As the proportion of the elderly population in the world increases, so will the prevalence of cognitive impairment as a result of normal ageing and neurodegenerative conditions such as Alzheimer's disease (AD)( Reference Raffai and Weisgraber 1 ), which is one of the most common forms of neurodegenerative disease. Patients who suffer from cognitive deficits exhibit memory loss, delusions and problems carrying out activities associated with normal daily life. The pathologic characteristics of AD include the presence and accumulation of β-amyloid (Aβ), neurofibrillary tangles, inflammation, oxidative stress and cell loss in the brain( Reference Glenner and Wong 2 ). There are two subtypes of AD. One is caused by genetics (early-onset AD), and the other, more common, subtype is called late-onset AD. Factors that cause late-onset AD include ageing, diabetes mellitus, hypercholesterolaemia and oxidative stress( Reference Puglielli, Tanzi and Kovacs 3 ).
Studies have examined the relationship between increased plasma cholesterol concentrations and AD( Reference Notkola, Sulkava and Pekkanen 4 ). They have also indicated that the cholesterol level in the brain is one of the factors that regulates amyloid precursor protein (APP) processing and Aβ formation( Reference Wolozin 5 ). The accumulation of cholesterol in hippocampal neurons, which results in accelerated cleavage of the APP into amyloidogenic components, has been observed in cell culture results( Reference Kolsch, Lutjohann and Tulke 6 ). In the brain, cholesterol almost exclusively originates from in situ synthesis, whereas circulating cholesterol is normally prevented from entering the central nervous system by the blood–brain barrier( Reference Bjorkhem 7 ). Because cholesterol cannot be eliminated from the central nervous system and may be toxic to neurons when it is present in excess, it is converted to 24-hydroxycholesterol (24-OHC) by 24-hydroxylase (also called Cyp46) before being transported out of the brain. This mechanism constitutes the major pathway of cholesterol homoeostasis in the brain. Epidemiological studies have indicated that 24-OHC concentrations in cerebrospinal fluid are increased in AD patients( Reference Schonknecht, Lutjohann and Pantel 8 ).
The Oriental plum (Prunus salicina Lindley), also called the Chinese plum or Japanese plum, is rich in polyphenols and anthocyanins. It has been shown that consuming polyphenol-rich foods may prevent the onset of AD( Reference Lau, Shukitt-Hale and Joseph 9 ). In addition, the accumulation of Aβ in the brain was reduced and cognitive degradation was slowed in streptozotocin-induced diabetic rats that were fed a powered plum diet( Reference Kao-Ting, Yue-Hwa and Ching-I 10 ). It has also been shown that blood cholesterol concentrations in rats can be reduced in a dose–response manner by consuming a polyphenol-rich diet( Reference Boyer and Liu 11 , Reference Osada, Suzuki and Kawakami 12 ). The purpose of the present study was therefore to examine the effects of consuming Oriental plums on cognitive performance and cerebral neurodegeneration-related protein expression in C57BL/6 mice that were fed a cholesterol-enriched diet.
Materials and methods
Animals and diets
The present experiment was approved by the Institutional Animal Care and Use Committee or Panel (IACUC/IACUP) of Taipei Medical University. A total of sixty 4-week-old male C57BL/6-strain mice were purchased from BioLASCO. Before the experiment began, the mice had access to food and water ad libitum and were housed at 23 ± 2°C. After being allowed to acclimatise to these conditions for 2 weeks, the mice were randomly divided into four groups of twenty mice each and kept on a 12 h light–12 h dark cycle. Each group received one of the following treatments for 20 weeks (5 months): the control (Ctrl) group was fed the American Institute of Nutrition (AIN)-93M diet; the high cholesterol (HC) group was fed the AIN-93M diet with 5 % cholesterol; the high cholesterol+low Oriental plum (LOP) group was fed the AIN-93M diet with 5 % cholesterol and 2 % Oriental plum powder; and the high cholesterol+high Oriental plum (HOP) group was fed the AIN-93M diet with 5 % cholesterol and 5 % Oriental plum powder. Oriental plums (P. salicina) were purchased from a local market. Dried plum powder was produced by freeze-drying and then grinding the plums. The experimental diets were prepared according to the AIN-93M formulation with or without 5 % cholesterol and/or dried plum powder. The high-cholesterol diet supplemented with 5 % cholesterol for inducing hypercholesterolaemia in mice was modified from a previous study( Reference Lu, Wu and Zheng 13 ). Maize starch powder in the HC group was partly substituted with dried plum powder in the LOP and HOP groups. The amounts of dried plum powder used in the experimental diets were based on our earlier findings, which showed that Oriental plums had beneficial effects on cognitive function( Reference Kao-Ting, Yue-Hwa and Ching-I 10 ). The total phenolic and anthocyanin contents of the dried powder were 24·09 (sem 0·11) mg gallic acid equivalent/g dried weight of powder and 2·28 (sem 0·01) mg cyanidin-3-glucoside equivalent/g dried weight of powder, respectively. The measurements of the antioxidant contents of the dried plum power were described in our previous work( Reference Kao-Ting, Yue-Hwa and Ching-I 10 ). Diet compositions are shown in Table 1. All mice consumed food and water ad libitum for 20 weeks before cognitive testing was conducted. The body weights of the mice were measured every week. Blood samples were collected every month to measure thiobarbituric acid-reactive substances and cholesterol concentrations. After 5 months of treatment, the mice were starved for 12 h and then killed using anaesthesia with an intraperitoneal injection of a solution of Zoletil 50 (Virbac) and 2 % Rompun (1:1 ratio, 1 ml/kg body weight; Bayer). The mice were then transcardially perfused with ice-cold 0·1 m-PBS (pH 7·4), and their brains were rapidly removed. The cortices and hippocampi were immediately collected, snap-frozen in liquid N2 and stored at − 80°C until analysis.
Table 1 Diet composition of each group
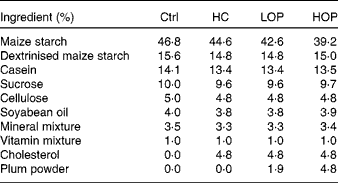
Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum.
Morris water maze task
The Morris water maze task has been used extensively in investigations related to cognitive function in rodents. In the present study, it was performed after 5 months of treatment according to the method described by Vorhees & Williams( Reference Vorhees and Williams 14 ). The apparatus (TSE Systems) consisted of a circular pool (diameter: 150 cm, height: 100 cm) filled with water (depth: 60 cm, 24 ± 2°C) that was placed in a room that had consistently located spatial cues. An escape platform (diameter: 14 cm) was placed in the middle of one of the quadrants 1 cm below the water surface and equidistant from the side wall and the middle of the pool. The platform provided the only escape from the water and was located in the same quadrant in every trial. Four different starting positions were equally spaced along the perimeter of the pool. On each training day during the acquisition trial, all four starting positions were used one time in a random sequence (i.e. four training trials per day). The trial began by placing the animal in the water facing the wall of the pool at one of the starting points. If the mouse failed to escape within 60 s, it was gently conducted to the platform. It was allowed to stay on the platform for 15 s. The inter-trial interval was 15 min. After each trial, the mice were dried, and they were returned to their cages at the end of the session. The animals were trained for 3 d. At 24 h after the last training session, the mice were submitted to a probe trial. Before this session, the submerged platform was removed. The retention test consisted of placing the mice in the water for 60 s. Performances were evaluated and analysed with image tracking software (FG34PATH; HaSoTec), which measured the number of crossings over the original platform location, the latency of arrival at the original platform location and the time spent in the target quadrant.
Serum cholesterol concentrations
Serum was separated from blood samples by centrifugation at 3000 g for 10 min at 4°C. Concentrations of total cholesterol were determined by the colorimetric enzymatic method using an autoanalyser (Roche Modular P800).
Serum thiobarbituric acid-reactive substances
A method modified from that described by Yagi( Reference Yagi 15 ) was used. Serum (100 μl) was reacted with 800 μl of 0·22 % H2SO4, 100 μl of 10 % phototungstic acid and 200 μl of a 0·61 % thiobarbituric acid solution. The samples were heated to 95°C for 1 h before the addition of 400 μl of 1-butanol. The samples were then vigorously stirred for 2 min and centrifuged at 800 g for 15 min. The absorbance of the organic phase was measured at excitation of 515 nm and emission of 555 nm, and the samples were compared to a blank.
Brain cholesterol and 24-hydroxycholesterol concentrations
After the Morris water maze test, the mice were killed. The cortices and hippocampi of the mice were excised and minced. Cholesterol was extracted using the procedure described by Folch et al. ( Reference Folch, Lees and Sloane Stanley 16 ). Total cholesterol was determined using a commercial kit (Roche). Brain 24-OHC content was determined using a 24(S)-hydroxycholesterol ELISA kit (Enzo Life Sciences). The resulting yellow colour was read at 450 nm. The signal was inversely proportional to the concentration of 24-OHC in the sample.
Cyp46 mRNA analysis
Total RNA was isolated from the cortical and hippocampal samples using TRIzol reagent according to the manufacturer's protocol (Life Technologies). Complementary DNA was synthesised by reverse transcription using 3 μg of total RNA. Complementary DNA was synthesised using 1 μg of oligo dT, 1 μl of an RNase inhibitor, 2 μl of 10 mm-dNTP mixture, 4 μl of 5 × reaction buffer and 1 μl of Moloney murine leukaemia virus (MMLV) (RT) in an 8-μl reaction mixture. After allowing the oligo dT to anneal further at 65°C for 5 min, reverse transcription was carried out at 45°C for 60 min. The enzyme, RT, was heat-inactivated at 70°C for 5 min. To quantify the mouse Cyp46 mRNA, 1–3 μg of complementary DNA was used as a temperate for a real-time PCR analysis based on SYBR Green I with the LightCycler® Carousel-Based System (Roche Diagnostics). A 285-bp PCR product containing Cyp46 was amplified using specific primers (forward primer: 5′-AAC TTT GTC ACC TTC TTC ATT GC-3′; reverse primer: 5′-CCA TCA CTG TGA ATG CCA GA-3′). Amplification and detection were done on a LightCycler 480 System (Roche Diagnostics) under the following conditions: an initial 5 min denaturation step at 94°C, followed by thirty-five cycles of denaturation at 94°C for 10 s, annealing at 60°C for 5 s and extension at 72°C for 8 s. The specificity of the amplified PCR product was inspected by performing a melting curve analysis on the LightCycler 480 System. The gene expression of Cyp46 was calculated based on crossing point values obtained by the second derivative maximum procedure (with β-actin as the housekeeping gene).
β-Secretase 1 and β-amyloid protein analysis
Proteins were extracted from cortical and hippocampal samples using radioimmunoprecipitation assay (RIPA) lysis buffer (50 mm-Tris–HCl at pH 7·6, 150 mm-NaCl, 0·1 % SDS, 0·5 % sodium deoxycholate and 1 % Triton X-100) with protease and a phosphatase inhibitor cocktail (Thermo Fisher Scientific). Extracted proteins were separated by 12 % SDS-PAGE. Following electrophoresis, the separated proteins in the gel were transferred onto polyvinylidene difluoride membranes (Millipore) at 80 mA for 2 h. Once the transfer was complete, the membranes were immediately incubated in blocking buffer for 1 h. To detect the target proteins, blotted membranes were incubated overnight at 4°C with primary antibodies against Aβ1-42 (mice anti-Aβ; Millipore), BACE1 (rabbit anti-BACE; Abcam) and secondary antibodies (goat anti-mouse IgG and goat anti-rabbit IgG (Sigma). Before quantifying the expression of the target proteins, a peroxidase-based detection of the blot was performed with a Western blotting luminal reagent and was visualised with a Biospectrum AC imaging system (UVP). Quantification of the target proteins was analysed using Image-Pro Plus software (Media Cybernetics).
Statistical analysis
Data are expressed as means with their standard errors. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc.) and SPSS version 18.0 (SPSS, Inc.). The correlation between serum and brain cholesterol concentrations was analysed by Pearson's correlation coefficient analysis. A repeated measures ANOVA was performed on the Morris water maze test data. A one-way ANOVA followed by Duncan's post hoc test was performed on the other analyses. Statistical significance was set at P <0·05 for all of the tests.
Results
Body weight changes and serum cholesterol concentrations
Mean body weight among the groups did not differ significantly throughout the 5-month experiment period (averages of 23–36 g in each group, data not shown). Fig. 1 shows that the mice that consuming a 5 % high-cholesterol diet (the HC group) exhibited a significantly higher concentration in serum cholesterol at the end of the experiment than the Ctrl and HOP groups did (P= 0·029). No significant differences in cholesterol concentrations were found among the Ctrl, LOP and HOP groups after 5 months of treatment.

Fig. 1 Serum cholesterol concentrations at 1 month and 5 month during the experimental period. Values are means (n 20 at 0 months; n 15 at 5 months), with their standard errors represented by vertical bars. * Mean values were significantly different (P= 0·029; Duncan post hoc analysis). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum. To convert cholesterol from mg/dl to mmol/l, multiply by 0·0258.
Cortical and hippocampal cholesterol concentrations
After five months of consuming a 5 % high-cholesterol diet, the HC group had significantly higher cholesterol concentrations (P= 0·008) in the cortex and hippocampus than the other groups did (Fig. 2). The cholesterol concentration in the cortex and hippocampus in the HOP group was not significantly different from that in the Ctrl group at the end of the experiment.

Fig. 2 Cholesterol concentrations in the hippocampus (![]() ) and cortex (
) and cortex (![]() ) at the end of month 5. Values are means (n 6–8 per group), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different from the matched tissue (P= 0·008; Duncan post hoc analysis). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum. To convert cholesterol from mg/dl to mmol/l, multiply by 0·0258.
) at the end of month 5. Values are means (n 6–8 per group), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different from the matched tissue (P= 0·008; Duncan post hoc analysis). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum. To convert cholesterol from mg/dl to mmol/l, multiply by 0·0258.
Correlation between serum and brain cholesterol concentrations in mice
Fig. 3 shows a significantly positive correlation between serum and cortical (Fig. 3(A), r 0·73, P= 0·0001)/hippocampal (Fig. 3(B), r 0·79, P= 0·0001) cholesterol concentrations in the mice from all of the groups.

Fig. 3 Correlation between serum and brain cholesterol levels in mice (n 4–6 per group). (A) Cortex: r 0·73, P= 0·0001; (B) hippocampus: r 0·79, P= 0·0001. To convert cholesterol from mg/dl to mmol/l, multiply by 0·0258. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Lipid peroxidation in serum
The results of measuring lipid peroxidation in the serum, which is expressed as thiobarbituric acid-reactive substances, showed that the thiobarbituric acid-reactive substances concentration in the serum of the HOP group was significantly lower (P= 0·051) than that of the HC group and was not significantly different from that of the Ctrl group at the end of the experiment (Fig. 4).
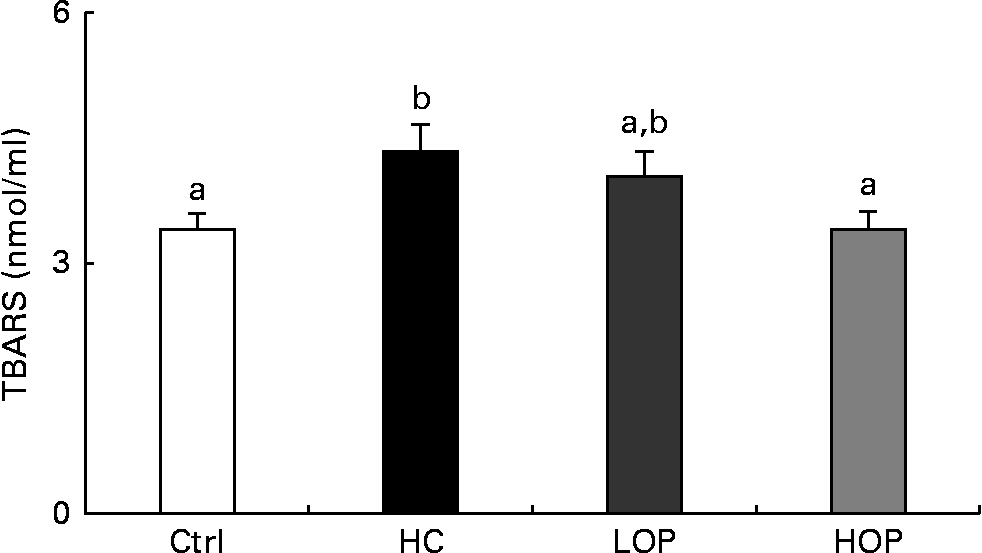
Fig. 4 Serum thiobarbituric acid-reactive substance (TBARS) concentrations in mice. Values are means (n 11–13 per group), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P= 0·051; Duncan post hoc analysis). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum.
Cognition analysis with the Morris water maze
Fig. 5(A) shows that after consuming a high-cholesterol diet for 5 months, on day 1 of the task, mice in the HC group had a longer escape latency than those in the Ctrl and HOP groups (P= 0·006). On day 3, mice in the HOP, LOP and Ctrl groups spent significantly shorter time finding the target than those in the HC group did (P= 0·0012). On the other hand, on day 4 (Fig. 5(B)), the time spent in the previously learned target quadrant by the HOP group tended to be closer to that of the Ctrl group and longer than those of the HC and LOP groups (P= 0·054).

Fig. 5 Cognitive function of mice as assessed by the Morris water maze at month 5. Values are means (n 6–8 per group), with their standard errors represented by vertical bars. (A) Spatial learning and memory trial (latency time to find a hidden platform during a 3 d acquisition session in four trial blocks). (B) Spatial memory trial (% time in the target quadrant in the probe trial). a,b,cMean values with unlike letters were significantly different (P= 0·006 for day 1; P= 0·0012 for day 3; P= 0·054 for day 4; Duncan post hoc analysis). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Expression of Cyp46 mRNA and 24-hydroxycholesterol concentrations in the brain
Cyp46 plays an important role in the elimination of brain cholesterol and the conversion of brain cholesterol into 24-OHC. Fig. 6(A) and (B) shows the expression of Cyp46 mRNA in the cortex and hippocampus at the end of the experiment. In the cortex, the expression of Cyp46 mRNA in the HOP and LOP groups was lower than that of the HC group (P= 0·05). The concentration of 24-OHC in the cortex and hippocampus was also lower in the HOP group than that of the HC group (P= 0·05) (Fig. 7).

Fig. 6 Expression of 24-hydroxylase (Cyp46) mRNA in (A) the cortex and (B) the hippocampus in mice. Values are means (n 6–8 per group), with their standard errors represented by vertical bars. Total RNA was extracted from the brains of mice and quantified in real time. a,bMean values with unlike letters were significantly different (P= 0·05; Duncan post hoc analysis). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum.

Fig. 7 24-Hydroxycholesterol (24-OHC) contents in (A) the cortex and (B) the hippocampus in mice. Values are means (n 6–8 per group), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P= 0·05). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum. To convert 24-OHC from mg/dl to mmol/l, multiply by 0·0248.
Expression of β-secretase 1 and β-amyloid proteins in the brains of mice
BACE1 and Aβ expressions in the brains of the mice in each group are shown in Figs. 8 and 9. In the cortex and hippocampus, the expression of both BACE1 and Aβ in the HOP group was significantly lower than that of the HC group (P= 0·05).

Fig. 8 Expression of the β-secretase 1 (BACE1) protein in (A) the cortex and (B) the hippocampus in mice. Values are means (n 3 per group) of the percent relative density (with the control set to 100 %), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P= 0·05; Duncan post hoc analysis). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum.

Fig. 9 Expression of β-amyloid (Aβ) protein in (A) the cortex and (B) the hippocampus in mice. Values are means (n 3 per group) of the percent relative density (with the control set to 100 %), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P= 0·05; Duncan post hoc analysis). Ctrl, control; HC, high cholesterol; LOP, high cholesterol+low Oriental plum; HOP, high cholesterol+high Oriental plum.
Discussion
In the present study, we investigated the protective effect of polyphenol-rich Oriental plums on cognitive function and cerebral neurodegeneration-related protein expression in mice that were fed a high-cholesterol diet. A loss of short-term memory is commonly observed among AD patients( Reference Glenner and Wong 2 ). We found that mice that consumed a high-cholesterol diet for a fairly long experimental period (5 months) had elevated cholesterol concentrations in the serum and brain as well as elevated levels of Aβ in the brain when the diet was not supplemented with Oriental plum powder. The accumulation of Aβ is one of the pathological features of AD. Evidence from earlier studies has shown that a high-cholesterol diet induces oxidative stress and inflammatory response and might be related to neurodegeneration and cognitive deficits( Reference Thirumangalakudi, Prakasam and Zhang 17 ). Recent findings have indicated that consuming a diet high in cholesterol causes some cognitive decline in mice. In the present study, the addition of 2 and 5 % Oriental plum powder to a high-cholesterol diet ameliorated the increased cholesterol concentrations in mice. In addition, we also found that there was a positive correlation between serum and brain cholesterol concentrations, which indicates that the blood–brain barrier function might be impaired under a long-term high plasma cholesterol concentration and that cholesterol levels in the brain are thus affected.
The pathogenesis of cognitive deficits caused by elevated cholesterol levels in the brain is still not completely understood( Reference Refolo, Malester and LaFrancois 18 ). However, factors such as increased BACE1, 24-OHC and Aβ levels in the brain have been found to be associated with neurodegeneration( Reference Schonknecht, Lutjohann and Pantel 8 ). The Aβ protein is derived from cleaving APP by BACE1 through the β pathway. BACE1 is located in cholesterol-rich regions (i.e. in lipid rafts). Thus, elevated cholesterol concentrations may increase the size of lipid rafts and the activity of BACE1( Reference Raffai and Weisgraber 1 ). Some clinical observations provided in vivo and in vitro evidence indicating that cholesterol plays a role in APP processing and Aβ generation( Reference Bouillot, Prochiantz and Rougon 19 ). It has also been shown that when rabbits were fed a diet enriched with cholesterol, they had increased levels of Aβ in the brain( Reference Reiss and Voloshyna 20 ). In transgenic mice that expressed a mutant human APP, Aβ deposits increased in the brain along with plasma cholesterol concentrations( Reference Umeda, Tomiyama and Kitajima 21 ). In the present study, expressions of BACE1 and Aβ in the HC group were significantly higher than those in the Ctrl and HOP groups, which suggests that cognitive declines in the HC group may have been caused by higher BACE1 and Aβ levels.
The conversion of cholesterol into 24-OHC by Cyp46 appears to be one of the most important mechanisms in the central nervous system for transporting cholesterol out of the brain( Reference Bjorkhem 7 ). The present results showed that Cyp46 mRNA expression significantly increased in the cortices and hippocampi of the mice in the HC group. Its expression at transcriptional level was shown to be stable and not affected by steroid hormones or other factors, such as statins, cholesterol or oxysterols, but it can be regulated by oxidative stress( Reference Ohyama, Meaney and Heverin 22 ). Thus, the present findings suggest that the increased Cyp46 mRNA expression in cholesterol-treated mice may be a consequence of oxidative stress, which is indicative of the cholesterol accumulation in the brain( Reference Aytan, Tamtürk and Kartal-özera 23 ). In the cortex but not the hippocampus, the elevations were significantly blunted or ameliorated when compared with data from the LOP and HOP groups, which suggests that OP might act as an antioxidant to attenuate brain cholesterol oxidation. The increased Cyp46 mRNA expression might reflect an increase in its protein expression, which in turn might lead to the increased production of 24-OHC( Reference Ohyama, Meaney and Heverin 22 ). Although we did not measure the protein level of Cyp46, this assumption is supported by the fact that significantly increased 24-OHC was found in the cortex and hippocampus in the HC group. It has been shown that the rate of transporting 24-OHC out of the brain is slower than that of forming 24-OHC from cholesterol; this might have caused too much 24-OHC and cholesterol in the brain in the present study. The raised 24-OHC might represent a higher catalytic efficiency of Cyp46. It should be noted that this increase seemed to be higher in the hippocampus than in the cortex, even though both areas appeared to have a similar degree of Cyp46 mRNA expression. Because Cyp46 mRNA and protein expression is neuron-specific( Reference Russell, Halford and Ramirez 24 ), we speculated that the difference in 24-OHC levels between the cortex and the hippocampus after cholesterol administration might be the result of other independent pathways, for example, the regulation of Cyp46 at the post-translation level. Further studies are warranted to elucidate this assumption. In addition, 24-OHC has been reported to prevent against the formation of amyloid( Reference Björkhem 25 ), which confirms the present finding of lower levels of Aβ in the hippocampus than in the cortex in the HC group. Although a clinical study has shown that 24-OHC was higher in the frontal cortices of AD patients as compared to the controls( Reference Heverin, Bogdanovic and Lütjohann 26 ), there is a lack of evidence to support a correlation between the levels of 24-OHC in the different subregions (i.e. the cortex and the hippocampus) and cognitive impairments. The increased levels of 24-OHC observed in patients with neurodegenerative disorders were more likely the consequence of blood–brain barrier damage or neuronal cell death rather than the metabolic inactivity of a large number of neuronal cells( Reference Leoni, Masterman and Mousavi 27 , Reference Leoni, Masterman and Patel 28 ).
Polyphenols are natural antioxidants. It has been shown that polyphenols reduce serum cholesterol by inhibiting cholesterol absorption, increasing bile flow and inhibiting the activities of 3-hydroxy-3-methylglutaryl-CoA reductase and acyl-CoA:cholesterol acyltransferase( Reference Krecman, Skottova and Walterova 29 , Reference Gorinstein, Leontowicz and Leontowicz 30 ). In addition, polyphenols are able to penetrate the blood–brain barrier and hence to protect neurons from oxidative stress( Reference Williams and Spencer 31 ). In the present study, those mice that consumed diets containing plum powder had lower peripheral plasma cholesterol and brain cholesterol, which may be the reason why they performed better in the cognitive tests than those that did not consume plum-containing diets. Furthermore, the present findings demonstrated that consuming a diet with 5 % Oriental plum powder significantly decreased plasma and brain cholesterol concentrations, the mRNA expression of Cyp46, BACE1 protein, Aβ protein and 24-OHC in the cortex and the hippocampus and improved the cognitive function of mice that had cognitive declines as the result of a high-cholesterol diet. This suggests that a plum powder-containing diet may be beneficial for preventing hypercholesterolaemia-related cognitive impairment. Additionally, 2 and 5 % Oriental plum powder-supplemented diets are equivalent to 32·4 and 81 g of plum powder for a 60 kg human per d, respectively, based on the body surface area formula( Reference Reagan-Shaw, Nihal and Ahmad 32 ). According to the United States Department of Agriculture food consumption database, the standard serving of dried plums is 42 g (five dried plums)( Reference Stacewicz-Sapuntzakis 33 ). Consequently, the dose of daily plum powder (approximately 1 or 2 servings) used in the present study was similar to the typical suggested adult intake. The larger dose (i.e. 5 % plum powder supplementation) proved to be more effective in delaying hypercholesterolaemia-related cognitive declines. Accordingly, a higher-percentage plum powder diet is theoretically consumable and more practical for further clinical studies to determine whether the same significant effect is found in human subjects with hypercholesterolaemia.
Acknowledgements
We thank Kao-Ting Lee of Sentosa Company Limited for providing technical support during the present experiment. We also thank Professor Si-Chuan Shen of National Taiwan Normal University for providing suggestions.
P.-H. K. contributed to the lab work. P.-H. K. and C.-I. L. drafted the manuscript. W.-C. C. provided advice on several aspects of the study. Y.-H. C. helped design the experiment, discussed the results and commented on the manuscript. S.-H. L. conducted the experiment, interpreted the data, supervised the research project and completed the manuscript.
The authors declare no conflicts of interest.















