Glucagon-like peptide-1 (GLP-1) is secreted from the endocrine L cells in the small and large intestine. GLP-17–36 (the most biologically active form) is rapidly broken down by protease enzymes, such as dipeptidyl peptidase-IV, to GLP-19–36. GLP-1 has been well characterised as having two main roles as a satiety hormone and as an incretin. As a satiety hormone, GLP-1 has been shown to both enhance satiety and reduce food intake, and has been proposed to be useful as a treatment for weight management. The role of GLP-1 as an incretin has been well defined, and it is believed that the action of incretins may account for at least 50 % of insulin that is released in response to oral glucose(Reference Kim and Egan1). As a result, analogues of GLP-17–36, which are resistant to dipeptidyl peptidase-IV, are being used as a therapeutic treatment for type 2 diabetes.
Dietary carbohydrates that pass through the small intestine to the colon can be classified as dietary fibre; these can act as a substrate for the gut microbiota, leading to the production of SCFA. It is these bioactive SCFA that in turn have been proposed to increase the production of GLP-1. This is thought to be mediated through SCFA binding to G-protein-coupled receptors (free fatty acid receptor 2/3; FFAR2/3) on GLP-1-secreting L cells in the colon(Reference Tolhurst, Heffron and Lam2). Therefore, the products of the fermentation of fibres may cause an increase in endogenous GLP-1 concentrations and thus may potentiate the effects of GLP-1 as an incretin as well as a satiety signal.
Resistant starch (RS), a non-viscous fermentable dietary fibre, has been shown in many animal studies to increase GLP-1 production(Reference Zhou, Martin and Tulley3–Reference Shen, Keenan and Martin5). Studies in human subjects where GLP-1 has been measured following increased RS feeding are limited(Reference Robertson, Currie and Morgan6–Reference Raben, Tagliabue and Christensen8), with only one of these being an acute feeding study(Reference Raben, Tagliabue and Christensen8), and these have predominately shown no effect of RS on GLP-1 concentrations. However, the beneficial effects that have been observed following increased RS consumption, ranging from 24 h to 12 weeks, have been an improvement in insulin sensitivity(Reference Robertson, Currie and Morgan6, Reference Robertson, Bickerton and Dennis7, Reference Johnston, Thomas and Bell9, Reference Maki, Pelkman and Finocchiaro10), insulin secretion(Reference Bodinham, Smith and Wright11), a reduction in food intake(Reference Bodinham, Frost and Robertson12) and increased satiety(Reference Willis, Eldridge and Beiseigel13, Reference Liljeberg, Akerberg and Bjorck14). The only potential candidate that could fulfil all of these roles would be GLP-1.
Our group has previously demonstrated a significant reduction in food intake following increased RS intakes (from high-amylose maize type 2 RS (HAM-RS2))(Reference Bodinham, Frost and Robertson12). The present study therefore aimed to follow a similar protocol but specifically measuring the endogenous secretion of GLP-1 and postprandial insulin and glucose concentrations. The study aimed to determine whether the previously observed reductions in food intake and changes to postprandial insulin concentrations could be due to increases in endogenous GLP-1 concentrations.
Experimental methods
Participants
A total of thirty healthy adult males, with a mean age of 24·5 (sem 0·7) years and a mean BMI of 26·2 (sem 0·9) kg/m2, participated in the present study. Participants were excluded if they had a history of gastrointestinal or endocrine disease. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Surrey Ethics Committee. Written informed consent was obtained from all participants.
Study design
The study design was based on that described previously(Reference Bodinham, Frost and Robertson12). The study was a single-blind, cross-over study in which participants visited the clinical unit on two occasions at least 1 week apart. On these clinical visits, participants consumed either 80 g Hi-Maize 260® (comprising 60 % type 2 RS (HAM-RS2) and 40 % rapidly digestible starch (RDS), therefore providing 48 g HAM-RS2 as measured by the Association of Official Analytical Chemists for total dietary fibre method 991.43) or 32 g of placebo (Amioca®, comprising 100 % RDS) supplying no dietary fibre. Both starches were manufactured and supplied by Ingredion Inc. Half of the starch was provided at breakfast and the other half at lunch, incorporated into a mousse, therefore providing 24 g dietary fibre or 0 g dietary fibre at each meal.
On arrival at the clinical unit, a cannula was placed into the antecubital fossa and two fasted blood samples were taken. At time zero, participants consumed the standardised breakfast (1587 kJ, 9·6 g protein, 67·1 g carbohydrate, 8·0 g fat and 0·5 g dietary fibre (24·5 g dietary fibre for the HAM-RS2 supplement)). Following the breakfast, blood samples were taken every 30 min for 3 h. Participants were then provided with the standardised lunch (3888 kJ, 36·1 g protein, 112·1 g carbohydrate, 37·0 g fat and 5·0 g dietary fibre (29·0 g dietary fibre for the HAM-RS2 supplement)). Further blood samples were collected for the following 4 h. Bowel habit and symptom diaries were completed on the day of the study and the following day to assess gastrointestinal tolerance of the starches.
Biochemistry
Whole blood was collected into fluoroxalate tubes for glucose analysis, potassium EDTA tubes for insulin analysis and potassium EDTA tubes with 200 kallikrein-inhibiting units aprotinin per ml of blood for C-peptide and total GLP-1 concentrations. All samples were centrifuged for 10 min at 3000 rpm and plasma stored at − 20°C until batch analysis. Plasma glucose concentrations were measured enzymatically using a commercially available kit for the ILab650 (Instrumentation Laboratory) and inter-assay variation was < 2 %. Plasma insulin and C-peptide concentrations were measured by RIA using commercially available kits (Millipore) with inter-assay variations of 18·2 and 17·7 %, respectively, and intra-assay variations of 9·2 and 9·5 %, respectively. Plasma total GLP-1 concentrations were measured by ELISA using a commercially available kit (Millipore) with an inter-assay variation of 15·0 % and an intra-assay variation of 4·5 %.
A priori power calculation and statistics
The thirty participants in the present cross-over study (α = 0·05) would yield an 80 % probability of detecting a 540 pmol/l per 420 min change in postprandial GLP-1 response (AUC, based on a treatment sd of 1018). All postprandial time course data were analysed using repeated-measures ANOVA. The incremental AUC (iAUC) was calculated for each meal using the trapezoid method, and then the iAUC was compared between the treatments using paired t tests. All statistical analyses were carried out using SPSS version 18 for Windows (SPSS, Inc.) with significance assumed as P <0·05. All results presented are means with their standard errors.
Results
Both HAM-RS2 and placebo were well tolerated by the participants with a similar frequency of bowel movements recorded and no adverse gastrointestinal effects reported on the day of the study or the following day. Participants' weight did not differ between the two study days.
Glucagon-like peptide-1 concentrations
There was a significant treatment × time interaction between HAM-RS2 and placebo for postprandial GLP-1 concentrations (P =0·020; Fig. 1).

Fig. 1 Postprandial plasma glucagon-like peptide-1 (GLP-1) concentrations. Values are means for thirty participants following 48 g high-amylose maize type 2 resistant starch (●) or placebo (○) consumption; consumed in two equal portions, one at time zero and the other at 180 min, with their standard errors represented by vertical bars. There was a significant treatment × time interaction using repeated-measures ANOVA (P =0·020).
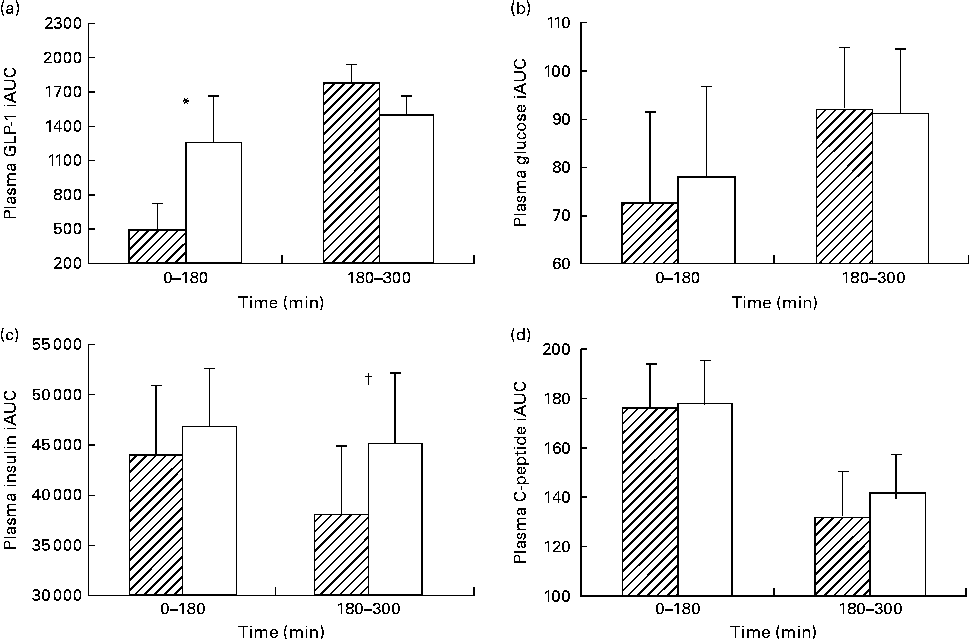
The iAUC for each meal showed a significantly lower GLP-1 response with HAM-RS2 compared with placebo following breakfast (P =0·025; Fig. 2(a)). However, following the lunch meal, there were no differences between the starches (Fig. 2(a)).

Fig. 2 Incremental AUC (iAUC) at each meal for plasma (a) glucagon-like peptide-1 (GLP-1), (b) glucose, (c) insulin and (d) C-peptide. Values are means for thirty participants following 48 g high-amylose maize type 2 resistant starch (HAM-RS2, ![]() ) or placebo (□) consumption; consumed in two equal portions at breakfast (time zero) and lunch (180 min), with their standard errors represented by vertical bars. * Mean value was significantly different for GLP-1 iAUC at time 0–180 min with HAM-RS2 compared with placebo (P =0·025) but not significantly different at time 180–300 min. † Mean value was significantly different for insulin iAUC at time 180–300 min with HAM-RS2 compared with placebo (P =0·034) but not significantly different at time 0–180 min. Mean values were not significantly different for glucose or C-peptide concentrations.
) or placebo (□) consumption; consumed in two equal portions at breakfast (time zero) and lunch (180 min), with their standard errors represented by vertical bars. * Mean value was significantly different for GLP-1 iAUC at time 0–180 min with HAM-RS2 compared with placebo (P =0·025) but not significantly different at time 180–300 min. † Mean value was significantly different for insulin iAUC at time 180–300 min with HAM-RS2 compared with placebo (P =0·034) but not significantly different at time 0–180 min. Mean values were not significantly different for glucose or C-peptide concentrations.
Postprandial metabolite concentrations
Over the whole 7 h postprandial period, there were no differences between HAM-RS2 and placebo for concentrations of plasma glucose, insulin or C-peptide.
The iAUC for insulin following breakfast (0–180 min) were not different between HAM-RS2 and placebo; however, after lunch (180–300 min), there was a significantly lower response following HAM-RS2 than with placebo (P =0·034; Fig. 2(c)).
There were no significant differences in iAUC between the RS and placebo for glucose or C-peptide (Fig. 2(b) and (d), respectively).
Discussion
To our knowledge, this is the first study that has investigated the effects of increased HAM-RS2 intake on endogenous GLP-1 concentrations when compared with a placebo matched for both energy and glycaemic load, in human subjects. The present study has shown that during the 3 h following a breakfast meal containing HAM-RS2, there was a significant reduction in GLP-1 concentrations compared with placebo. However, after a further HAM-RS2-containing meal, there was no difference in GLP-1 concentrations between the supplements.
In the present acute postprandial study, increased intakes of 48 g HAM-RS2, a non-viscous fermentable fibre, did not increase plasma GLP-1 concentrations and, indeed, following the breakfast meal, GLP-1 concentrations were significantly lower with the RS than compared with the placebo. There is little previous direct evidence for an effect of RS on GLP-1 in human subjects. A postprandial study comparing 27 g RS (from raw potato starch) with a placebo (that was not matched for glycaemic carbohydrate) also found lower GLP-1 with the RS(Reference Raben, Tagliabue and Christensen8). In the present study, there was a significant treatment × time interaction, translating as the RS-containing meal giving a lower acute GLP-1 response with higher post-lunch levels, when compared with placebo (Fig. 1). This may suggest that the increase in GLP-1 concentrations was due to the fermentation of HAM-RS2, and investigating the effects later on in the day or even the subsequent morning may have produced significant differences in GLP-1 concentrations. However, one study in which 60 g HAM-RS2 was consumed for 24 h before the investigation compared with the same placebo found no effect of HAM-RS2 on GLP-1 concentrations(Reference Robertson, Currie and Morgan6). Another study looking at carry-over effects of evening meals varying in indigestible carbohydrate content found no effect of evening meals on fasting GLP-1 concentrations the following day, but did demonstrate a significantly higher GLP-1 AUC (0–120 min) following one treatment (barley kernels containing 20·2 g of total dietary fibre) compared with the control product (3·9 g of total dietary fibre)(Reference Nilsson, Ostman and Holst15). Additionally, a study in which 30 g HAM-RS2 was consumed daily for 4 weeks compared with a matched glycaemic carbohydrate placebo again found no effect of HAM-RS2 on GLP-1(Reference Robertson, Bickerton and Dennis7), although, unlike the present study, this was not an acute response to the fibre. Overall, the data from the present study and from the previous studies would suggest that HAM-RS2 does not cause an increase in endogenous GLP-1, particularly when HAM-RS2 is consumed acutely.
While the GLP-1 data in human subjects following increased RS intake are limited, there is much animal work that has shown consistent increases in GLP-1 blood concentration following higher levels of RS feeding(Reference Zhou, Martin and Tulley3–Reference Shen, Keenan and Martin5) as well as increases in proglucagon gene expression in the caecum and colon(Reference Zhou, Martin and Tulley3, Reference Keenan, Zhou and McCutcheon4, Reference Zhou, Hegsted and McCutcheon16). However, it is difficult to directly translate these findings as the studies in rodents have not been acute (days rather than hours), and therefore it may be that prolonged exposure to HAM-RS2 in human subjects is required to affect GLP-1 concentrations. A previous study in human subjects has shown an increase in GLP-1 concentrations following wheat fibre intake, although a year of increased wheat fibre feeding was required before the effect was observed(Reference Freeland, Wilson and Wolever17).
Other acute studies using increased fibre feeding have shown inconsistent results. A 2 h postprandial study feeding a galactose and guar gum-mixed drink compared with a control drink found increased GLP-1 concentrations(Reference Adam and Westerterp-Plantenga18); another 6 h postprandial study comparing an inulin drink with high-fructose maize syrup drinks found GLP-1 concentrations to be higher with inulin at 30 min(Reference Tarini and Wolever19). However, other studies where dietary fibres have been given acutely, as in the present study, have found no effect on GLP-1 concentrations(Reference Barone Lumaga, Azzali and Fogliano20, Reference Frost, Brynes and Dhillo21). Overall, a recent review has concluded that the current research on the effects of fibres on GLP-1 demonstrates that fibres do not increase GLP-1 concentrations compared with control; however, the authors have acknowledged that there are few studies that have actually assessed the effects of fibre on gut hormones directly(Reference Klosterbuer, Greaves and Slavin22).
Previously, we have shown that acute ingestion of HAM-RS2 reduced postprandial insulin concentrations(Reference Bodinham, Frost and Robertson12), which we have demonstrated again in the present study following the second dose of HAM-RS2, but not following the first dose, or over the whole 7 h period. In the present study, the lower insulin response observed after the second dose was found in conjunction with a higher GLP-1 concentration, which is not in agreement with GLP-1 acting as an incretin following fibre feeding. This would therefore suggest that following increased RS intakes, GLP-1 is unlikely to have a role as an incretin. In many experimental designs, RS has replaced portions of digestible starch, or ratios of amylose:amylopectin have been manipulated (changing glycaemic index and/or glycaemic load), thus making comparisons problematic. A recent review of the literature related to RS and glycaemia/insulinaemia highlighted this disparity(Reference Robertson23). In our previous work, we hypothesised that a reduced postprandial insulin response often attributed to RS intake may be due to increased hepatic insulin clearance(Reference Bodinham, Frost and Robertson12); however, we found no evidence to support this in the present study.
The limitations of the present study are that we only measured total as opposed to active GLP-17–36 concentrations and therefore the effects of GLP-1 as a satiety signal may be masked. As recently reviewed, gut hormones may not act independently, and it may be the additive effects of several gut hormones that result in observed changes to satiety(Reference Klosterbuer, Greaves and Slavin22). Indeed, it is established that the postprandial levels of GLP-1 attained during feeding would have minimal effects on food intake(Reference Flint, Raben and Ersboll24, Reference Verdich, Toubro and Buemann25), when considered in isolation. However, GLP-19–36 may still have a role as an incretin, as some studies have suggested that it may reduce glycaemia to a small degree independently to any effects on insulin secretion(Reference Elahi, Egan and Shannon26, Reference Meier, Gethmann and Nauck27). Therefore, the relevance of measuring total GLP-1 concentrations needs to be considered in an attempt to understand the health benefits of dietary fibre intake.
Overall, it would appear from the present study and that of the literature that GLP-1 is not a candidate signal for the acutely reduced food intake and enhanced insulin sensitivity previously attributed to HAM-RS2.
Acknowledgements
We thank all of the participants for their time. The present study was funded by Ingredion Inc. but the industrial funder had no involvement in the study design or data analysis and interpretation. N. M. A.-M. was funded by an Educational Scholarship from the Saudi Arabian Government, King Saud University for Health Science. The supplements were supplied by Ingredion Inc. C. L. B. was involved in the designing of the study protocol, recruitment of the volunteers, conducting of the study days, analysis of the data, statistical analysis and writing of the paper. N. M. A.-M. was involved in the recruitment of the volunteers, and the conducting of the study days and analysis of the data. L. S. was involved in the recruitment of the volunteers, and the conducting of the study days and analysis of the data. M. D. R. was involved in the designing of the study protocol, assistance on the study days, analysis of the data, statistical analysis and refining of the paper.