaction, while the lowest fat content was observed in meat of kids fed HR-rpCLA compared with the other groups (P < 0·01). Regardless of dietary fat supplementation, meat ash (11·2 v. 9·5 g/kg) and protein (221·8 v. 206 g/kg) contents were higher while moisture (744·6 v. 756·3 g/kg) and fat (22·35 v. 28·25 g/kg) contents were lower in kids fed the HR diets than those fed the LR diets (P < 0·01). Dietary rpCLA supplementation had no effect on meat moisture and protein contents of growing goat kids (P > 0·05), whereas rpCLA-fed kids had the higher meat ash (12·6 v. 8·1 g/kg) and the lower meat fat (21·9 v. 28·65 g/kg) contents compared with HSO-fed kids (P < 0·01). The redness (a*), yellowness (b*) and lightness (L*) values of meat were not affected by dietary RUP level, rpCLA supplementation and their interaction (P > 0·05).
Table 4. Effect of dietary rumen undegradable protein (RUP) and rumen-protected conjugated linoleic acid (rpCLA) level on meat chemical composition and colour characteristics of growing goat kids
(Mean values with pooled standard errors)
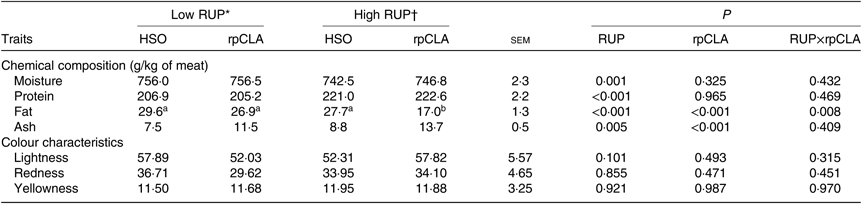
HSO, hydrogenated soyabean oil; CP, crude protein.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Diets containing low RUP level (250 g/kg CP).
† Diets containing high RUP level (350 g/kg CP).
Meat fatty acid profile
Effect of dietary RUP level, rpCLA supplementation and their interaction on meat FA concentration in growing kids is shown in Table 5. The concentrations of total SFA, 10 : 0, 12 : 0, 18 : 0, 20 : 0, 22 : 0, total MUFA, cis-9-14 : 1, cis-9-17 : 1, cis-9-18 : 1, trans-11-18 : 1, total PUFA, cis-9,12-18 : 2, cis-9,12,15-18 : 3, trans-10, cis-12-CLA, and 14 : 1, 16 : 1 and 18 : 1 desaturase index of meat were not influenced by dietary RUP level and rpCLA interaction (P>0·05). Kids fed the HR-HSO diet had the highest meat 14 : 0, 16 : 0 and cis-9-16 : 1 contents compared with the other groups (P<0·05). The highest total CLA and cis-9, trans-11-CLA concentrations and CLA index were observed in meat of kids fed LR-rpCLA and HR-rpCLA compared with the other groups (P < 0·05). Increasing dietary RUP level had no effect on meat total SFA, 12 : 0, 20 : 0, 22 : 0, total MUFA, cis-9-14 : 1, cis-9-17 : 1, cis-9-18 : 1, trans-11-18 : 1, total PUFA, cis-9,12-18 : 2, total CLA, cis-9, trans-11-CLA concentrations, and 14 : 1, 16 : 1 and 18 : 1 desaturase index (P > 0·05). Meat concentrations of 10 : 0 (0·34 v. 0·25 g/100 g of FA), 14 : 0 (5·08 v. 4·44 g/100 g of FA), 16 : 0 (29·61 v. 26·55 g/100 g of FA), 17 : 0 (1·29 v. 1·19 g/100 g of FA), cis-9-16 : 1 (3·54 v. 2·61 g/100 g of FA), cis-9,12,15-18 : 3 (0·95 v. 0·68 g/100 g of FA) and trans-10, cis-12-CLA (0·045 v. 0·025 g/100 g of FA) were higher while 18 : 0 (12·19 v. 17·26 g/100 g of FA) was lower in kids fed the HR diets compared with those fed the LR diets (P < 0·05). Addition of rpCLA to the diet of growing kids had no effect on their meat 10 : 0, 14 : 0, 17 : 0, 20 : 0, total MUFA, cis-9-14 : 1, cis-9-17 : 1, cis-9-18 : 1 and trans-11-18 : 1 concentration, and 14 : 1, 16 : 1 and 18 : 1 desaturase index (P > 0·05). Kids fed diets supplemented with rpCLA had lower meat concentrations of total SFA (48·60 v. 52·65 g/100 g of FA) and 16 : 0 (25·76 v. 30·40 g/100 g of FA) and higher concentrations of 12 : 0 (0·79 v. 0·46 g/100 g of FA), 18 : 0 (15·19 v. 14·26 g/100 g of FA), 22 : 0 (0·65 v. 0·48 g/100 g of FA), total PUFA (5·56 v. 4·04 g/100 g of FA), cis-9,12-18 : 2 (4·82 v. 3·15 g/100 g of FA), total CLA (0·65 v. 0·41 g/100 g of FA), cis-9, trans-11-CLA (0·605 v. 0·385 g/100 g of FA) and trans-10, cis-12-CLA (0·045 v. 0·025 g/100 g of FA) than those fed diets supplemented with HSO.
Table 5. Effect of dietary rumen undegradable protein (RUP) and rumen-protected conjugated linoleic acid (rpCLA) level on meat fatty acid (FA) profile of growing kids
(Mean values with pooled standard errors)
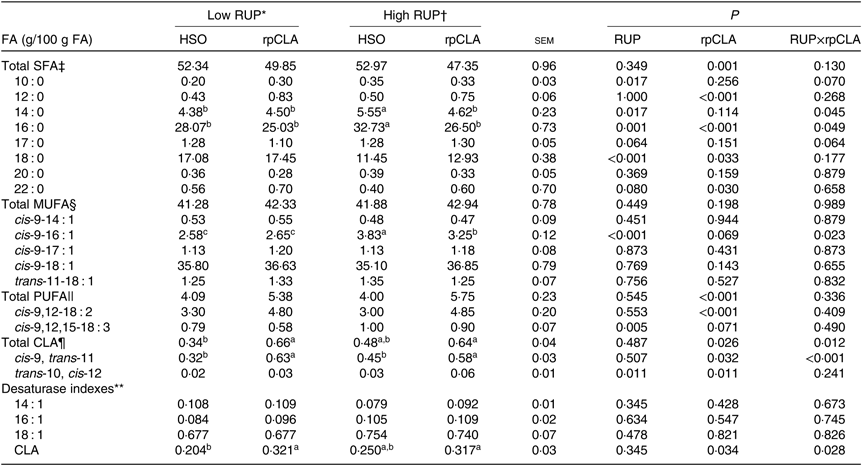
HSO, hydrogenated soyabean oil; CLA, conjugated linoleic acid; CP, crude protein.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P<0·05).
* Diets containing low RUP level (250 g/kg CP).
† Diets containing high RUP level (350 g/kg CP).
‡ Total SFA = 12 : 0 + 14 : 0 + 16 : 0 + 17 : 0 + 18 : 0 + 20 : 0 + 22 : 0.
§ MUFA = cis-9-14 : 1 + cis-9-16 : 1 + cis-9-17 : 1 + cis-9-18 : 1 + trans-11-18 : 1.
|| PUFA = cis-9,12-18 : 2 + cis-9,12,15-18 : 3.
¶ Total CLA = cis-9, trans-11-CLA + trans-10, cis-12-CLA.
** Desaturase indexes based on 14 : 1, 16 : 1, 18 : 1 and CLA were computed as (cis-9-14 : 1/(14 : 0 + cis-14 : 1)), (cis-9-16 : 1/(16 : 0 + cis-9-16 : 1)), (cis-9-18 : 1/(18:0 + cis-9 18 : 1)) and (cis-9, trans-11-CLA/(trans-11-18 : 1 + cis-9, trans-11-CLA)), respectively.
Discussion
In the present experiment, diets were formulated with two different RUP levels and supplemented with HSO or rpCLA in order to study meat quality in growing goat kids. The hypothesis that addition of rpCLA to diets containing higher RUP level increases meat beneficial FA profile and reduces its fat content in growing goat kids is supported by the results of this experiment.
Based on our information, there is no report on interaction of rpCLA and RUP in growing ruminants. In contrast to our results, there was no interaction between rpCLA and dietary CP level on DMI, ADG and FE in growing bulls(Reference Schiavon, Tagliapietra and Dal Maso36). However, dietary supplementation of growing beef bulls(Reference Schiavon, Tagliapietra and Dal Maso36) and young Holstein bulls(Reference Alberti, Gómez and Mendizabal37) with rpCLA had no effect on DMI, ADG and FE, but supplemental rpCLA resulted in higher ADG and FE in growing beef heifers during days 57–89 of an experiment(Reference Gillis, Duckett and Sackmann7). Increasing dietary RUP level in growing Holstein calves(Reference Kazemi-Bonchenari, Mirzaei and Jahani-Moghadam38) and finishing heifers(Reference Stelzleni, Froetsche and Pringle39) had no effect on DMI, ADG and FE. However, higher FE and lower DMI reported in growing beef steers fed diets containing a higher RUP level(Reference Gorocica-Buenfil, Fluharty and Reynolds40). In a recent study(Reference Silva, Detmann and Dijkstra41) increasing RUP level from 38 to 51 % of dietary CP increased ADG and FE in growing Holstein heifers, but had no effect on DMI. These discrepancies between our results and those reported by others may be due to the physiological state, animal species and concentrate ratio in the diets. Provision of adequate amount of rumen-degradable protein ensures optimum microbial activity and proliferation, which increases DM intake(Reference Westwood, Lean and Garvin42). In the present study, greater ADG in kids fed the HR-rpCLA diet could be attributed to higher RUP level, which may consequently increase essential amino acid absorption in the small intestine(Reference Harstad and Prestlokken43). Some theories that have been put forward to explain the effects of CLA on lipid metabolism in adipose tissue, included increased BMR and energy expenditure, increased mobilisation and oxidation of FA from adipose tissue, reduced pre-adipocytes proliferation and/or differentiation, and increased adipocytes apoptosis(Reference Pariza16, Reference Brown and McIntosh44, Reference Wang and Jones45). Overall, these mechanisms may explain the better performance of kids fed the HR-rpCLA diet in the present experiment. Kids fed the HR-rpCLA diet had lower DMI and higher ADG compared with other groups (Table 3), which could explain the higher FE in these animals.
The present results showed no effect of dietary RUP level, rpCLA supplementation or their interaction on hot carcass weight and dressing percentage of growing kids. We did not find any published data regarding the interaction between dietary RUP level and rpCLA in growing ruminants to compare with these results. However, similar to the results of the present study, increasing dietary RUP level in beef steers(Reference Stelzleni, Froetsche and Pringle39, Reference Gorocica-Buenfil, Fluharty and Reynolds40) and supplemental rpCLA in beef bulls(Reference Schiavon, Tagliapietra and Dal Maso36) or beef heifers(Reference Schlegel, Ringseis and Shibani10) had no effect on their hot carcass weight and dressing percentage.
In the present experiment, we observed lower meat fat content when rpCLA was added to diet containing higher RUP level than those with lower RUP level. These findings were in contrast with the results of previous studies that reported no effect of increasing dietary RUP level(Reference Gorocica-Buenfil, Fluharty and Reynolds40) or rpCLA supplementation(Reference Wynn, Daniel and Flux9, Reference Schiavon, Marchi and Tagliapietra17) on carcass protein and fat contents in growing ruminants. However, administration of CLA mixtures has been found to strongly reduce body fatness in monogastric growing animals(Reference Pariza16). Such reduction, mainly due to the biological action of the trans-10, cis-12-CLA isomer, appears to be caused mostly by a reduction in body fat accretion and not to a mobilisation of body fat that had already accumulated before the experiment(Reference Pariza16). Also, abomasal infusion of CLA or feeding rpCLA(Reference Baumgard, Sangster and Bauman46) resulted in a strong reduction in milk fat content in dairy cows.
The reduction of meat fat content in the present study may be also related to preserved or enhanced muscle mass by rpCLA supplementation. Park et al.(Reference Park, Albright and Liu47) and Pariza et al.(Reference Pariza, Park and Cook21) suggested that CLA induced changes in regulation of some cytokines that affect skeletal muscle catabolism and immune function. Furthermore, CLA blocks adipose tissue development by inhibiting preadipocyte proliferation and differentiation, including de-differentiation of mature adipocytes, and stimulating programmed cell death of adipogenic cells(Reference Mersmann11). The reduction of meat fat content in kids fed the HR-rpCLA diet could be attributed to effect of rpCLA on stimulation of protein accretion(Reference Schiavon, Tagliapietra and Dalla Montà22) and inhibition of fat synthesis(Reference Wynn, Daniel and Flux9, Reference Schlegel, Ringseis and Shibani10, Reference Schiavon, Marchi and Tagliapietra17). In this regard, it has been reported that supplemental rpCLA has a sparing effect on energy repartitioning, which is supported by higher milk protein and lower milk fat content in lactating dairy cows fed a diet containing rpCLA(Reference von Soosten, Meyer and Piechotta23). The possible rpCLA effects on protein anabolism might be mediated by anabolic hormones like IGF-I. This growth factor is part of the somatotropic axis, which describes the interaction of growth hormone, growth hormone receptors in the liver, and IGF-I synthesised and secreted by the liver(Reference Lucy, Jiang and Kobayashi24). Increased plasma IGF-I concentrations was reported during supplementation of rpCLA in lactating dairy cows(Reference Castañeda-Gutiérrez, Benefield and de Veth48). Such a similar mechanisms may be happen in kids fed the HR-rpCLA diet in the present study.
The higher protein content in meat of kids fed HR diets compared with those fed LR diets in the present study was in contrast to Gorocica-Buenfil et al.(Reference Gorocica-Buenfil, Fluharty and Reynolds40) who found no effect of dietary RUP level on meat protein and fat contents in beef steers. The higher protein content in the meat of kids fed HR diets compared with those fed LR diets in the present study may be related to absorption of more essential amino acids due to increased RUP at the small intestine, which might have promoted muscle protein synthesis(Reference Harstad and Prestlokken43). Therefore, animals fed the LR diets synthesised more fat, whereas those fed the HR diets synthesised more protein in their carcass, which suggests that a greater dietary RUP promotes the rate of protein synthesis in growing ruminants.
To our knowledge, there is no report about the effect of dietary RUP level on meat ash content. The ash content reported for fresh goat meat ranges between 0·8 and 1·7 %. The mineral content in meat is affected by many factors such as animal species, breed, climate, diet composition and type of tissue. The most abundant minerals in goat meat are K, P, Na, Mg and Ca (757, 530, 108, 49 and 13 mg/100 g DM, respectively). The lower meat ash content in kids fed LR diets compared with those fed HR diets in the present study may be attributed to higher plasma urea concentration in these animals (unpublished data). Urea excretion in the kidney is accompanied by Na excretion(Reference Reece, Erickson and Goff49). Since Na is one the most abundant minerals in goat’s meat, therefore this may explain the lower ash content in meat of kids fed LR diets as a result of higher urinary Na excretion. In contrast to our results, supplemental rpCLA had no effect on meat ash content in growing bulls(Reference Schiavon, Marchi and Tagliapietra17). However, dietary CLA supplementation increased body ash content in mice(Reference Park, Albright and Liu47) and bone mineral density in growing rats(Reference Roy, Bourgeois and Rodriguez50). Supplemental CLA has been suggested to possibly enhance absorption of some minerals(Reference Jewell and Cashman51). This mechanism may explain the higher meat ash content in kids fed rpCLA supplementation in the present study.
It could be postulated from these results that dietary rpCLA could be involved in a metabolic regulation that increases efficiency of N retention, particularly under conditions of higher dietary RUP level.
We found that meat colour parameters in growing kids were not influenced by supplemental rpCLA in diets with different RUP level, which was similar to previous findings in growing ruminants(Reference Schlegel, Ringseis and Shibani10, Reference Schiavon, Marchi and Tagliapietra17, Reference Stelzleni, Froetsche and Pringle39).
Results showed lower total SFA and 16 : 0 concentrations in meat of kids fed rpCLA supplementation compared with those fed HSO. This differs from previous reports in which researchers failed to detect any effect of rpCLA on total SFA content in meat of growing ruminants(Reference Schlegel, Ringseis and Shibani10, Reference Schiavon, Marchi and Tagliapietra17, Reference Gómez, Mendizabal and Sarriés18). However, similar to this result, Terré et al.(Reference Terré, Nudda and Boe52) reported lower meat 16 : 0 content in growing lambs fed rpCLA supplementation. The reduction of total SFA content in meat of kids fed supplemental rpCLA in our study can be attributed to lower 14 : 0 and 16 : 0 and higher trans-10, cis-12-CLA isomer contents of meat in these animals (Table 5). The majority of SFA from 4 to 14 carbon length and half of the 16 : 0 produce in intramuscular fat tissues of growing ruminants by de novo synthesis(Reference Wood, Enser and Fisher2). Trans-10, cis-12-CLA reduces fat synthesis in ruminant tissues through down-regulation of lipogenic capacity and key lipogenic enzymes and factors(Reference Urrutia, Toledo and Baldin53). The increase in PUFA content of meat fat in kids fed diets containing supplemental rpCLA in the present study may be attributed to a higher cis-9,12-18 : 2 concentration in carcass of these animals. Furthermore, the higher PUFA content of meat from kids fed diets containing rpCLA compared with those fed HSO supplementation may be explained by lower meat fat content in these animals (Table 4). The PUFA in intramuscular fat is captured mainly by phospholipids. The amount of phospholipids within the muscle is quite constant if expressed as proportion of fresh tissue, whereas it decreases with increasing level of fatness if expressed as proportion of total FA, this is because neutral lipids, especially TAG, which are the large majority of fat depots, contain mainly SFA and MUFA(Reference Wood, Enser and Fisher2).
The reduction of meat SFA concentration by dietary rpCLA supplementation in the present study could have beneficial effects on human health as the adverse effects of excessive consumption of medium-chained SFA, especially 14 : 0 and 16 : 0, on human health have been found(Reference Shingfield, Bonnet and Scollan4). Excessive intakes of SFA may also be associated with lower insulin sensitivity and development of the metabolic syndrome and diabetes(Reference Kennedy, Martinez and Chuang54). The higher 16 : 0 and cis-9-16 : 1 concentrations in meat of kids fed the HR-HSO diet may be contributed to higher concentration of these FA in their diet (Table 2). In our study, data on meat FA profile showed a higher 18 : 0 concentration for rpCLA-fed kids compared with HSO-fed groups. This finding was consisted with the results of Schiavon et al.(Reference Schiavon, Marchi and Tagliapietra17) and Gomez et al.(Reference Gómez, Mendizabal and Sarriés18) in growing beef bulls and young calves, respectively. However, in contrast to the results of the present study, they reported the lack of effect of dietary rpCLA on meat total PUFA and cis-9,12-18 : 2 concentrations. A higher meat 18 : 0 concentration in kids fed LR diets (higher rumen degradability) compared with those fed HR diets (lower rumen degradability) in the present study might be due to a more complete biohydrogenation of UFA by the rumen micro-organisms. Kids fed LR diets probably would have resulted in higher rumen concentration of ammonia and ammonia is the main source of nitrogen for cellulolytic bacteria(Reference Bach, Calsamiglia and Stern55), which in turn are the main micro-organisms contributing to biohydrogenation of UFA in the rumen(Reference Jenkins, Wallace and Moate56).
Our results indicated that meat cis-9, trans-11-CLA was higher in kids fed LR-rpCLA and HR-rpCLA diets. Analogous to our data, previous researchers(Reference Schiavon, Marchi and Tagliapietra17, Reference Gómez, Mendizabal and Sarriés18, Reference Terré, Nudda and Boe52) found higher meat cis-9, trans-11-CLA concentration in growing ruminants by supplemental rpCLA. CLA, in particular cis-9, trans-11-CLA, has been suggested to have a role in the prevention of cancer and atherosclerosis in humans(Reference Belury57). This isomer of CLA can be produced endogenously by the action of Δ9-desaturation of trans-11-18 : 1, which is active in several tissues including skeletal muscle and adipose tissue(Reference Bauman, Baumgard and Corl5, Reference Jiang, Michal and Tobey58). Our findings confirm the results of aforementioned studies that rpCLA supplementation is an effective strategy for increasing tissue CLA content in ruminants. The higher trans-10, cis-12-CLA concentration in meat from rpCLA-fed kids compared with those fed diets containing HSO in the present experiment agreed with Terre et al.(Reference Terré, Nudda and Boe52) and Schiavon et al.(Reference Schiavon, Marchi and Tagliapietra17) in growing lambs and bulls, respectively.
The trans-10, cis-12-CLA isomer was previously found to reduce body fatness, which appeared to be caused by a reduction in gene expression and activity of FA synthase and acetyl-CoA carboxylase in tissues(Reference Mersmann11, Reference Pariza16), which may be a reason for the lower meat fat content in the present study with rpCLA supplementation. In the present study, despite feeding goat kids with equal amounts of trans-10, cis-12-CLA and cis-9, trans-11-CLA, the concentration of trans-10, cis-12-CLA was lower compared with cis-9, trans-11-CLA, which was in agreement with the previous studies(Reference Wynn, Daniel and Flux9, Reference Schiavon, Marchi and Tagliapietra17). This may be related to a faster oxidation and clearance of trans-10, cis-12-CLA than cis-9, trans-11-CLA from tissues(Reference Martin, Gregoire and Siess59). The lack of effect of dietary RUP level on desaturase index of 14 : 1, 16 : 1 and 18 : 1 in the present study was in contrast to the finding of Gorocica-Buenfil et al.(Reference Gorocica-Buenfil, Fluharty and Reynolds40) who reported a lower desaturase index when RUP level in the diet of beef steers was increased. This discrepancy between the present experiment and the Gorocica-Buenfil et al.(Reference Gorocica-Buenfil, Fluharty and Reynolds40) study may be due to the different concentrations of cis-9-16 : 1 and cis-9-18 : 1, and these two UFA have not same trend in response to the dietary RUP level in these two experiments. In our study, the meat concentration of cis-9, trans-11-CLA and CLA desaturase index had the same trend, which probably indicates the conversion of the same amount of trans-11-18 : 1 to cis-9, trans-11-CLA by the action of Δ9-desaturase. The lack of effect of the administration of the rpCLA mixture on desaturase indexes of 14 : 1, 16 : 1 and 18 : 1 in the present study reflects no or the same inhibition of the Δ9-desaturase enzyme activity by the trans-10, cis-12-CLA isomer, which reflected by similar meat trans-10, cis-12-CLA content (Table 5). Based on the result of Baumgard et al.(Reference Baumgard, Matitashvili and Corl60), it was expected that increasing amounts of trans-10, cis-12-CLA isomer should reduce the endogenous synthesis of cis-9, trans-11-CLA by desaturation of trans-11-18 : 1. However, in the present experiment, addition of rpCLA to HR diets increased the cis-9, trans-11-CLA and had no effect on the trans-11-18 : 1 concentration, and the resulting cis-9, trans-11-CLA/(trans-11-18 : 1+cis-9, trans-11-CLA) ratio was increased. This suggests that the exogenous supply of cis-9, trans-11-CLA has probably more effective than the inhibitory effect by trans-10, cis-12-CLA on this parameter.
In conclusion, feeding HR diets supplemented with rpCLA had no effect on meat protein content, colour parameters, total SFA, MUFA and PUFA concentrations, hot carcass weight, dressing percentage and internal fat weight. However, supplementation of diets containing high RUP level with rpCLA not only decreased meat fat content but also increased its beneficial FA concentration such as cis-9, trans-11-CLA, which could have beneficial effects on human health.
Acknowledgements
The authors thank Dr L. MacLaren (Department of Animal Science and Aquaculture, Haley Institute, Agricultural Campus, Dalhousie University, Truro, Canada) for recommendations and edition of the final version of this manuscript.
The authors would like to acknowledge Ilam Agriculture and Natural Resources Research and Education Center (IANRREC) for their corporation and financially supporting this research (grant no. 4-39-13-051-950731). In addition, IANRREC and Ilam University contributed to design, conduct, analysis of samples and data, and writing of this article.
A. P. is a PhD student of Ilam University and this paper is extracted from his dissertation. F. F. and H. J. are supervisors of the dissertation and contributed in formulating the research questions, designing the study, carrying it out and writing the article. A. A., S. V. and G. T. are advisors of the dissertation and contributed in analysing the data and writing the article.
There are no conflicts of interest.










