Age-related osteoporosis mainly affects people older than 70 years of age and results, in most cases, in both vertebral and hip fractures. Given the magnitude of this public health problem and the dramatically increased proportion of older individuals predicted in the next decades, it is necessary to evaluate the potential of every preventive intervention.
In addition to the well-recognised risk factors such as Ca and vitamin D deficiencyReference Heaney and Weaver1, other nutritional aspects have been suggested to contribute to the increased incidence of osteoporosis in the elderly. Indeed, ageing has been correlated with a physiological decline in appetite and food intake. This change predisposes the elderly to a poor nutritional statusReference Donini, Savina and Cannella2, resulting in a compromised musculoskeletal system characterised by lower muscle mass and a decrease in bone mineral contentReference Wilkins and Birge3.
The impact of low energy and protein intake on the skeleton has been investigated in the elderlyReference Lumbers, New, Gibson and Murphy4–Reference Ilich and Kerstetter6. Bonjour et al. Reference Bonjour7 revisited the concept of AlbrightReference Albright, Smith and Richardson8 who hypothesised that ‘a diet inadequate in protein might lead to a negative nitrogen balance’ and consequently affect bone formation. Several studies have established a positive correlation between bone mineral density (BMD) and both energy and protein intakeReference Hannan, Tucker, Dawson-Hughes, Cupples, Felson and Kiel9–Reference Coin, Sergi, Beninca, Lupoli, Cinti, Ferrara, Benedetti, Tomasi, Pisent and Enzi11, and such oral supplementation seemed to improve the clinical outcome in elderly patients with femoral neck fracturesReference Delmi, Rapin, Bengoa, Delmas, Vasey and Bonjour12, Reference Schurch, Rizzoli, Slosman, Vadas, Vergnaud and Bonjour13.
In animals, energy restriction has been demonstrated to adversely affect bone status. McCay et al. Reference McCay, Crowell and Maynard14 first reported that bones became fragile after long-term energy restriction in rats and that ‘some crumbled with the course of dissection’. In this particular study, the fragility most likely was due to the extreme dietary deprivation, including Ca insufficiency. However, subsequent works have demonstrated altered femoral bone mineral contentReference Lee, Panemangalore and Wilson15, Reference Sanderson, Binkley, Roecker, Champ, Pugh, Aspnes and Weindruch16, BMD and biomechanical properties in old rats under energy restrictionReference Talbott, Cifuentes, Dunn and Shapses17, Reference LaMothe, Hepple and Zernicke18.
Protein is a major component of the bone organic matrix and consequently, dietary proteins contribute the essential amino acids necessary for new matrix synthesis. In rats, protein under-nutrition has been associated with lower bone mass and strength, modulated by the growth hormone–insulin-like growth factor 1 (IGF-1) axisReference Ammann, Bourrin, Bonjour, Meyer and Rizzoli19, and in animals fed a low protein diet, bone strength was increased by dietary supplementation with essential amino acidsReference Ammann, Laib, Bonjour, Meyer, Ruegsegger and Rizzoli20. Consequently, modulation of the amount and quality of dietary protein intake represents an interesting approach to preventing bone loss during ageing.
Whey protein (WP) contains a relatively high proportion of essential amino acids and can effectively modulate whole body protein anabolismReference Boirie, Dangin, Gachon, Vasson, Maubois and Beaufrere21 and prevent body protein loss in elderly subjectsReference Dangin, Boirie, Guillet and Beaufrere22. However, few studies have examined the effect of WP intake on bone status. The administration of WP was shown to effectively increase bone strengthReference Takada, Kobayashi, Kato, Matsuyama, Yahiro and Aoe23 and enhance bone formationReference Kelly, Cusack and Cashman24 in young animals. On the other hand, casein, which is widely used in experimental dietsReference Reeves25, has some interesting properties. Casein intake leads to the formation of casein phosphopeptides during digestion and the casein phosphopeptides have been suggested to promote Ca absorptionReference Etcheverry, Wallingford, Miller and Glahn26, Reference Sato, Noguchi and Naito27 and stimulate bone mineralisationReference Scholz-Ahrens and Schrezenmeir28. Therefore, both casein and WP potentially have an impact on bone quality and/or status. However, further studies are required to specifically examine how these proteins modulate skeletal metabolism and status during ageing.
This study was designed to test if an adequate protein intake, provided by casein or WP, could prevent the alteration of bone status induced by energy deficiency in the elderly. Using old male rats, two specific questions were addressed:
i. What are the long-term effects on bone status of protein and energy restriction (PER) and energy restriction (ER) alone, compared to normal protein and energy supply (N)?
ii. How does the protein source (casein or WP) influence bone status in restricted and unrestricted rats?
Experimental methods
Experimental design
The study was conducted in accordance with the regional Ethics Committee (France).
Male Wistar rats were purchased from Janvier (Le Genest St Isle, France) and housed individually. Male rats were studied in order to eliminate the potential confounding effect of hormonal fluctuations present with an oestrus cycle. Animals were subjected to 12–12 h light–dark cycles and had free access to water.
At the beginning of the study, the rats were 16 months old and the experiment continued for 5 months. During a 2-week adaptation period, the animals were maintained on a standard chow diet to record their daily food intake and body weight. Then, they were randomly assigned to one of six dietary groups (n 10 animals per group). Two control groups received a normal diet (N): a standard semi-purified diet containing 69 % carbohydrate, 6 % fat and either 17 % casein (N-C) or 17 % WP (N-WP) as protein source (Table 1). Rats of the N groups were fed a measured amount, corresponding to 90–95 % of the average ad libitum intake, to match energy intake and to facilitate the study of healthy, non-obese control animalsReference Pugh, Klopp and Weindruch29. The mean energy intake was about 451 kJ/d (108 kcal/d). The protein and energy-restricted groups (PER-C and PER-WP) were limited to 60 % of the intake of the N groups (i.e. 10·2 % protein and 272 kJ/d (65 kcal/d), respectively). The energy-restricted groups (ER-C and ER-WP) received 60 % of the energy of the controls (i.e. 272 kJ/d), but had their protein intake maintained at the level of the N groups. All diet-restricted rats were normalised to the N animals with respect to lipid, fibre, mineral and vitamin intake. Thus, all the animals consumed the same amounts of dietary Ca and P (Ca:P ratio = 1·45). During the experiment, body weight was recorded twice weekly.
Table 1 Daily ration composition
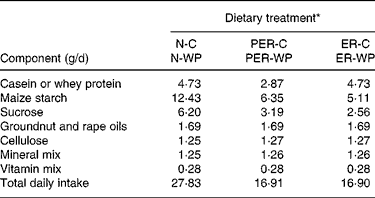
* N, ER and PER, treatment with normal, energy-restricted, and protein and energy-restricted diet respectively; -C, and -WP, casein and whey protein additions to ER, PER and N diets.
At the end of the experiment, rats were fasted for 12 h and sacrificed. Blood as well as prior 24-h urine samples were collected to assay the biochemical parameters. Femurs were cleaned from adjacent tissues. Left femurs were harvested in saline solution (9 g NaCl/l) and frozen ( − 20°C) until mechanical testing. Right femurs were placed in 80 % alcohol until BMD was measured.
Physical measurements
Bone mineral density
BMD was assessed by dual-energy X-ray absorptiometry, using a Hologic QDR-4500 A X-ray bone densitometer (Hologic, Massy, France). Total femoral BMD, metaphyseal BMD and diaphyseal BMD were determined. For metaphyseal BMD and diaphyseal BMD measurements, scans were cut and analysed as follows: the first cut of the femur was performed at the upper third, and the next cut was made at the lower third. Diaphyseal BMD, which is rich in cortical bone, corresponded to the density of the second third of the femur. Metaphyseal BMD, which mainly contains cancellous bone, was calculated as the mean of the femoral proximal metaphysis density and the femoral distal metaphysis density.
Femoral mechanical testing
Femoral length and mean diaphyseal diameter were measured with a precision caliper (Mitutoyo, Shropshire, UK). The femoral failure load was determined using a three-point bending testReference Turner and Burr30, with a Universal Testing Machine (Instron 4501, Instron, Canton, MA, USA). The two lower supports were separated by a 20 mm distance and an upper crosshead roller was applied in front of the middle of the bone until failure at a speed of 0·5 mm/min to guarantee that 85–90 % of the bone flexure was due to bending.
Static histomorphometry
After BMD measurements, distal right femurs were dehydrated in a graded series of ethanol solutions for 5 d prior to embedding in methyl methacrylate (Sigma, L'Isle d'Abeau, France). Blocks were then polished with a grinder (Metaserv 2000, Buehler, Coventry, UK) and 10 μm frontal sections were cut using a RM2165 Leica microtome (Leica Microsystems Nussloch GmbH, Nussloch, Germany). Sections were stained using the Von Kossa silver method (AgNO3; Sigma). Four sections were analysed per femur. To characterise static cancellous bone, image acquisition was carried out with an Axioplan EE microscope (Zeiss, Göttingen, Germany) and image analysis performed in the secondary spongiosa of the distal femur metaphysis with the OsteoLab software (Biocom, Paris, France). This allows an evaluation of cancellous bone volume (bone volume:total tissue volume, %), trabecular number, trabecular thickness (μm) and trabecular separation (mm). Cortical bone was assessed at the femoral diaphysis. Cross sections were analysed with the ImageJ 1·34 s software (National Institutes of Health, Bethesda, MD, USA) to measure tissue, marrow and cortical areas (mm2). All histomorphometric parameters were determined according to Parfitt et al. Reference Parfitt, Drezner, Glorieux, Kanis, Malluche, Meunier, Ott and Recker31.
Biochemical analysis
Osteoblastic activity
Plasma osteocalcin (OC) was measured by RIA, using rat 125I-labeled OC, a goat anti-rat OC antibody and a donkey anti-goat secondary antibody (Biochemical Technologies, Stoughton, MA, USA). The sensitivity was 0·01 ng/ml. The intra- and interassay precisions were 6·8 and 8·9 %, respectively.
Bone resorption
The urinary deoxypyridinoline (DPD) excretion rate (nmol/24 h) was determined by competitive RIA, using a rat monoclonal anti-DPD antibody adsorbed to the inner surface of a polystyrene tube and 125I-labeled DPD (Pyrilinks-D RIA kit, Metra Biosystems, Mountain View, CA, USA). The sensitivity was 2 nmol/l. The intra- and interassay precisions were 4 and 6 %, respectively.
Leptin
Plasma leptin concentrations were assessed by RIA using an anti-rat leptin antibody and a rat leptin as standard (Rat Leptin RIA kit; Linco Research Inc., Missouri, USA). The lowest limit of sensitivity was 0·5 ng/ml, and the intra- and interassay variations were 1·5 and 2·5 %, respectively.
Insulin-like growth factor I
IGF-1 concentrations were measured in serum samples using a two-site immunoenzymometric assay (OCTEIA Rat/Mouse IGF-1 kit, IDS, Paris, France). The sensitivity of the assay was 82 ng/ml. Intra- and interassay variations were 5·7 and 10·7 %, respectively.
Urinary calcium excretion
Urinary Ca was determined by atomic absorption spectrophotometry (Perkin Elmer 400, Norwalk, CT, USA). Each sample was diluted appropriately with distilled water and lanthanum chloride (0·1 %) for atomisation. The urinary Ca excretion was calculated using the volume of the 24 h urine samples collected.
Statistical methods
Results are expressed as means with their standard errors and were analysed with XLSTAT (Addinsoft, Paris, France). The BMD, biomechanical and histomorphometric variables were subjected to a two-way analysis of covariance (ANCOVA) with body weight as the covariate to ensure the assessment of dietary restriction and protein type independently of body weight variationsReference Lane, Black, Handy, Shapses, Tilmont, Kiefer, Ingram and Roth32. Other parameters were analysed using a two-way ANOVA, testing for any difference among groups. Thus, the main effects assessed were dietary restriction (N / PER / ER), protein (C / WP) and their interaction (dietary restriction × protein). If a result was found significant (P < 0·05), the Student–Newman–Keuls multiple comparison test was used to determine specific differences between means. Linear regressions were also performed, to study internal correlations among variables, and the Pearson test was carried out to assess their significance.
Results
Body weight
Changes in body weight are shown in Fig. 1. As expected, animals on dietary restriction exhibited a significant decrease in body weight (P < 0·0001) at the completion of the study, compared to rats fed normal diets. The PER and ER rats weighed about 150 g less than N groups. There was no significant difference between the four restricted groups, whatever the level or the quality of dietary protein. Plasma leptin concentrations are known to correlate with adiposity in mammalsReference Maffei, Halaas and Ravussin33. Here, the leptin levels (ng/ml) were markedly lower in the PER and ER groups than in the N groups (N-C: 11·94 (sem 1·54); N-WP: 12·31 (sem 1·45) v. PER-C: 3·46 (sem 0·39); PER-WP: 4·35 (sem 0·50); ER-C: 2·28 (sem 0·31); ER-WP: 3·62 (sem 0·63)). This suggests a fat-mass reduction in the restricted animals.
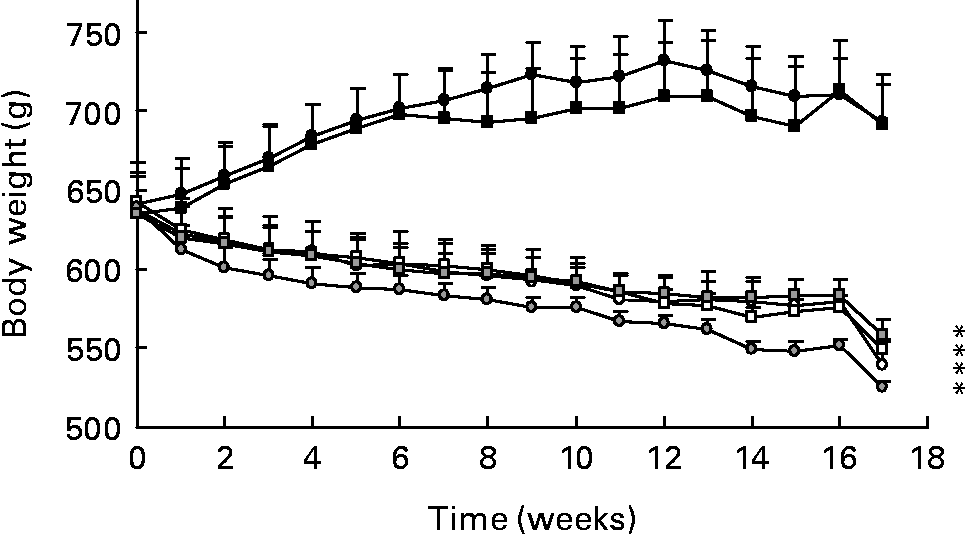
Fig. 1 Body weight of rats fed normal diets (with casein (C), N-C (●) and with whey protein (WP), N-WP (■)), protein and energy restricted diets (PER-C (○), PER-WP (□)), and energy restricted diets (ER-C (![]() ), ER-WP (
), ER-WP (![]() )). Values are expressed as means with their standard errors indicated by vertical bars. Two-way ANOVA indicates a significant effect of dietary restriction (P < 0·001), a non-significant effect of protein type and no interaction between the two variables. * Mean values significantly different from the N groups.
)). Values are expressed as means with their standard errors indicated by vertical bars. Two-way ANOVA indicates a significant effect of dietary restriction (P < 0·001), a non-significant effect of protein type and no interaction between the two variables. * Mean values significantly different from the N groups.
Bone mineral density
The BMD was consistently reduced by both types of dietary restriction (PER and ER) in total femur (P = 0·020), as well as at the diaphyseal (P = 0·016) and the metaphyseal (P = 0·064) sites (Fig. 2(a), (b) and (c) respectively)). The casein-fed rats tended to have a higher BMD than those fed the WP diet (P = 0·073).
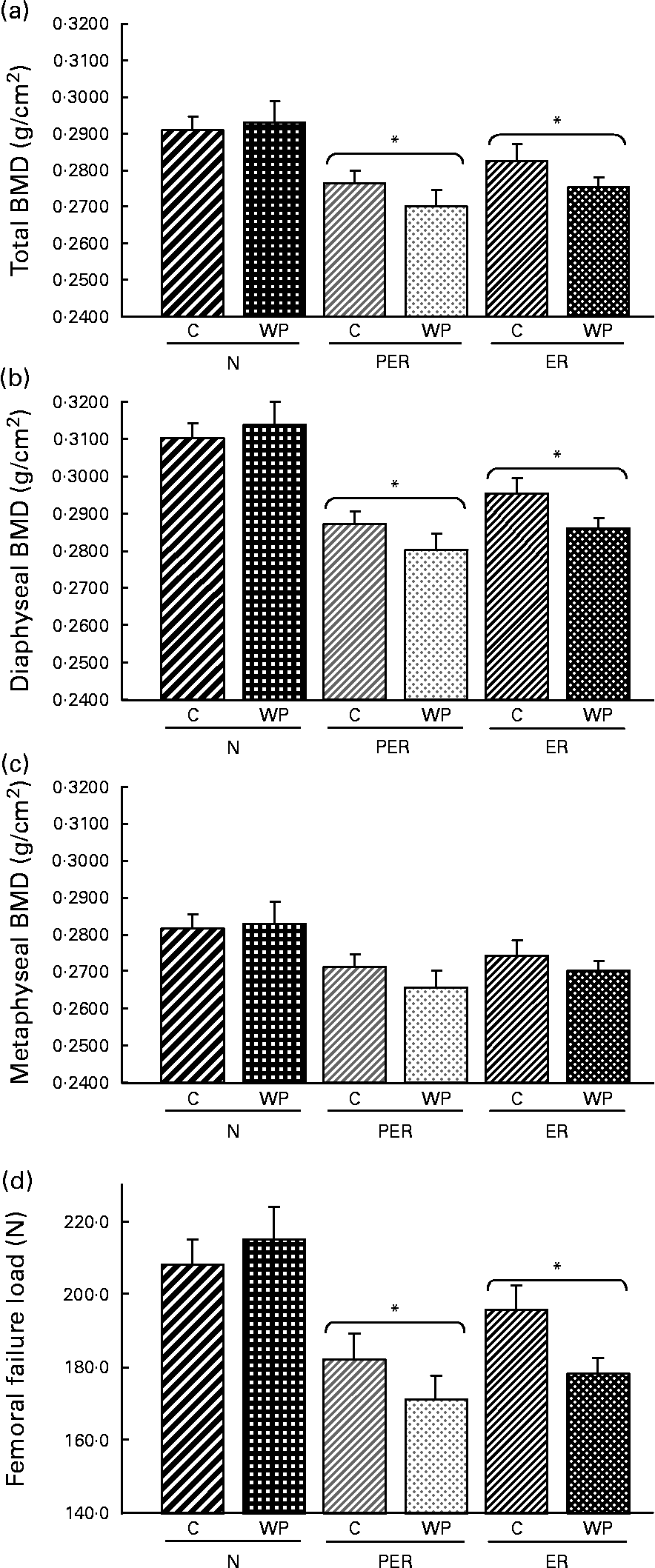
Fig. 2 Effect of dietary restrictions (normal(N)/protein and energy restricted (PER)/energy restricted (ER)) and the type of protein provided in the diet (casein (C)/whey protein (WP)) on (a) total, (b) diaphyseal and (c) metaphyseal femoral BMD and femoral biomechanical resistance (d). Values are expressed as means with their standard errors indicated by vertical bars. Two-way ANCOVA indicates a significant effect of dietary restriction on total and diaphyseal BMD (P = 0·020 and P = 0·016 respectively) and on femoral failure load (P = 0·013), a non-significant effect of protein type and no interaction between the two variables. * Mean values significantly different from the N groups.
Biomechanical properties
Femurs from restricted animals (PER and ER) had a lower resistance to fracture compared to those from the N groups (P = 0·013), but resistance to fracture did not differ between the PER and ER groups (Fig. 2(d)). The type of dietary protein had no significant effect. However, femoral biomechanical resistance tended to be higher in the casein groups (P = 0·089) than the WP groups.
Static histomorphometry
Histomorphometric data of the distal femur are shown in Table 2. The trabecular bone volume to total volume ratio (bone volume: total tissue volume; P = 0·043) and trabecular thickness (P = 0·009) were lower in the energy restricted groups (ER), compared to the protein-energy restricted (PER) groups. Casein intake was associated with an increase in bone volume (P = 0·045), as well as an elevated trabecular number (P = 0·009) compared to the WP diets, in both restricted and non-restricted rats. Cortical bone parameters were only affected by the dietary restriction factor. Tissue area in the femoral diaphysis decreased in the PER and ER groups compared to the N groups (P = 0·053). The same pattern was observed for cortical area (P = 0·022).
Table 2 Effect of dietary restrictions (normal (N)/protein-energy restricted (PER)/energy restricted (ER)) and the type of protein provided in the diet (casein (C)/whey protein (WP)) on histomorphometry of cancellous bone at the distal femoral metaphysis and of cortical bone at the femoral diaphysis
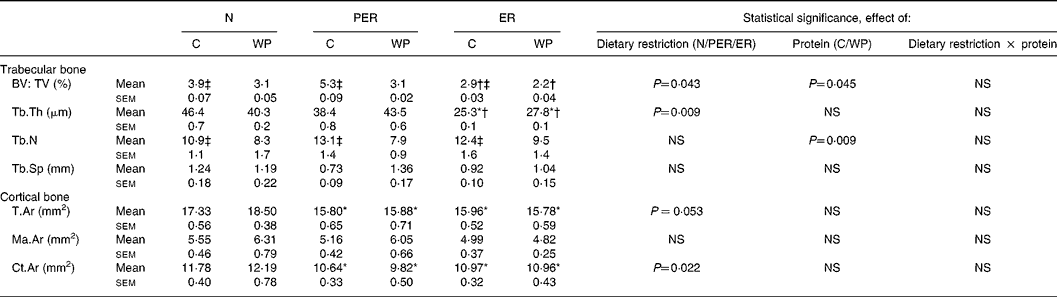
BV, bone volume; TV, tissue volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation; T.Ar, tissue area; Ma.Ar, marrow area; Ct.Ar, cortical area. Two-way ANCOVA was performed. Comparison between dietary restrictions (N/PER/ER): * significantly different from the N groups; † significantly different from the PER groups. Comparison between protein types (C/WP): ‡ significantly different from the WP groups.
Bone biomarkers
Fig. 3 shows the levels of bone formation (OC) and bone resorption (DPD) markers at the end of the experiment. Plasma OC was reduced in the ER groups (P = 0·029) compared to N groups. A similar trend was observed in the PER animals. Moreover, OC levels tended to be higher with casein consumption (P = 0·072) than with WP intake. The urinary DPD excretion rate was decreased with both dietary restrictions, compared to the N diets (P < 0·0001), and ER animals excreted significantly less DPD than PER rats. Furthermore, using linear regression analysis, a positive correlation (r 0·543, P < 0·0001) was established between formation and resorption markers.
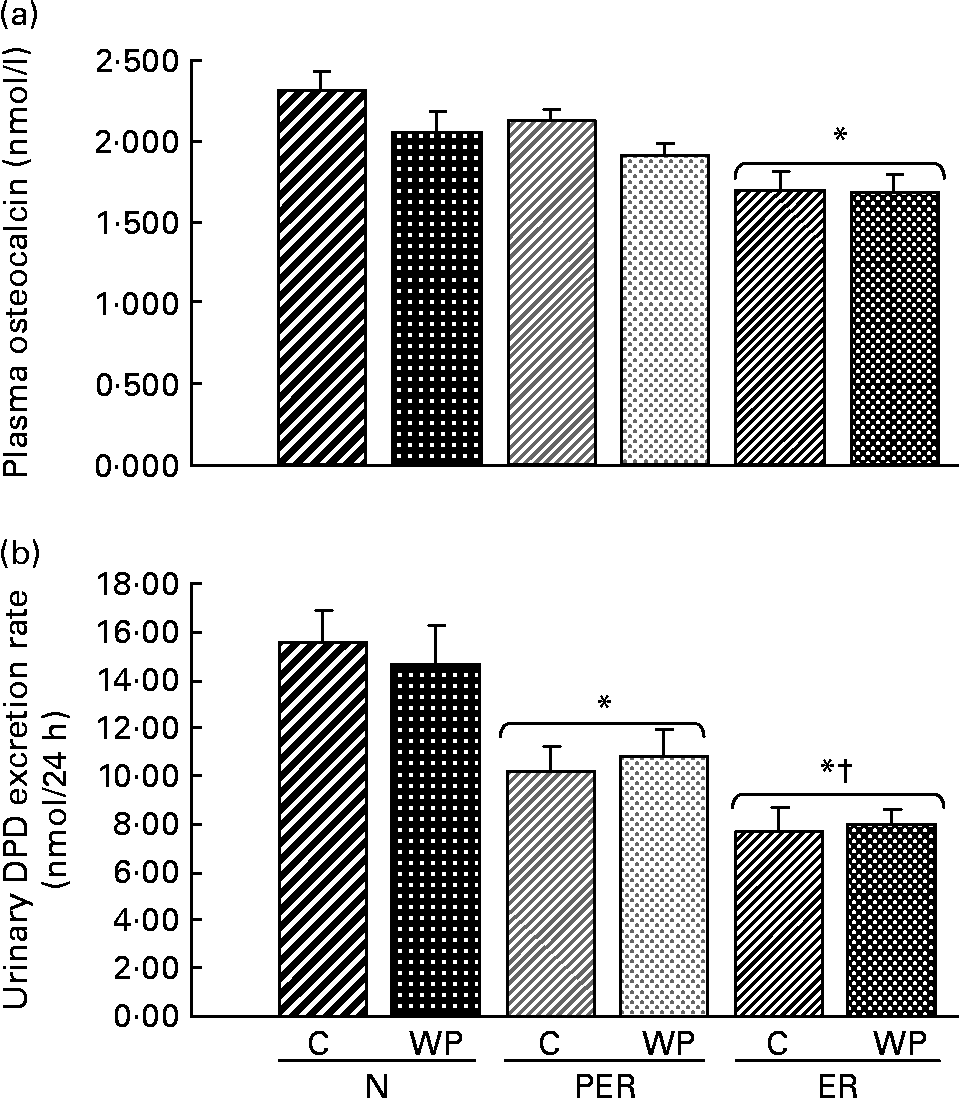
Fig. 3 Effect of dietary restrictions (normal(N)/protein and energy restricted (PER)/energy restricted (ER)) and the type of protein provided in the diet (casein (C)/whey protein (WP)) on plasma osteocalcin (a) and the urinary deoxypyridinoline (DPD) excretion rate (b). Values are expressed as means with their standard errors indicated by vertical bars. Two-way ANOVA indicates a significant effect of dietary restriction on osteocalcin and DPD levels (P = 0·029 and P < 0·001 respectively), a non-significant effect of protein type and no interaction between the two variables. * Mean values significantly different from the N groups. † Mean values significantly different from the PER groups.
Plasma Insulin-like growth factor-1
Plasma IGF-1 concentrations (Fig. 4) were lower in the PER and ER groups compared to the N groups (P = 0·001). This decrease in IGF-1 levels was not correlated with the amount of dietary protein. The interaction between dietary restriction and protein effects was significant (P = 0·014), with the following relative ranking: (N-WP) = (N-C) = (ER-WP) = (PER-C)>(ER-C) = (PER-WP).
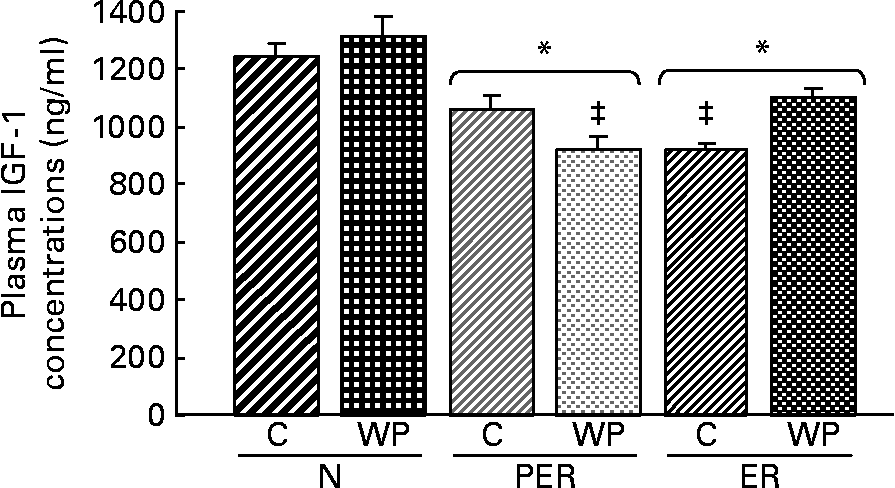
Fig. 4 Effect of dietary restrictions (normal(N)/protein and energy restricted (PER)/energy restricted (ER)) and the type of protein provided in the diet (casein (C)/whey protein (WP)) on plasma insulin-like growth factor 1 (IGF-1) concentrations. Values are expressed as means with their standard errors indicated by vertical bars. Two-way ANOVA indicates a significant effect of dietary restriction on IGF-1 levels (P = 0·001), a non-significant effect of protein type and a significant effect of the interaction between the two variables (P = 0·014). * Mean values significantly different from the N groups. ‡ Mean values significantly different from the other groups.
Calcium excretion
No statistical change in urinary Ca excretion was recorded in the groups (data not shown).
Discussion
Nutritional deficiencies often occur in the elderly and energy and protein undernutrition have been suggested to alter bone health and to increase the risk of osteoporotic fractures. Several studies have assessed the impact of dietary restrictions on boneReference McCay, Crowell and Maynard14–Reference LaMothe, Hepple and Zernicke18, Reference Lane, Black, Handy, Shapses, Tilmont, Kiefer, Ingram and Roth32, Reference Talbott, Rothkopf and Shapses34–Reference Black, Allison, Shapses, Tilmont, Handy, Ingram, Roth and Lane38. However, these studies differ widely in their experimental design, duration, age at onset of restriction and diet composition. Thus, based on these, it is difficult to interpret the effects of dietary restrictions on the skeleton during ageing and to dissociate the respective effects of protein and energy deficiency. To our knowledge, this study is the first to test the effects of protein quality and quantity on bone status during ageing in rats.
Our experimental model was the aged male Wistar rat, which has been established as a relevant model for age-related bone loss in human subjectsReference Schapira, Lotan-Miller, Barzilai and Silbermann39. The severity of dietary restriction (40 %) was based on previous rodent studiesReference Talbott, Cifuentes, Dunn and Shapses17, Reference Talbott, Rothkopf and Shapses34, Reference Brochmann Murray, Beamer, Duarte, Behnam, Grisanti and Murray35, Reference Kalu, Hardin, Cockerham, Yu, Norling and Egan40. During the experimental period, body weight markedly decreased with both dietary restrictions (a 23 % change compared to the controls; Fig. 1). In the statistical analysis, body weight was included as an independent variable to ensure the assessment of dietary restriction and protein type independently of its variations. Weight loss has been demonstrated to result in decreased BMD as a consequence of reduced mechanical loading, altered hormone levels and dietary factors, and changes in bone compositionReference Sanderson, Binkley, Roecker, Champ, Pugh, Aspnes and Weindruch16, Reference Lane, Black, Handy, Shapses, Tilmont, Kiefer, Ingram and Roth32, Reference Talbott, Rothkopf and Shapses34, Reference Brochmann, Duarte, Zaidi and Murray36, Reference Black, Allison, Shapses, Tilmont, Handy, Ingram, Roth and Lane38. Some authors express bone parameters per 100 g body weightReference Sanderson, Binkley, Roecker, Champ, Pugh, Aspnes and Weindruch16, Reference LaMothe, Hepple and Zernicke18, Reference Lambert, Lamothe, Zernicke, Auer and Reimer37, which skews the data. Therefore, it remains unclear if there is a direct relationship between body weight and BMD, and whether this link is age-dependent and similar at weight-bearing and non-weight-bearing sites.
Femoral BMD was significantly lower in energy and protein-energy restricted animals (Fig. 2). This result is consistent with the observations in old rats of Talbott et al. Reference Talbott, Cifuentes, Dunn and Shapses17 and Lee et al. Reference Lee, Panemangalore and Wilson15 who reported decreased BMD and bone mineral content in response to dietary restriction. Sanderson et al. Reference Sanderson, Binkley, Roecker, Champ, Pugh, Aspnes and Weindruch16 and Black et al. Reference Black, Allison, Shapses, Tilmont, Handy, Ingram, Roth and Lane38 also observed a detrimental effect of dietary restriction on bone in rats and monkeys, but this effect was only attributed to body weight variation. In contrast to these results, our data support the view that BMD variations are not related to body weight reduction, as demonstrated by the ANCOVA analysis. Our results are consistent with those published by LaMothe et al. Reference LaMothe, Hepple and Zernicke18, who demonstrated that the impaired tibia structural properties associated with energy restriction were independent of body mass. Therefore, dietary-induced modulation of hormonal factors is likely to contribute to these variations.
BMD changes may be explained by several factors. At the metaphyseal site, the trend recorded for metaphyseal BMD was associated with a decrease in bone volume in the ER animals (Table 2). This decrease in bone volume:total tissue volume seems to be the result of a lower trabecular thickness, whereas trabecular number and separation were unchanged. Surprisingly, no changes were seen in the PER groups. Thus, energy restriction alone seems to have a more pronounced effect than simultaneous protein and energy restriction. In contrast, Bourrin et al. Reference Bourrin, Toromanoff, Ammann, Bonjour and Rizzoli41 reported a decrease in trabecular thickness in the tibia proximal metaphysis in response to protein restriction, as well as a decrease in BMD. However, the applied restriction was far more severe (protein level 2·5 %) than in the present study (protein level 10·2 %). In this study, the amount of dietary protein modulated the trabecular volume, but was not correlated with the BMD data. At the diaphyseal site (Table 2), the low BMD recorded in the restricted animals is consistent with a lower cortical tissue area. This indicates a decreased diaphyseal width and a lower diaphyseal cortical area. Both parameters are highly correlated with diaphyseal BMD (r 0·416, P = 0·005 and r 0·716, P < 0·0001, respectively). Cortical area changes resulted in altered femoral biomechanical properties in the restricted rats (r 0·586, P < 0·0001). NnakweReference Nnakwe42 and Talbott et al. Reference Talbott, Cifuentes, Dunn and Shapses17 similarly identified a decline in ultimate bone strength in rats fed a 40 % restricted diet.
Our results clearly indicate that energy and protein undernutrition affect bone status in aged rats. Nevertheless, adequate protein intake did not prevent the detrimental effects of energy restriction. Indeed, no difference was noted between PER and ER with respect to femoral BMD and the corresponding mechanical data (Fig. 2(a)). Similarly, Bourrin et al. Reference Bourrin, Toromanoff, Ammann, Bonjour and Rizzoli41 reported a decrease in BMD and bone strength with protein restriction in aged male rats. Ammann et al. Reference Ammann, Laib, Bonjour, Meyer, Ruegsegger and Rizzoli20 showed that bone strength was reduced by a low protein diet (only 2·5 % protein in the diet) in adult female ovariectomised rats. However, the consumption of an isoenergic essential amino acid supplement corrected these variations.
The values for the physical bone measurements were associated with a decrease in both bone formation and resorption markers (Fig. 3). The plasma OC levels tended to decrease in the PER groups and reached significant values in the ER groups, indicating a reduced bone formation rate. Similarly, lower urinary DPD excretion rates were recorded in the restricted animals (PER and ER), suggesting a reduced bone resorption. The bone metabolism data were not consistent with the BMD values, and did not explain the decrease in femoral BMD. Indeed, there was no bone remodelling imbalance. This difference might be due to the fact that the DPD and OC assays were carried out on samples collected on the last day of the experiment. Thus, these reflect the bone status at this specific time point, whereas the BMD variations reflect effects accumulated over the entire duration of the experiment. The exact impact of dietary restrictions on bone biomarkers will require further studies as previous reports have shown conflicting resultsReference Lane, Black, Handy, Shapses, Tilmont, Kiefer, Ingram and Roth32, Reference Talbott, Rothkopf and Shapses34, Reference Ndiaye, Cournot, Pelissier, Debray and Lemonnier43.
Protein restriction (10·2 %, compared to the normal level of 17 %) did not change the plasma OC levels (Fig. 3), whereas the DPD levels were significantly lower in the PER groups than in the ER groups. It seems that the PER diets induced more resorption than the ER conditions, but this was not correlated with the BMD values. Using different nutritional conditions, Bourrin et al. Reference Bourrin, Toromanoff, Ammann, Bonjour and Rizzoli41 demonstrated that protein deprivation (2·5 % v. 15 %) was associated with a decrease in OC levels from the first week of deficiency, while urinary DPD remained unchanged throughout the experiment.
Dietary restrictions (PER and ER) were associated with lower plasma IGF-1 levels (Fig. 4). Nutritional status (especially energy and dietary protein intake) is a critical factor in the regulation of circulating IGF-1 levelsReference Thissen, Ketelslegers and Underwood44. Considering the bone anabolic effect of IGF-1Reference Clemmons and Underwood45, this decrease might explain, at least in part, the changes in femoral BMD and bone biomarkers. This is supported by the positive correlations between IGF-1 levels and BMD (r 0·350, P = 0·025), cortical area (r 0·353, P = 0·032) and biomechanical properties (r 0·317, P = 0·043), respectively.
In this study, the IGF-1 levels did not vary significantly between the two protein intake levels (PER and ER) (Fig. 4). Yet, dietary proteins are known to influence both the production and action of IGF-1Reference Isley, Underwood and Clemmons46. Plasma IGF-1 levels have been shown to decrease with protein restrictionReference Bourrin, Toromanoff, Ammann, Bonjour and Rizzoli41, Reference Thissen, Ketelslegers and Underwood44, Reference VandeHaar, Moats-Staats, Davenport, Walker, Ketelslegers, Sharma and Underwood47 and Ammann et al. Reference Ammann, Bourrin, Bonjour, Meyer and Rizzoli19 suggested that an impaired IGF-1 system leads to decreased bone mineral mass and fragility under protein deprivation. However, these conclusions are based on data from animals fed a 2·5 % casein diet, which is a drastic deprivation. No significant changes were detected in bone parameters and plasma IGF-1 levels in rats fed diets containing more than 5 % protein. This is in agreement with our observations.
The PER-WP and ER-C groups exhibited lower plasma IGF-1 levels. This could be attributed to time-dependent variations between casein and WP digestionReference Boirie, Dangin, Gachon, Vasson, Maubois and Beaufrere21, Reference Mahe, Roos, Benamouzig, Davin, Luengo, Gagnon, Gausserges, Rautureau and Tome48. The IGF-1 levels were most likely reduced in the PER-WP group because of the faster absorption rate of WP compared to casein.
Overall, protein quality had little impact on bone status. Nevertheless, rats fed the casein diets exhibited a high number of trabeculae than the WP-fed animals, resulting in an increased bone volume (Table 2). A parallel response was seen in total BMD (P = 0·073) and plasma OC (P = 0·072), even if the trends were not statistically significant. In previous studies of rats, dietary casein was demonstrated to stimulate bone mineralisation by improving Ca deposition in bone and inhibiting bone resorptionReference Sato, Noguchi and Naito27, Reference Matsui, Yano, Awano, Harumoto and Saito49. In contrast, in mini-pigs casein-derived casein phosphopeptides had only marginal effects on bone mineral contentReference Scholz-Ahrens and Schrezenmeir28.
According to our data, WP consumption did not improve bone status more effectively than casein. Paradoxically, Takada et al. Reference Takada, Kobayashi, Kato, Matsuyama, Yahiro and Aoe23 found that WP consumption increased the breaking strength and suppressed bone resorption in ovariectomised female rats. Similarly, Kelly et al. Reference Kelly, Cusack and Cashman24 demonstrated that WP intake increased alkaline phosphatase activity and IGF-1 mRNA levels in young rats, suggesting enhanced bone formation. In the present study, protein quality had no effect on the OC and plasma IGF-1 levels. These differences can be attributed to the age of our experimental animals (21 months), because ageing is associated with impaired IGF-1 secretionReference Hammerman50, resulting from perturbations to the hypothalamo–adenohypophysial–somatotrope axisReference Thissen, Ketelslegers and Underwood44, Reference Sonntag, Lenham and Ingram51.
Urinary Ca excretion was unchanged with the different types of protein. However, casein was previously shown to enhance Ca absorption, due to the bioactive casein phosphopeptides resulting from the digestive breakdown of caseinReference Scholz-Ahrens and Schrezenmeir28. In contrast, other studies found no stimulating effect of casein phosphopeptides on intestinal Ca absorption, neither in ratsReference Brommage, Juillerat and Jost52 nor in human subjectsReference Teucher, Majsak-Newman, Dainty, McDonagh, FitzGerald and Fairweather-Tait53. Zhao et al. Reference Zhao, Martin, Wastney, Schollum and Weaver54 reported a Ca absorption-enhancing effect of WP intake, but it was absent during long-term WP-feeding. The lack of variation in Ca absorption in this study could be due to adaptation, which would eliminate the stimulating effect of dietary casein and WP. As suggested in the Zhao study, this effect could be consistent with a down regulation of active Ca absorption, through a suppression of the parathyroid hormone–vitamin D axis, in response to the initial increase in Ca absorption during chronic feeding. In our opinion, the lack of variation in this study may be due to modulation of passive and active Ca transport during ageing. Indeed, it is well-established that ageing often is associated with impaired Ca absorption as well as vitamin D deficiency and this can result in secondary hyperparathyroidismReference Lips55.
To summarise, protein–energy restriction and energy restriction alone induced lower femoral BMD and impaired biomechanical properties, compared to controls, independently of body weight variations. Our study confirms that nutritional deficiencies may contribute to age-related bone loss, since lower BMD and biomechanical resistance are associated with an increased risk of bone fracture. These changes could be attributed to a decrease in IGF-1 levels, but the exact mechanisms need to be identified. No bone-sparing effect has been reported when energy restriction is associated with an adequate protein intake. Under our experimental conditions, neither casein nor WP appear to prevent the detrimental effects of dietary restrictions on bone mass. Nevertheless, diets providing casein seem to preserve bone health more efficiently than those containing WP, as judged by BMD and histomorphometry. In this study, mineral intake was standardised in every group, which is important because energy and protein undernutrition often are associated with Ca deficiency in the elderly. It is conceivable that disruption in dietary Ca intake, in addition to energy and protein restriction, could have a more pronounced effect on bone metabolism.










