Pomegranate (POM) or Punica granatum is an ancient fruit originating from the Middle East( Reference Kalaycıoğlu and Erim 1 ). The POM fruit is berry-like with a leathery rind enclosing many seeds surrounded by the juicy arils, which comprise the edible portion of the fruit( Reference Stover and Mercure 2 ). This edible part represents approximately 52 % of total fruit weight, comprising 78 % juice and 22 % seeds( Reference EL-Nemr, Ismail and Raga 3 ). Dietary supplementation with POM fruit has traditionally been consumed as pomegranate juice (POMj) obtained from the first-press (partial pressing) squeezing of whole POM fruits( Reference Seeram, Zhang and McKeever 4 ). More recently, pomegranate extract (POMe) has been developed in liquid and dry powder forms to provide alternative convenient sources for obtaining the bioactive polyphenols found in POMj. The liquid POMe is produced by extraction of the remaining fruit residue obtained, after an additional pressing, and the powdered POMe is obtained from further resin purification and drying (solid-phase extraction) to produce a powder with a high concentration of polyphenols( Reference Seeram, Zhang and McKeever 4 ).
Dietary supplementation with POMj or POMe, which are both rich in polyphenols, has been reported to promote several beneficial health effects( Reference Basu and Penugonda 5 , Reference Zarfeshany, Asgary and Javanmard 6 ). In particular, POM supplementation appears to be effective at enhancing physiological responses in individuals exhibiting physiological stress such as CVD( Reference Aviram, Rosenblat and Gaitini 7 ), oxidative stress( Reference Kaplan, Hayek and Raz 8 ), cellular inflammation( Reference Adams, Seeram and Aggarwal 9 ) or joint or muscle damage( Reference Shukla, Gupta and Rasheed 10 , Reference Ammar, Chtourou and Turki 11 ). Indeed, POM consumption has been reported to lower CVD morbidity by enhancing myocardial blood flow (BF, 17 %)( Reference Sumner, Elliott-Eller and Weidner 12 ) and antioxidant status (130 %)( Reference Aviram, Rosenblat and Gaitini 7 ), and lowering LDL-cholesterol oxidation (−90 %), systolic blood pressure (SBP, −12 %), and carotid artery thickness (−30 %)( Reference Aviram, Rosenblat and Gaitini 7 ). Similarly, POM has been shown to attenuate oxidative stress by lowering free radical production and lipid peroxidation (−65 %)( Reference Kelawala and Ananthanarayan 13 ), and to inhibit some cellular inflammation transcripts( Reference Afaq, Malik and Syed 14 , Reference Huang, Yang and Harada 15 ) such as NF-κB, TNF-α and cyclo-oxygenase-2 (COX-2). Since, these positive physiological effects afforded by POM supplementation have the potential to prevent or treat various disease risk factors, POM has been described as a ‘super fruit’( Reference Afaq, Malik and Syed 14 ). In this context, and compared with other purported nutraceuticals (e.g. green tea, red wine, orange, blueberry and cranberry juices), POM supplements have been reported to confer the most potent antioxidant and anti-inflammatory effects( Reference Kelawala and Ananthanarayan 13 , Reference Seeram, Aviram and Zhang 16 ). Indeed, compared to the aforementioned foods, POMj is more effective in attenuating LDL oxidation and inhibiting cellular oxidative stress in macrophages. Moreover, POMj exhibits a high capacity to neutralise free radicals with a reported antioxidant activity three times higher than of red wine and green tea (Trolox equivalent antioxidant capacity=18–20 v. 6–8)( Reference Seeram, Aviram and Zhang 16 ). POM also possesses a higher antioxidant activity compared to other food stuffs such as turmeric, ragi, amla, amaranth, rajmah, sesame, wheat and flaxseed( Reference Kelawala and Ananthanarayan 13 ). Although the underlying mechanisms for the beneficial physiological (e.g. antioxidant, anti-damaging and anti-inflammatory) effects of POM supplementation, are not yet clear( Reference Trombold, Reinfeld and Casler 17 – Reference Ammar, Turki and Hammouda 19 ), its efficacy has been attributed to the high bioavailability of its constituent polyphenols compared to other polyphenol-rich foods( Reference Basu and Penugonda 5 , Reference Seeram, Aviram and Zhang 16 ).
Physical exercise is a potent and multifaceted physiological stressor, as evidenced by an immediate increase in markers of muscle damage( Reference Ammar, Chtourou and Trabelsi 20 – Reference Ammar, Chtourou and Souissi 23 ), inflammation and oxidative stress( Reference Ammar, Chtourou and Souissi 23 , Reference Ammar, Chtourou and Hammouda 24 ) and a protracted period of muscle weakness and soreness during the post-exercise recovery period( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 ). Since POM supplementation appears particularly effective at improving numerous physiological responses in individuals manifesting symptoms of physiological stress( Reference Aviram, Rosenblat and Gaitini 7 , Reference Sumner, Elliott-Eller and Weidner 12 – Reference Afaq, Malik and Syed 14 ), POM supplementation might have potential as an ergogenic and recovery aid. Notwithstanding this potential for enhanced exercise performance and post-exercise recovery following POM supplementation, studies assessing the effects of POM supplementation on exercise performance and recovery are limited and yield equivocal findings( Reference Trombold, Reinfeld and Casler 17 – Reference Ammar, Turki and Hammouda 19 , Reference Trombold, Barnes and Critchley 25 – Reference Mazani, Fard and Baghi 27 ).
The aims of the present systematic review were (i) to examine the effect of POM intake on exercise performance and recovery, as well as its acute and delayed effects on muscle damage, oxidative stress, inflammation and cardiovascular function following exercise in healthy individuals and (ii) to outline how aspects of the study design (e.g. fitness status of participants, biomarkers assessed, supplement dose and exercise protocol) can influence the potential ergogenic and recuperative effects of POM supplementation. The recommendations from this review will have the potential to inform POM supplementation guidelines to optimise exercise performance and recovery practices in athletes and sports nutritionist.
Methods
This systematic review was conducted and reported in accordance with the guidelines of the preferred reporting items for systematic reviews and meta-analyses statement, which is an evidence-based protocol describing a set of items for reporting in systematic reviews and meta-analyses( Reference Moher, Liberati and Tetzlaff 28 ).
Data sources and search strategy
To inform our review, a comprehensive systematic search of studies was performed electronically in the following databases: PubMed/Medline, Web of Science and science direct from inception to January 2018. The search was limited to English language. The following search terms and Medical Subject Headings (MeSH) were used to source articles from pertinent peer-reviewed journals: Pomegranates (MeSH) OR Pomegranates (All Fields) OR Pomegranate (All Fields) OR Punicagranatum (All Fields) OR Punicagranatums (All Fields) OR granatum, Punica (All Fields) AND exercise (MeSH) OR exercise (All Fields) OR exercises (All Fields). The search was supplemented by manually cross-matching reference lists, key author searches, and citation searching of all retrieved papers to potentially identify additional studies. The search strategies were combined, and duplicates were removed by Endnote and manually by two of the authors. Once all relevant articles had been located, the researcher individually considered each article for its appropriateness for inclusion based on the pre-determined inclusion criteria described below. Where there was uncertainty with regard to inclusion, discussion with a third researcher determined the final inclusion or exclusion of the article.
Inclusion and exclusion criteria
To be included in the systematic review, individual studies needed to fulfill the following inclusion criteria: (i) primary research published in peer-reviewed journals in English, (ii) research conducted with healthy human participants (sedentary, active or trained subjects), (iii) original studies that had investigated an acute or long-term POM supplementation intervention (juice or extract) on performance and/or physiological responses, (iv) no severe methodological deficiencies (e.g. absence of placebo (PLA) control, participant were not blinded, inappropriate statistical analysis procedures) and (v) published before February 2018. Exclusion criteria were: (i) studies written in languages other than English, (ii) data from congress or workshop publications, (iii) animal studies, (iv) studies in which no supplementation was given, (v) studies which administered multiple supplements in addition to POM as this thwarted clear separation of the effects of POM from the other supplements, (vi) studies in which no exercise was performed and (vii) studies in which exercise was performed in extreme environments (e.g. altitude, heat, etc.). No limits were set for the year of publication. Case studies, encyclopaedia, book chapters and reviews were excluded, although the bibliographies of the latter were consulted to refine article searches.
Study selection
Following the removal of duplicate studies from the different search engines, inclusion or exclusion of the remaining articles was performed by applying the above criteria on the title and abstract to determine eligibility in a preliminary independent screening. Selected papers were then read in full to finalise eligibility or exclusion. A summary of this process is outlined in Fig. 1. The university’s library, hand searches, electronical databases and contact with the authors were used to obtain full copies of the published manuscripts.
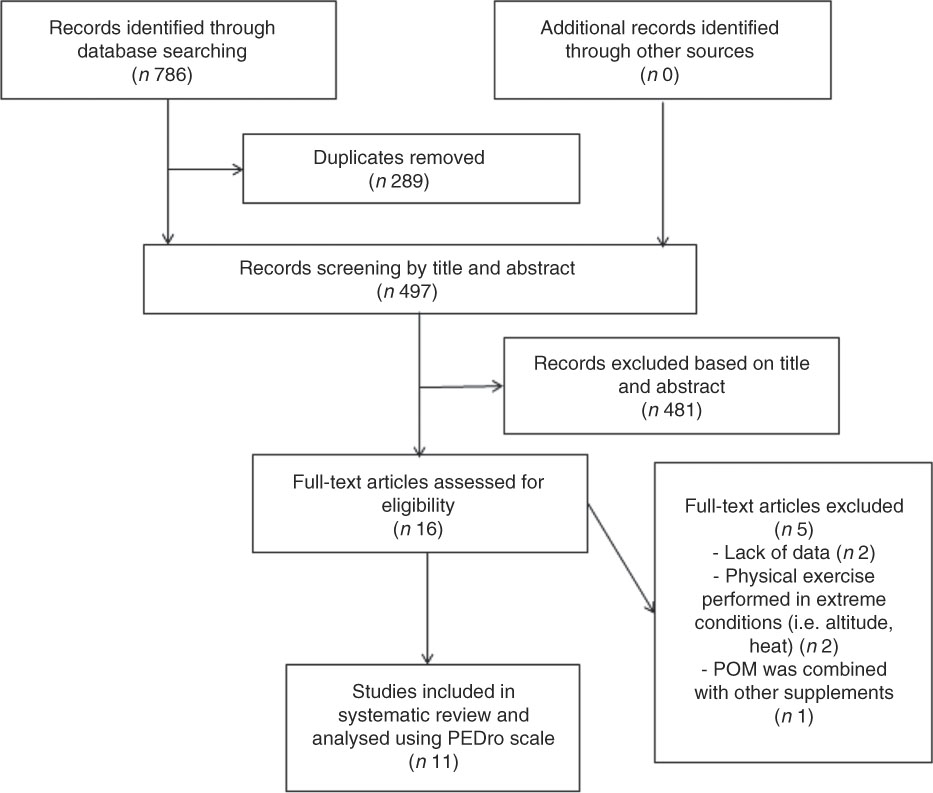
Fig. 1 Flowchart of study selection. PEDro, physiotherapy evidence database scale; POM, pomegranate.
Data extraction
Data were extracted using a standardised form. The primary outcomes extracted in this review were the effects of POM supplementation on physical performance, fatigue, and perception of pain and soreness (e.g. rating of perceived exertion (RPE), delayed onset muscle soreness (DOMS), pain scale) during and/or following exercise. These outcomes are presented in Table 1. All data concerning the effect of POM supplementation on muscle damage (e.g. the concentrations of creatine kinase (CK), lactate dehydrogenase (LDH), myoglobin (Mb), aspartate aminotransferase (ASAT)), oxidative stress (e.g. thiobarbituric acid-reactive substances (TBARS), malondialdehyde (MDA), protein carbonyl (PC), total antioxidant capacity (TAC), glutathione peroxidase (GPX), superoxide dismutase (SOD), catalase (CAT), uric acid (UA), arylesterase), inflammatory (e.g. C-reactive protein (CRP), high-sensitivity C-reactive protein (hs-CRP), IL-6, matrix metalloproteinases (MMP), sE-selectin, leucocytes) and cardiovascular (e.g. heart rate (HR), blood pressure, BF, vessel diameter (VD), oxygen saturation) responses following exercise were extracted from the research papers and are shown in Table 2. For all extracted performance and physiological data (Tables 1 and 2), the effects of POM supplementation were separated into data collected (i) during and immediately (up to 2 h) after exercise, which we classified as acute responses and (ii) after a period of at least 24 h/48 h following exercise, which we classified as delayed responses( Reference Trombold, Reinfeld and Casler 17 – Reference Ammar, Turki and Hammouda 19 , Reference Ammar, Chtourou and Turki 22 – Reference Trombold, Barnes and Critchley 25 ).
Table 1 Effect of pomegranate (POM) on physical performance and fatigue and muscle soreness responses following exercise (ex)

POMj, pomegranate juice; PLA, placebo; ↑, significant increase using POM supplementation compared with PLA; DOMS, delayed onset muscle soreness; ↓, significant decrease using POM supplementation compared with PLA; ↔, no significant difference between POM and PLA conditions; POMe, pomegranate extract; TTE, treadmill runs to exhaustion; PV, peak velocity; RPE, rating of perceived exertion; RSA, repeated sprint ability; RTF, repetitions to fatigue.
Table 2 Effect of pomegranate (POM) on muscle damage, oxidative stress, inflammatory and cardiovascular responses following exercise (ex)

POMj, pomegranate juice; PLA, placebo; ↔, no significant difference between POM and PLA conditions; CK, creatine kinase; MB, myoglobin; hs-CRP, high-sensitivity C-reactive protein; TBARS, thiobarbituric acid-reactive substances; ↓, significant decrease using POM supplementation compared with PLA; SBP, systolic blood pressure; DBP, diastolic blood pressure; POMe, pomegranate extract; PV, peak velocity; ↑, significant increase using POM supplementation compared with PLA; HR, heart rate; GPX, glutathion peroxidase; SOD, superoxide dismutase; MDA, malondialdehyde; MMP, matrix metalloproteinases; ARE, arylesterase; TAC, total antioxidant capacity; ASAT , aspartate aminotransferase; ALAT, alanine aminotransferase; PC, protein carbonyl; LDH, lactate dehydrogenase; CAT, catalase; UA, uric acid; Tbil, total bilirubin; RSA, repeated sprint ability; RTF, repetitions to fatigue; SPO2, oxygen saturation; BP, blood pressure.
Quality assessment
To assess the methodological quality of the selected studies, the Physiotherapy Evidence Database (PEDro) scale was used( Reference Maher, Sherrington and Herbert 29 ). The PEDro scale is based on the Delphi list developed by Verhagen et al.( Reference Verhagen, de Vet and de Bie 30 ) at the Department of Epidemiology, University of Maastricht. The PEDro scale is a reliable and objective tool that helps identify which of the randomised clinical trials from the same areas of physiotherapy practice are likely to be externally (criteria 1) and internally (criteria 2–9) valid and could have sufficient statistical information to make their results interpretable (criteria 10 and 11)( Reference Maher, Sherrington and Herbert 29 ). Each paper was independently assessed twice by two independent reviewers using the eleven-item checklist to yield a maximum score of 10 (the sum of awarded points for criteria 2–11). Points are only awarded when a criterion is clearly satisfied. In case of disagreements concerning trial scoring, a discussion with a third reviewer was conducted. The level of agreement between reviewers was calculated via the κ values with k=0·91 indicating an excellent agreement( Reference Landis and Koch 31 ).
Results
A total of eleven studies( Reference Trombold, Reinfeld and Casler 17 – Reference Ammar, Turki and Hammouda 19 , Reference Trombold, Barnes and Critchley 25 – Reference Mazani, Fard and Baghi 27 , Reference Trexler, Smith-Ryan and Melvin 32 – Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ) met the inclusion criteria and were included in the current systematic review. The studies examined either the effects of POM intake on exercise performance and/or exercise-induced fatigue, soreness, muscle damage, oxidative stress, inflammation and cardiovascular function. All studies used a statistical significance threshold of P<0·05.
Study selection and characteristics
Study selection
The predefined search strategies yielded a preliminary pool of 786 possible papers. Removal of duplicates resulted in a selection of 497 published papers. A first screening of titles and abstracts for eligibility against inclusion and exclusion criteria let to a provisional list of sixteen published studies. The full texts of fourteen articles were retrieved, while two studies were excluded because insufficient data were published. After a careful review of the fourteen full texts, three articles were excluded (two studies investigated physical exercises performed in extreme conditions (i.e. altitude, heat) and one study used POM combined with other supplements). Therefore, eleven studies met our inclusion criteria for determining the effects of POM supplementation on exercise performance, recovery and a variety of physiological outcome measurements.
Study characteristics
The characteristics of each study, and the performance and the physiological changes following POM supplementation compared to PLA supplementation, are respectively summarised in Tables 1 and 2. In all, four papers examined the effect of POM supplementation on physical performance and physiological responses, such as muscle damage and inflammation, following strength exercise( Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 ); and cardiovascular responses following running( Reference Trexler, Smith-Ryan and Melvin 32 ), cycling( Reference Roelofs, Smith-Ryan and Trexler 35 ) and strength( Reference Ammar, Turki and Chtourou 18 , Reference Roelofs, Smith-Ryan and Trexler 35 ) exercise. In all, two studies only examined the change in physical performance without physiological measurements( Reference Trombold, Reinfeld and Casler 17 , Reference Machin, Christmas and Chou 33 ), while the remaining five studies only assessed the effect of POM supplementation on the physiological responses to exercise such as muscle damage( Reference Fuster-Muñoz, Roche and Funes 26 ), oxidative stress( Reference Ammar, Turki and Hammouda 19 , Reference Mazani, Fard and Baghi 27 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ), inflammation( Reference Fuster-Muñoz, Roche and Funes 26 , Reference Mazani, Fard and Baghi 27 ) and cardiovascular function( Reference Tsang, Wood and Al-Dujaili 34 ). Different exercise models were employed in the studies included in the current systematic review. Specifically, four studies included strength exercises such as unilateral eccentric( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 ) and Olympic weightlifting( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 ) movements, three studies employed treadmill running( Reference Mazani, Fard and Baghi 27 , Reference Moher, Liberati and Tetzlaff 28 , Reference Tsang, Wood and Al-Dujaili 34 ), two studies used a combination of strength and running( Reference Machin, Christmas and Chou 33 ) or cycling( Reference Roelofs, Smith-Ryan and Trexler 35 ) exercise, while the two remaining trials used ultra-endurance exercises( Reference Fuster-Muñoz, Roche and Funes 26 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ). Further measures completed to assess the physiological effects of POM supplementation included RPE( Reference Ammar, Turki and Chtourou 18 ), perceptions of DOMS( Reference Trombold, Reinfeld and Casler 17 , Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 ) and pain and vitality scales( Reference Moher, Liberati and Tetzlaff 28 ), which are presented with performance in Table 1. Concerning the acute (up to 2 h) and delayed (at least 24/48 h) responses to exercise, four studies assessed the acute and delayed performance and/or physiological responses( Reference Trombold, Reinfeld and Casler 17 – Reference Ammar, Turki and Hammouda 19 , Reference Trombold, Barnes and Critchley 25 ), five studies only assessed the acute responses( Reference Mazani, Fard and Baghi 27 , Reference Trexler, Smith-Ryan and Melvin 32 , Reference Tsang, Wood and Al-Dujaili 34 – Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ), while two studies( Reference Fuster-Muñoz, Roche and Funes 26 , Reference Machin, Christmas and Chou 33 ) only assessed the delayed responses.
Subjects characteristics
The studies involved in this systematic review included a total of 230 participants (190 males, twenty females, with twenty not specified). The number of participants in each trial ranged from 9( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 ) to 45( Reference Hellsten, Nyberg and Jensen 37 ), with a mean sample size of 20·9(SD10) and a mean age ranging from 21( Reference Trombold, Reinfeld and Casler 17 – Reference Ammar, Turki and Hammouda 19 ) to 35( Reference Fuster-Muñoz, Roche and Funes 26 ) years. These eleven studies targeted healthy adult participants of varying fitness status. In all, four studies recruited recreationally-( Reference Trombold, Barnes and Critchley 25 , Reference Machin, Christmas and Chou 33 , Reference Tsang, Wood and Al-Dujaili 34 ) to highly-( Reference Trexler, Smith-Ryan and Melvin 32 ) active participants (total n 100 participants), four studies( Reference Trombold, Reinfeld and Casler 17 – Reference Ammar, Turki and Hammouda 19 , Reference Roelofs, Smith-Ryan and Trexler 35 ) recruited resistance trained participants (n 54 participants) and three studies( Reference Fuster-Muñoz, Roche and Funes 26 , Reference Mazani, Fard and Baghi 27 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ) recruited endurance trained athletes (total n 74).
Study design and supplement administration
As presented in Tables 1 and 2, the reviewed studies (nine out of eleven) implemented a double-blind, PLA-controlled experimental design. The majority of these studies (nine out of eleven) employed a randomised design where (i) two studies employed three experimental arms( Reference Fuster-Muñoz, Roche and Funes 26 , Reference Machin, Christmas and Chou 33 ) with at least one being POM treatment, (ii) three studies used two experimental arms( Reference Mazani, Fard and Baghi 27 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ) and (iii) four studies used one experimental arm (i.e. crossover design) with a 1 week( Reference Trexler, Smith-Ryan and Melvin 32 , Reference Roelofs, Smith-Ryan and Trexler 35 ) or a 2 week washout period( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 ). Concerning, the two remaining studies( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 ), the experimental protocol was completed during 1 week of an intensive training program in a group of elite weightlifters (i.e. one experimental arm) which necessitated a small washout period (48 h). Therefore, to avoid any possible protracted effect of POM supplementation on the physiological responses post training, the authors selected a non-randomised crossover design with the POM treatment administered first for all participants. The eleven trials included in this review employed one of two varieties of dietary POM supplementation with an intervention period that ranged from 30 min to 21 d. The majority (n 9) opted for POMj, with beverages ingested both before and following the training/exercises sessions. Indeed, in five studies, participants were supplemented for 4 d pre- and 4/5 d post-exercise with 0·5 litre POMj once or twice daily( Reference Trombold, Barnes and Critchley 25 , Reference Machin, Christmas and Chou 33 ); 7 d pre- and 8 d post-exercise with 0·25 litre twice daily( Reference Trombold, Reinfeld and Casler 17 ); 1 h (0·5 litre) pre- and 2 d (3×0·25 litre/d) post exercise( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 ). In the remaining four studies, POM was only ingested before exercises sessions with a treatment of: 0·5 litre/d during a period of 1 week( Reference Tsang, Wood and Al-Dujaili 34 ); 0·24 litre/d during a period of 2 weeks( Reference Mazani, Fard and Baghi 27 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ); or 0·2 litre/d during a period of 3 weeks( Reference Fuster-Muñoz, Roche and Funes 26 ). The two remaining studies( Reference Trexler, Smith-Ryan and Melvin 32 , Reference Roelofs, Smith-Ryan and Trexler 35 ) opted for an acute consumption of 1000 mg POMe 30 min before exercises sessions. With regard to the antioxidant capacity of the POM supplements administered in the selected studies, the total phenolic content ranged from 0·65 g/0·5 litre( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 , Reference Machin, Christmas and Chou 33 ) to 2·56 g/0·5 litre( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 ).
Methodological quality of studies
All reviewed studies scored a moderate to high score of 7 and above with a mean PEDro score of 8·9 (SD 0·9). Of the eleven studies included, three investigations( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 , Reference Roelofs, Smith-Ryan and Trexler 35 ) received a perfect score of 10, five investigations( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 , Reference Trexler, Smith-Ryan and Melvin 32 – Reference Tsang, Wood and Al-Dujaili 34 ) scored 9 out of 10 as they failed to randomly allocate subjects to a group or failed to achieve similar baseline values for the primary outcome measure, two investigations( Reference Fuster-Muñoz, Roche and Funes 26 , Reference Mazani, Fard and Baghi 27 ) scored 8 out of 10 as they failed to blind therapists and achieve similar baseline values for the primary outcome measure, and the remaining investigation( Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ) scored 7 out of 10 as the authors failed to achieve similar baseline values for the primary outcome measure and to blind the experimenters to the supplement order. Overall, the study quality was deemed to be good to excellent.
Effect of pomegranate on acute and delayed physical performance
A total of six studies assessed the effect of POM supplementation on exercise performance( Reference Trombold, Reinfeld and Casler 17 , Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 , Reference Trexler, Smith-Ryan and Melvin 32 , Reference Machin, Christmas and Chou 33 , Reference Roelofs, Smith-Ryan and Trexler 35 ). In all, three of these studies evaluated the change in acute (i.e. immediately and up to 2 h) physical performance after treadmill( Reference Trexler, Smith-Ryan and Melvin 32 ), repeated sprint ability (RSA)( Reference Roelofs, Smith-Ryan and Trexler 35 ) and strength( Reference Ammar, Turki and Chtourou 18 , Reference Roelofs, Smith-Ryan and Trexler 35 ) exercise with the remaining three studies assessing the delayed (i.e. after a period of at least 24/48 h following exercise) effect of POM on strength recovery following unilateral( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 ) and bilateral eccentric exercise( Reference Machin, Christmas and Chou 33 ).
Effect on acute physical performance
In highly active participants, ingestion of 1000 mg of POMe (2×500 mg capsules) 30 min before exercise (Table 1) was reported to improve time to exhaustion during treadmill running at 90 % (388 (SD 199) v. 346 (SD 163) s) and 100 % (171 (SD 66) v. 159 (SD 62) s) but not 110 % (108 (SD 45) v. 104 (SD 40) s) of the peak velocity (PV) obtained in a graded treadmill test continued until exhaustion( Reference Trexler, Smith-Ryan and Melvin 32 ). Moreover, the average and peak power output in sprint 5 during an RSA test on a friction-braked cycle ergometer (i.e. 6 s maximal sprints×ten repetitions with a load of 65 g/kg of body mass applied and a 30 s passive recovery separating intervals) was also enhanced following the same POMe ingestion procedures( Reference Roelofs, Smith-Ryan and Trexler 35 ). With regard to resistance exercise performance, POMe ingestion has been reported to have no effect on the number of repetitions to fatigue (RTF) during bench and leg press exercise( Reference Roelofs, Smith-Ryan and Trexler 35 ). Conversely, Ammar et al.( Reference Ammar, Turki and Chtourou 18 ) recently showed that consumption of 500 ml POMj 60 min before high-intensity weightlifting exercise enhanced the total (8·3 %) and maximal (3·26 %) load lifted in two Olympic movements (snatch and clean and jerk) compared to the PLA condition (Table 1). The discrepancies between studies could be linked to inter-study differences in the supplementation procedures employed. Therefore, the existing findings suggest that ingestion of 500 ml of POMj 60 min before exercise is more likely to enhance resistance exercise performance than 1000 mg of POMe ingested 30 min before exercise.
The enhanced performance following POM ingestion might be linked to increased muscle BF. Indeed, Trexler et al.( Reference Trexler, Smith-Ryan and Melvin 32 ) observed enhanced performance in association with an increase in post exercise VD and brachial artery BF after POMe ingestion. However, given that BF was only investigated post-exercise at the brachial artery in this study, it still unclear whether POM increases arm and/or leg BF during exercise. The beneficial effect of POM on BF could be due to its high content of polyphenols (e.g. flavonoids) which can promote nitric oxide (NO) synthesis (an important contributor to exercise-induced vasodilation( Reference Hellsten, Nyberg and Jensen 37 ) by enhancing NO synthase activity, and NO bioavailability, through limiting NO scavenging by reactive oxygen species (ROS)( Reference Ignarro, Byrns and Sumi 38 ).
Effects of pomegranate on muscle strength recovery
The performance of eccentric exercise has been shown to reduce maximal strength and increase the sensation of soreness in the exercising muscles, with muscle soreness peaking 24–48 h post such exercise( Reference Gibala, MacDougall and Tarnopolsky 39 ). Although, soreness scores return towards baseline after this point( Reference Newham, Mills and Quigley 40 ), strength can remain depressed compared to baseline even up to several days after undertaking eccentric exercise( Reference Clarkson and Hubal 41 ). It has been reported that full recovery of strength typically requires 7–14 d( Reference Stupka, Tarnopolsky and Yardley 42 ). To date, studies assessing the effect of POM supplementation on post exercise muscle recovery (Table 1) have shown that, in both untrained( Reference Trombold, Barnes and Critchley 25 ) and trained( Reference Trombold, Reinfeld and Casler 17 ) subjects, consumption of 500 ml POMj for 9–15 d before an intensified training session (two to three sets of twenty unilateral maximal eccentric elbow flexion) can expedite the recovery of strength assessed during the 2–168 h period post exercise. Indeed, compared to PLA, there was greater strength recovery following POMj supplementation at 48 h (85 v. 78 %) and 72 h (89 v. 84 %) post exercise( Reference Trombold, Barnes and Critchley 25 ). Concerning the effect of POMj supplementation on lower limb recovery, Trombold et al.( Reference Trombold, Reinfeld and Casler 17 ) showed that the recovery of knee extensor isometric strength was not affected by POMj after six sets of ten unilateral eccentric knee extension exercise performed by resistance trained men. Collectively, these initial studies suggested that POM supplementation can accelerate strength recovery in arm muscles but not leg muscles. More recently, however, Machin et al.( Reference Machin, Christmas and Chou 33 ) showed that consuming POMj either once-daily (650 mg/d) or twice-daily (1300 mg /d) improved strength recovery in both leg and arm muscles after completing unaccustomed eccentric exercise in recreationally active men (Table 1). These conflicting results could be explained by the training status of the participants (untrained v. resistance trained subjects) and/or the composition of the eccentric exercise protocols. Specifically, the eccentric exercise protocol employed by Machin et al.( Reference Machin, Christmas and Chou 33 ) was based on 20 min of downhill running, thereby engaging both sets of leg muscles and provoking a greater degree of physiological perturbation( Reference Costa, Moreira and Cavalcanti 43 ), whereas Trombold et al.( Reference Trombold, Reinfeld and Casler 17 ) used a protocol comprising six sets of ten eccentric unilateral knee extension exercises. The beneficial effect of POM on muscle strength recovery has recently been related to its antioxidant and anti-inflammatory properties( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 ) and its ability to enhance vasodilation and BF( Reference Trexler, Smith-Ryan and Melvin 32 , Reference Hellsten, Nyberg and Jensen 37 ).
Effect of pomegranate on muscle fatigue, pain and soreness
A total of four studies have examined the effects of POM on muscle fatigue, pain and soreness following physical exercise( Reference Trombold, Reinfeld and Casler 17 , Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 , Reference Trexler, Smith-Ryan and Melvin 32 ). In all, three of these studies analysed the change in muscle fatigue and soreness acutely and up to 48 h( Reference Ammar, Turki and Chtourou 18 ) or 96 h( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 ) post strength exercise, while only one study focused on the effect of POM on muscle pain immediately following treadmill runs session( Reference Trexler, Smith-Ryan and Melvin 32 ).
Effect on acute muscle fatigue, pain and soreness
In untrained subjects (Table 1), a daily drink of POMj before (4 d) and following (4 d) intense upper body eccentric exercise has been reported to lower the perception of muscle soreness in the elbow-flexors 120 min post-exercise( Reference Trombold, Barnes and Critchley 25 ). Similarly, POMj consumed 1 h before and over the 48 h following a resistance training session (Table 1) has been reported to blunt the acute perception of muscle fatigue with lower ratings of perceived exertion (RPE) values reported (−4·37 %) following POMj supplementation( Reference Ammar, Turki and Chtourou 18 ). The immediate lowering of post-exercise muscle fatigue and soreness following POMj supplementation might be explained by blunted tissue oedema and/or a lower accumulation of metabolic by-products which relay information to the central nervous system via groups III and IV muscle afferents( Reference Cheung, Hume and Maxwell 44 ). This reduction in muscle soreness and fatigue following POMj supplementation might be expected to translate into less fatigue in a subsequent training session, which may have implications for enhancing physical performance during a training programme( Reference Ammar, Chtourou and Turki 22 , Reference Ammar, Chtourou and Hammouda 24 ). With regard to the effect of POM supplementation on the perception of muscle soreness and fatigue following intermittent exercise, it has been reported that pain, as assessed using the visual analogue pain scale, was not significantly affected by POMe treatment( Reference Trexler, Smith-Ryan and Melvin 32 ). However, the following statement on the vitality scale, ‘At this moment I feel alive and vital’, was found to be greater 30 min following POMe ingestion( Reference Trexler, Smith-Ryan and Melvin 32 ). Taken together, these results indicate that POM supplementation appears to acutely attenuate the sensation of fatigue and soreness post exercise with potential implications for performance in subsequent training sessions.
Effect on delayed onset muscle soreness
Exhaustive or unaccustomed intense exercise can cause muscle damage, which results in pain, tenderness, swelling and stiffness. Given the delayed nature of these symptoms, they are collectively referred to as DOMS( Reference Allen 45 ). Trombold et al.( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 ) were the first to assess the effect of POMj supplementation on the DOMS provoked by a bout of intense eccentric exercise (Table 1). These studies showed that consumption of 250–500 ml POMj twice daily could attenuate elbow-flexor muscle soreness at 48 and 72 h post exercise in resistance trained males( Reference Trombold, Reinfeld and Casler 17 ), but not in recreationally active males( Reference Trombold, Barnes and Critchley 25 ). However, knee extensor muscle soreness was not significantly affected by POMj in either population( Reference Trombold, Reinfeld and Casler 17 ). Therefore, in response to unilateral eccentric exercise, these authors concluded that POMj supplementation can alleviate exercise-induced soreness of the arm muscles, but not leg muscles, with this beneficial effect more likely to occur in resistance training individuals. Conversely, POMj supplementation has been reported to lower the perception of muscle soreness (i.e. at 48 h) in knee extensors, but not the elbow flexors, in elite weightlifters completing whole body resistance exercise( Reference Ammar, Turki and Chtourou 18 ). The authors of this study ascribed the absence of a lower soreness perception in elbow flexors after POMj supplementation to the lower soreness provoked by the weightlifting exercises in the arms compared to the legs. Accordingly, the lower muscle pain in the upper compared to the lower body musculature likely lowered the scope for a POMj-mediated attenuation in muscle soreness in the former compared to the latter. Therefore, it appears that the blunting of muscle soreness post POMj supplementation might be linked to the degree of soreness evoked by a given exercise task.
In addition to inter-study differences in limb-specific muscle soreness responses post POMj supplementation, the studies of Ammar et al.( Reference Ammar, Turki and Chtourou 18 ) and Trombold et al.( Reference Trombold, Reinfeld and Casler 17 ) yielded contrasting results on the effects of POMj on muscle soreness of the same muscle group (knee extensors) in response to whole body( Reference Ammar, Turki and Chtourou 18 ) or unilateral( Reference Trombold, Reinfeld and Casler 17 ) resistance exercise. The blunting in knee extensor muscle soreness in the study by Ammar et al.( Reference Ammar, Turki and Chtourou 18 ), but not Trombold et al.( Reference Trombold, Reinfeld and Casler 17 ), might be linked to the higher polyphenol content of the POMj administered by Ammar et al.( Reference Ammar, Turki and Chtourou 18 ) (2·56 g/500 ml v. 650 mg/480 ml, respectively). Alternatively, or in conjunction with the different polyphenol doses administered, the disparate effects of POMj supplementation in these studies could be a function of differences the muscle mass engaged (large muscle mass exercise v. one limb knee extensor) or the exercise tasks completed (combination of eccentric and concentric v. eccentric only exercises) in Ammar et al.( Reference Ammar, Turki and Chtourou 18 ) compared to Trombold et al.( Reference Trombold, Reinfeld and Casler 17 ), respectively. Therefore, the potential for POM supplementation to blunt muscle soreness appears to be positively related to the dose of polyphenols administered and the volume of muscle mass engaged. Thus, dietary supplementation with POM containing a sufficient dose of polyphenols could be an effective treatment to improve the recovery of muscles strength and weakness which might result in a lower fatigue perception and higher performance in the subsequent training session( Reference Ammar, Chtourou and Turki 22 , Reference Ammar, Chtourou and Hammouda 24 ).
Effect of pomegranate supplementation on muscle damage responses
The mechanisms that underpin muscle damage are believed to involve both mechanical and metabolic processes( Reference Torres, Ribeiro and Alberto Duarte 46 ). Since, mechanical and metabolic demands on the skeletal muscles are influenced by the nature of physical activity, it was suggested that the magnitude and the level of muscle damage are affected by the mode, intensity and duration of exercise( Reference Ebbeling and Clarkson 47 ). To date, two general phases have been proposed to describe the damage responses during and following physical exercise. The first phase is initiated during exercise and involves mechanical and metabolic responses which are collectively referred to as primary or acute damage( Reference Ebbeling and Clarkson 47 ), while the second phase is associated with an ensuing inflammatory response which develops following exercise (i.e. days to weeks) and is termed delayed damage( Reference Howatson and Van Someren 48 ). Of the eleven studies conducted to date, three studies have investigated the effect of POM supplementation on exercise-induced muscle damage with two studies assessing both acute (3 min to 2 h) and delayed (1–4 d) responses to strength exercises( Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 ) and one study assessing only the delayed effect of POM following an endurance training session( Reference Fuster-Muñoz, Roche and Funes 26 ).
Effect on acute muscle damage responses
In recreationally-active males (Table 2) Trombold et al.( Reference Trombold, Barnes and Critchley 25 ) showed that, compared to PLA, POMe supplementation had no effect on muscle damage markers 2 h post unilateral eccentric exercise. Indeed, in response to two sets of twenty maximal eccentric elbow flexion repetitions, CK was increased to a similar extent in both the PLA and POMe conditions with no change in Mb 2 h post exercise. In contrast, using Olympic weightlifting exercises (Table 2), Ammar et al.( Reference Ammar, Turki and Chtourou 18 ) reported a blunting in muscle damage following whole body resistance exercise in well-trained subjects after POMj supplementation. Specifically, consumption of POMj attenuated the acute increase of CK (−8·75 %) and LDH (−1·64 %), and blunted the increase of ASAT and alkaline phosphatase compared to the PLA condition. Similar to the muscle soreness results, these conflicting results could: (i) be explained by the lower volume of muscle mass engaged, and by extension experiencing damage, in the study of Trombold et al.( Reference Trombold, Barnes and Critchley 25 ), (ii) confirm that the magnitude of muscle damage responses (and therefore scope for recovery) are affected by the nature of exercise( Reference Ebbeling and Clarkson 47 ) and (iii) reflect an attenuated acute beneficial effect of POM on muscle damage following unilateral exercise.
Effect on delayed muscle damage responses
Similar to the results observed 2 h post exercise, Trombold et al.( Reference Trombold, Barnes and Critchley 25 ) did not observe a beneficial effect of POMj on the delayed damage responses 1–4 d following eccentric unilateral elbow exercise. Indeed at 24, 72 and 96 h post-exercise, no differences in CK and Mb were observed in untrained subjects between the POMe and PLA conditions. These findings were corroborated in trained endurance athletes by Fuster-Muñoz et al.( Reference Fuster-Muñoz, Roche and Funes 26 ) who showed that consumption of 200 ml of POMj did not affect ASAT and alanine aminotransferase (ALAT) responses during a 3 week training program (Table 2). The authors concluded that the 3 week intervention was not sufficient to elicit a blunting in ASAT and ALAT in trained endurance athletes after POMj supplementation and suggested that an extended supplementation period (i.e. intervention period >3 weeks) could result in significant differences between the POMj and PLA groups( Reference Fuster-Muñoz, Roche and Funes 26 ). However, POMj supplementation had a different effect in well-trained resistance subjects( Reference Ammar, Turki and Chtourou 18 ). Indeed, the consumption of POMj 48 h before and during the training session accelerated muscle damage recovery 48 h post a weightlifting training session by expediting the recovery kinetics of CK (11·43 %), LDH (5·08 %) and ASAT (4·94 %). These results indicated that 48 h POMj supplementation can be sufficient to restore muscle damage to baseline levels following an intense strength training session (Table 2). Therefore, a natural POMj with high polyphenol concentration (i.e. 2·56 g/500 ml) could be a practical and potent treatment to alleviate muscle damage following intense physical exercise, particularly in resistance training individuals.
Effect of pomegranate supplementation on oxidative stress responses
Oxidative stress reflects an imbalance between pro-oxidant and antioxidant status with the former outweighing the latter( Reference Leeuwenburgh and Heinecke 49 ). Strenuous exercise or intensified training has been shown to elicit acute oxidative stress during exercise and to exhibit a delayed recovery of oxidative stress biomarkers (lipid peroxidation and enzymatic antioxidant) following exercise cessation( Reference Powers and Jackson 50 ). It is well accepted that exercise provokes the development of oxidative stress by enhancing ROS production via increased phospholipase A2, NADPH oxidase and xanthine oxidase activities( Reference Powers and Jackson 50 ). It is recognised that increased ROS exposure can contribute to fatigue during exercise via the oxidation of critical redox-sensitive sites within skeletal muscle( Reference Powers and Jackson 50 ) and the resulting structural damage to lipids, protein and DNA oxidation. However, recent evidence suggests that ROS are also integral to the adaptive responses of muscle fibres to exercise stress via the activation of transcription pathways that regulate gene and protein expression within skeletal muscle( Reference Powers and Jackson 50 ). Despite the high antioxidant capacity of POM (i.e. rich in polyphenols such as anthocyanins, flavonols and certain ellagitannins such as punicalagin( Reference Gil, Tomás-Barberán and Hess-Pierce 51 ) and its resultant potential to mitigate exercise-induced oxidative stress, few studies (n 5) have assessed the effects of POM on post-exercise oxidative stress( Reference Ammar, Turki and Hammouda 19 , Reference Fuster-Muñoz, Roche and Funes 26 , Reference Mazani, Fard and Baghi 27 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ).
Effect on acute oxidative stress responses
To date, four studies have examined the effect of POM supplementation on oxidative stress biomarkers immediately following physical exercise( Reference Ammar, Turki and Hammouda 19 , Reference Mazani, Fard and Baghi 27 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ). Specifically, these studies aimed to evaluate the efficacy of POMj consumption to improve the immediate antioxidant responses to exhaustive exercise in young healthy males( Reference Mazani, Fard and Baghi 27 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ) and in elite weightlifters( Reference Ammar, Turki and Hammouda 19 ). Mazani et al.( Reference Mazani, Fard and Baghi 27 ), Naghizadeh-Baghi et al.( Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ) and Ammar et al.( Reference Ammar, Turki and Hammouda 19 ) reported that consumption of POMj before exercise (240 ml/d for 14 d( Reference Mazani, Fard and Baghi 27 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ) and 750 ml/d for 2 d( Reference Ammar, Turki and Hammouda 19 ) enhanced the activity of key antioxidant enzymes and attenuated lipid peroxidation immediately after treadmill running (70 % maximal HR), ultra-endurance exercise and a weightlifting training session. While lipid peroxidation markers and the activity of enzymatic antioxidants were increased at post exercise in both the POMj and PLA groups, the pre–post exercise change was higher for enzymatic (e.g. SOD, GPX and CAT) and non-enzymatic antioxidants (e.g. UA and total bilirubin) and lower for MDA in the POMj condition compared to the PLA condition( Reference Ammar, Turki and Hammouda 19 , Reference Mazani, Fard and Baghi 27 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ). These observations support the use of POMj consumption to enhance antioxidant status in humans completing intense exercise( Reference Matthaiou, Goutzourelas and Stagos 52 ). Indeed, these findings are consistent with those of Tsang et al.( Reference Tsang, Wood and Al-Dujaili 34 ) who showed that 1 week of POMj consumption (500 ml/d containing 1·69 g total phenolics/l) significantly lowered urinary lipid peroxidation levels in the POMj group immediately after 30 min of treadmill running (50 % W max). Consistent with these findings, previous studies in sedentary subjects reported the effectiveness of POMj supplementation to neutralise ROS( Reference Aviram, Dornfeld and Rosenblat 53 , Reference Guo, Wei and Yang 54 ). Collectively, the existing literature suggests that POMj supplementation has the potential to blunt exercise-induced oxidative stress.
Effect on delayed oxidative stress responses
The effect of POMj consumption on the delayed oxidative stress response following exercise has only been investigated in the studies of Fuster-Muñoz et al.( Reference Fuster-Muñoz, Roche and Funes 26 ) and Ammar et al.( Reference Ammar, Turki and Hammouda 19 ), which recruited adult endurance and resistance trained males, respectively. In endurance trained males, 22 d of POMj supplementation attenuated PC and MDA levels such that these biomarkers were only increased following endurance training sessions in the PLA group (1·1 v. 1·8 nmol/mg and 14·1 v. 10·9 nmol/g protein, respectively for PC and MDA), suggestive of a reduction in oxidative stress during the aerobic training session after POMj supplementation( Reference Fuster-Muñoz, Roche and Funes 26 ). Similarly, resistance trained males exhibited a delayed effect of POMj in response to a weightlifting training session. Indeed, 48 h following a resistance exercise session, Ammar et al.( Reference Ammar, Turki and Hammouda 19 ) reported expedited recovery kinetics of MDA (5·63 %) and the antioxidant enzymes, CAT (8·94 %) and GPX (10·21 %) markers with POMj compared to PLA supplementation. Therefore, POMj supplementation appears to be effective at blunting oxidative stress biomarkers following both endurance and resistance exercise sessions. Consistent with these findings, previous studies in healthy non-active subjects showed that 15 d of POMj consumption increased levels of GSH (22·6 %) and lowered levels of MDA (24·4 %) and PC (17·7 %) even 1 week after POMj administration has terminated( Reference Matthaiou, Goutzourelas and Stagos 52 ). Collectively, the existing literature suggests that, even after ceasing POMj consumption, some of its beneficial effects on antioxidant status prevail for at least a few days. Although the mechanisms for these delayed effects have not been resolved, they might be linked to a protracted radical scavenging, antioxidant recycling and modulation of antioxidant enzymatic activity( Reference Halliwell, Rafter and Jenner 55 ).
Effect of pomegranate supplementation on inflammatory responses
Intense physical exercise, has been shown provoke a rapid and pronounced local inflammatory response (i.e. invasion of muscle by inflammatory cells). Thereafter, a systemic inflammatory response, known as acute-phase response( Reference Pedersen and Hoffman-Goetz 56 ), becomes manifest that can persist for days to weeks( Reference Leeuwenburgh and Heinecke 49 ). The leucocytes are the major cellular mediators of inflammation( Reference Cannon and St Pierre 57 ). The increased prevalence of leucocytes after intense exercise( Reference Malm, Lenkei and Sjodin 58 ) is believed to be mainly due to the rise of neutrophils and monocyte/macrophage influx as determined by the expression of leucocyte adhesion molecules( Reference Cannon and St Pierre 57 ). In addition, the secretion of pro-inflammatory cytokines, such as TNF-α and IL-1β, and the inflammation responsive cytokine, IL-6, by the endothelial cells is believed to mediate exercise-induced inflammatory process( Reference Pedersen and Hoffman-Goetz 56 ). Moreover, during physically demanding exercise tasks, hs-CRP, ceruloplasmin and MMP have previously been classified as biomarkers of inflammation. Indeed, the contraction of skeletal muscle after intense physical activity has been shown to stimulate the local production of MMP( Reference Haas, Milkiewicz and Davis 59 ), which play a physiological role in muscle regeneration( Reference Zimowska, Brzoska and Swierczynska 60 ) and adaptation( Reference Carmeli, Moas and Lennon 61 ) to exercise training. Of the several MMP, previous studies have shown that MMP2 (gelatinase A) and MMP9 (gelatinase B) play critical roles in remodelling and regenerating skeletal muscle following exercise( Reference Gohji, Fujimoto and Hara 62 ). Therefore, in this section we will focus on the main results of studies (n 4) which have investigated the effect of POMj supplementation on the acute( Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 , Reference Mazani, Fard and Baghi 27 ) and delayed( Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 , Reference Fuster-Muñoz, Roche and Funes 26 ) responses of inflammatory markers (i.e. leucocytes, IL, CRP or hs-CRP, MM2 and MM9) following intensive exercises (Table 2).
Effect on acute inflammatory responses
In untrained subjects, Trombold et al.( Reference Trombold, Barnes and Critchley 25 ) observed no change in IL-6 and hs-CRP responses in either the PLA or POMj group immediately following exercise. In this study, the absence of inflammatory responses following unilateral eccentric exercise could be explained by the small volume of muscle mass recruited. Indeed, when a similar type of exercise has been completed with a larger muscle mass recruited, previous studies have found increases in systemic IL-6 and hs-CRP( Reference Phillips, Childs and Dreon 63 ) and local inflammation( Reference MacIntyre, Reid and Lyster 64 ) post exercise. Consistent with this interpretation, Mazani et al.( Reference Mazani, Fard and Baghi 27 ) showed a significant increase in inflammatory markers following exhaustive running exercise with higher pre- to post-exercise changes in MMP2, MMP9 and hs-CRP in the PLA group compared to a group receiving 14 d of POMj supplementation. These results indicate that regular intake of POMj before exercise significantly blunts inflammatory responses before and after exhaustive exercise. The acute anti-inflammatory effect of POMj observed in sedentary subjects in the study of Mazani et al.( Reference Mazani, Fard and Baghi 27 ) has been recently confirmed by Ammar et al.( Reference Ammar, Turki and Chtourou 18 ) using resistance trained subjects. Indeed, the consumption of POMj during the 48 h (1500 ml) and the last 1 h (500 ml) before a weightlifting training session, which recruited a large muscle mass, was found to attenuate the increase in hs-CRP post exercise. Collectively, these findings support the anti-inflammatory properties of polyphenol-rich POMj supplementation previously reported in sedentary healthy subjects and patient populations( Reference Adams, Seeram and Aggarwal 9 , Reference Afaq, Malik and Syed 14 , Reference Huang, Yang and Harada 15 , Reference Gohji, Fujimoto and Hara 62 ) and suggest that the beneficial effect of POM is influenced by the volume of skeletal muscle mass recruited during exercise. Although the underlying mechanisms of the anti-inflammatory effects of POM supplementation are not entirely clear, various explanations have been proposed. For example, Ammar et al.( Reference Ammar, Turki and Chtourou 18 ) suggested that the lower post-exercise level of hs-CRP following POMj supplementation could be due to the inhibition of some inflammatory markers such as NF-κB, TNF-α and COX-2. In addition, given that the inhibition of MMP by TNF has been reported to be dependent on reducing ROS production( Reference Ahmed, Wang and Hafeez 65 ), and since polyphenolic-compounds present in POM have been shown to confer antioxidant properties that inhibit ROS production( Reference Aviram, Dornfeld and Rosenblat 53 ), blunted ROS production following POM supplementation might contribute to its anti-inflammatory effects( Reference Mazani, Fard and Baghi 27 ).
Effect on delayed inflammatory responses
In endurance trained athletes, Fuster-Muñoz et al.( Reference Fuster-Muñoz, Roche and Funes 26 ) reported that the levels of sE-selectin and hs-CRP over 22 d following aerobic training sessions was not measurably impacted by the consumption of POMj. Similarly, in resistance trained athletes, Ammar et al.( Reference Ammar, Turki and Chtourou 18 ) observed no effect of POMj on the recovery kinetics of hs-CRP and leucocyte levels 48 h following a weightlifting training session. However, given that hs-CRP better reflects endothelial dysfunction and vascular inflammation than muscular function( Reference Fuster-Muñoz, Roche and Funes 26 ), future studies would benefit from evaluating the effect of POM supplementation on the profiles of cytokines such as TNF or IL-6 which better relate to exercise performance( Reference Kara, Ozal and Gunay 66 ).
Effect of pomegranate juice/pomegranate extract on cardiovascular parameters
It is well established that the demand for oxygen and metabolic substrates increases in the contracting skeletal muscles during physical exercise. To meet these elevated demands, BF to working musculature is increased during exercise( Reference Hellsten, Nyberg and Jensen 37 ). NO production has been shown to be an import contributor to exercise-induced skeletal muscle hyperaemia( Reference Barona, Aristizabal and Blesso 67 ). Polyphenols have also been reported to improve cardiovascular function during stressful situations( Reference Tangney and Rasmussen 68 , Reference Khurana, Venkataraman and Hollingsworth 69 ). Given that polyphenol-rich POMj has been reported to protect NO from oxidative scavenging and to enhance the biological actions of NO( Reference Ignarro, Byrns and Sumi 38 ), the beneficial effect of POMj supplementation on cardiovascular function might be NO-mediated. Although previous studies have investigated the effect of POM supplementation on HR, BF, vessel dilation and cardiovascular function in sedentary subjects( Reference Zarfeshany, Asgary and Javanmard 6 , Reference Aviram, Rosenblat and Gaitini 7 , Reference Sumner, Elliott-Eller and Weidner 12 , Reference Aviram, Dornfeld and Rosenblat 53 , Reference Barona, Aristizabal and Blesso 67 ), a limited numbers of studies have investigated the effect of POM supplementation on these parameters during exercise and to what extent this might contribute to a potential ergogenic effects of POM supplementation. This section will focus on the main findings of studies (n 4) that have investigated how the consumption of POM impacts cardiovascular responses immediately( Reference Ammar, Turki and Chtourou 18 , Reference Trexler, Smith-Ryan and Melvin 32 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Roelofs, Smith-Ryan and Trexler 35 ) and up to 48 h( Reference Ammar, Turki and Chtourou 18 ) following running or strength exercise (Table 2).
Effect on acute cardiovascular responses
To date, two studies have investigated the effect of POM on BF and VD responses immediately following physical exercise( Reference Moher, Liberati and Tetzlaff 28 , Reference Roelofs, Smith-Ryan and Trexler 35 ). In these studies the consumption of 1000 mg of POMe (2×500 mg capsules) 30 min before exercise was reported to increase VD and BF immediately, and up to 30 min, after exhaustive exercise compared to a PLA condition. Indeed, POMe supplementation has been reported to result in a larger VD (0·42(SD0·07) v. 0·39(SD0·07) cm) and higher BF (40·6(SD24·8) v. 29·6(SD24·9) ml/min) 30 min post-POMe ingestion and 30 min post three treadmill runs to exhaustion (at 90, 100 and 110 % PV)( Reference Moher, Liberati and Tetzlaff 28 ). Similarly, POMe ingestion lead to a larger VD immediately following leg press and bench press exercise at 80 % one repetition maximum (1 RM) continued to fatigue (mean difference=0·042 cm for leg press and 0·029 cm for bench press) and 30 min post-leg press (mean difference=0·029)( Reference Roelofs, Smith-Ryan and Trexler 35 ). This beneficial effect of POMe was also observed following a RSA test with higher BF and VD observed respectively immediately and 30 min post exercise following POMe ingestion( Reference Roelofs, Smith-Ryan and Trexler 35 ). Given that NO production is an important contributor to vasodilatation( Reference Hellsten, Nyberg and Jensen 37 ), and that polyphenols have been shown to phosphorylate and thereby activate endothelial NO synthase( Reference Ignarro, Byrns and Sumi 38 , Reference Cockcroft 70 ), the results of Roelofs et al.( Reference Roelofs, Smith-Ryan and Trexler 35 ) could be explained by the high content of polyphenols in the POMe supplement (3500 μmol/l). Moreover, the protective role of POM against ROS-mediated NO scavenging( Reference Ignarro, Byrns and Sumi 38 ) could also explain the enhanced vasodilation following exercise.
Assuming POM supplementation increases BF during exercise, the associated increase in nutrient delivery to, and efflux of noxious metabolic by-products from, skeletal muscle( Reference Hellsten, Nyberg and Jensen 37 , Reference Barona, Aristizabal and Blesso 67 ) might contribute to ergogenic effect of POM supplementation during exercise and the enhanced post-exercise recovery( Reference Trombold, Reinfeld and Casler 17 , Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 , Reference Trexler, Smith-Ryan and Melvin 32 ). In addition to effects on BF and VD( Reference Trexler, Smith-Ryan and Melvin 32 , Reference Roelofs, Smith-Ryan and Trexler 35 ), POM has been reported to lower blood pressure and HR during physical exercise( Reference Ammar, Turki and Chtourou 18 , Reference Tsang, Wood and Al-Dujaili 34 ). Indeed, daily consumption of POMj (500 ml, 1·69 g total phenolic/l) for 1 week before exercise( Reference Tsang, Wood and Al-Dujaili 34 ) was shown to lower the SBP and the diastolic blood pressure (DBP) before and immediately after 30 min of treadmill exercise (50 % W max). Likewise, the consumption of POMj (500 ml) 1 h before the training session was shown to attenuate the acute increase of SBP (−4·46 %) and HR (−1·81 %) immediately (i.e. 3 min) after intense weightlifting exercise( Reference Ammar, Turki and Chtourou 18 ). The reduction in post-exercise blood pressure and HR with POMj, if also observed during exercise, implies that POMj supplementation might improve performance and lower the perception of fatigue( Reference Trombold, Reinfeld and Casler 17 , Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 ) by improving aspects of cardiovascular function.
Effect on delayed cardiovascular responses
The effect of POM on the delayed recovery of cardiovascular responses following exercise is currently unclear. Indeed, only one study has examined SBP and HR responses 48 h post exercise after POMj supplementation( Reference Ammar, Turki and Chtourou 18 ). To our knowledge, the delayed responses of BF, VD and DBP have yet to be assessed. The consumption of POMj before an intensive weightlifting training session improved the recovery kinetics of SBP 48 h post-exercise in elite weightlifters( Reference Ammar, Turki and Chtourou 18 ). Given that the reduction of SBP following POMj has been linked to a reduction in the cortisol:cortisone ratio( Reference Tsang, Wood and Al-Dujaili 34 ), the beneficial effect of POMj on SBP during exercise could be the result of 11β-hydroxysteroid dehydrogenase type 1 inhibition( Reference Tsang, Wood and Al-Dujaili 34 ). However, further studies are necessary to resolve the mechanisms for the improved cardiovascular function following POM supplementation.
Discussion
This systematic review evaluated the existing literature assessing the effect of POM supplementation on physical performance, muscle soreness and physiological responses during and following different exercise sessions. Based on the studies assessed in this review, POM supplementation appears to hold potential as a nutritional aid to enhance performance during endurance( Reference Moher, Liberati and Tetzlaff 28 , Reference Roelofs, Smith-Ryan and Trexler 35 ) and strength( Reference Trombold, Reinfeld and Casler 17 , Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 , Reference Machin, Christmas and Chou 33 ) exercise, and to expedite enhanced post exercise recovery of skeletal muscle function( Reference Trombold, Reinfeld and Casler 17 , Reference Trombold, Barnes and Critchley 25 , Reference Machin, Christmas and Chou 33 ). These improvements in exercise performance and recovery have been linked to an attenuation of muscle damage( Reference Ammar, Turki and Chtourou 18 ); lowered oxidative stress( Reference Ammar, Turki and Hammouda 19 , Reference Fuster-Muñoz, Roche and Funes 26 , Reference Mazani, Fard and Baghi 27 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ) and inflammation( Reference Ammar, Turki and Chtourou 18 , Reference Mazani, Fard and Baghi 27 ); and enhanced cardiovascular function( Reference Ammar, Turki and Chtourou 18 , Reference Moher, Liberati and Tetzlaff 28 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ). This review has potential implications for improving the use of POM supplementation by athletes, nutritionists and coaches to enhance exercise performance and post-exercise recovery.
Dietary supplementation with POM has shown promising potential to enhance physiological responses in sedentary individuals and patient populations under conditions of physiological strain( Reference Aviram, Rosenblat and Gaitini 7 – Reference Ammar, Chtourou and Turki 11 ). Since physical exercise is a potent and multifaceted physiological stressor, as evidenced by an increase in muscle damage, oxidative stress, inflammation and cardiovascular strain( Reference Ammar, Chtourou and Trabelsi 20 – Reference Trombold, Barnes and Critchley 25 ), a number of recent studies have examined the potential for POM supplementation to enhance exercise performance and post exercise recovery. When the existing literature was systematically reviewed in the current study, POM was shown to enhance performance and alleviate muscle fatigue and soreness using intermittent running( Reference Moher, Liberati and Tetzlaff 28 , Reference Roelofs, Smith-Ryan and Trexler 35 ) and strength exercises( Reference Trombold, Reinfeld and Casler 17 , Reference Ammar, Turki and Chtourou 18 , Reference Trombold, Barnes and Critchley 25 , Reference Machin, Christmas and Chou 33 ); to blunt muscle damage following weightlifting exercises( Reference Ammar, Turki and Chtourou 18 ); to promote an antioxidant effect following exhaustive strength exercises( Reference Ammar, Turki and Hammouda 19 ) treadmill running( Reference Mazani, Fard and Baghi 27 , Reference Tsang, Wood and Al-Dujaili 34 ) and ultra-endurance exercise( Reference Fuster-Muñoz, Roche and Funes 26 , Reference Naghizadeh-Baghi, Mazani and Shadman-Fard 36 ); to confer an anti-inflammatory effect during exhaustive running exercise( Reference Mazani, Fard and Baghi 27 ); and to promote beneficial effects on the cardiovascular system during strength( Reference Ammar, Turki and Chtourou 18 , Reference Roelofs, Smith-Ryan and Trexler 35 ) and treadmill running exercise( Reference Moher, Liberati and Tetzlaff 28 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Roelofs, Smith-Ryan and Trexler 35 ).
The positive effects of POM supplementation are likely linked to its high content of polyphenols. Previous studies investigating the effect of polyphenol supplementation have reported increases in BF, vessel dilation( Reference Barona, Aristizabal and Blesso 67 ) and endothelial function( Reference García-Lafuente, Guillamón and Villares 71 ). This potential for enhanced vasodilation following polyphenol supplementation could improve nutrient delivery to and promote the efflux of noxious metabolic by-products from skeletal muscle which might have implications for accelerating muscle recovery( Reference Moher, Liberati and Tetzlaff 28 , Reference Hellsten, Nyberg and Jensen 37 ). In addition to enhanced cardiovascular function, polyphenol supplementation protects against the development oxidative stress( Reference Pandey and Rizvi 72 ) and inflammation( Reference García-Lafuente, Guillamón and Villares 71 ). Accordingly, POM supplementation might aid exercise performance and recovery by enhancing cardiovascular function and mitigating oxidative stress and inflammation. In particular, the ergogenic and recuperative effects of POM supplementation might be linked to the scavenging of free radicals( Reference Perron and Brumaghim 73 ). Specifically, polyphenols can attenuate oxidative damage through the rapid donation of an electronto a free radical from −OH groups( Reference Nijveldt, van Nood and van Hoorn 74 , Reference Castañeda-Ovando, Pacheco-Hernández and PáezHernández 75 ). Therefore, polyphenols are capable of reducing, stabilising and inactivating free radicals species, thereby inhibiting lipid peroxidation and preventing against atherosclerosis and long-lasting Ca2+ release events( Reference Kerry and Abbey 76 , Reference Shirokova, Kang and Fernandez-Tenorio 77 ). Furthermore, modulating antioxidant enzymes and chelating metal ions (Fe2+, Cu2+; involved in free radical production), and the associated blunting of free radical production, are reported to be among the most important mechanisms mediating the protective effect of polyphenol-rich foods( Reference Korkina and Afanas'ev 78 , Reference Fraga, Galleano and Verstraeten 79 ). Other possible mechanisms by which polyphenol-rich supplements exert their beneficial effects are thought to include the inhibition of leucocyte immobilisation and xanthine oxidase activity( Reference Nijveldt, van Nood and van Hoorn 74 ); enhanced endothelial and mitochondrial function( Reference Scalbert, Manach and Morand 80 ); and the recycling of antioxidant and reducing agents to boost antioxidant defence systems (e.g. vitamins E and C)( Reference Perron and Brumaghim 73 , Reference Fraga, Galleano and Verstraeten 79 ).
The potential significance of polyphenols in mediating the positive physiological effects of POM supplementation is supported by observations that the variable polyphenol content of the POM supplements administered and the daily dose of POM consumed (presented in Tables 1 and 2) might influence the inter-study disparity in the efficacy of POM supplementation. For example, the consumption of natural POMj containing 2·56 g total polyphenols/0·5 litre three times per d (3×250 ml) during the 48 h period prior exercise has been reported to confer anti-damaging effects (i.e. acute and delayed) in responses to intense weightlifting exercise( Reference Ammar, Turki and Chtourou 18 ). Conversely, the consumption of a commercially produced POM (Wonderful Bottle) that contained only 0·65 g total polyphenols/0·5 litre two times per d (2×250 ml), did not influence muscle damage following unilateral eccentric exercise( Reference Trombold, Barnes and Critchley 25 ). These results imply that 750 ml of polyphenol-rich POMj (>0·7 g/0·5 litre) could be an important dosing threshold for POMj supplementation to confer anti-damaging effects during exercise. Similarly, the nature of exercise was also identified as an important mediator of the positive physiological effects of POM. Indeed, based on the existing evidence it appears that despite the enhanced physiological strain (large degree of muscle, cellular and oxidative damage) with eccentric exercise( Reference Camus, Felekidis and Pincemail 81 – Reference Clarkson and Hubal 84 ) and the potential for POM to attenuate this, benefits of POM supplementation were minimised following eccentric exercise compared to weightlifting one. Nevertheless, it should be acknowledged that many other factors could underlie the disparate inter-study results including the training status of the subjects (untrained v. trained), the type of exercise assessed (unilateral eccentric, weightlifting and running treadmill), and the duration of the investigation (30 min, 48 h, >1 week). Therefore, standardising these factors in future studies is important to resolve the potential efficacy of POM supplementation to enhance exercise performance, physiology and recovery and to optimise recommendations for best practice with POM supplementation.
Although consumption of polyphenol-rich beverages (e.g. polyphenols specific to POM, including flavonols, ellagitannins and anthocyanins) can modulate oxidative stress, muscle damage, inflammation and improve cardiovascular function and exercise recovery and performance( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 , Reference Tsang, Wood and Al-Dujaili 34 , Reference Barona, Aristizabal and Blesso 67 , Reference Tangney and Rasmussen 68 , Reference Perron and Brumaghim 73 ), it has been reported that a long-term (8 weeks) consumption of the polyphenol, trans-resveratrol (250 mg/d), can blunt the beneficial effects of exercise on the lowering blood pressure, and blood concentrations of several cardiovascular risk factors in elderly men( Reference Gliemann, Schmidt and Olesen 85 ). While the exact mechanism mediating the absence of a potential complementary synergy between exercise and resveratrol was not addressed in this study( Reference Gliemann, Schmidt and Olesen 85 ), the authors suggested that enhanced antioxidant defence in the resveratrol group may have retarded the exercise-induced increase in maximal oxygen uptake by abrogating ROS( Reference Baar 86 , Reference Radak, Chung and Koltai 87 ) which are now recognised as important signalling molecules that contribute to the adaptations to exercise training( Reference Jackson 88 ). Taken together, the results of the available studies indicate that, while the powerful antioxidant effect of polyphenols can blunt redox perturbation and muscle damage, and accelerate the recovery of skeletal muscle force production post-strenuous exercise in the short term, the long-term effects of continuous polyphenol supplementation and the accompanying antioxidant effect could disrupt some of the physiological adaptations elicited by a training program. These findings suggest a balance exists between the beneficial and undesirable effects of polyphenol supplementation which requires consideration in future research. Specifically, it is unclear whether the polyphenol blend that comprises POM promotes a similar blunting in exercise training adaptations as the polyphenol, resveratrol and what supplementation strategy with POM might optimise the balance between promoting recovery from specific training sessions without attenuating the exercise-induced redox signalling that provokes the physiological adaptations to exercise training. This requires addressing in future studies to optimise POMj supplementation guidelines.
Comparison between the effect of pomegranate and other nutritional interventions during exercise
It has been well established in sedentary individuals that POM possesses a higher antioxidant capacity compared to other supplement such as red wine, blueberry juice, cranberry juice, orange juice, green tea and wine vinegars( Reference Seeram, Aviram and Zhang 16 , Reference Gil, Tomás-Barberán and Hess-Pierce 51 , Reference Budak and Guzel-Seydim 89 ). Similarly, POM supplementation has shown potential to enhance muscle performance as evidenced by reduced DOMS, muscle damage, oxidative stress and inflammation, and improved cardiovascular responses during and following exercise( Reference Trombold, Reinfeld and Casler 17 – Reference Ammar, Turki and Hammouda 19 , Reference Trombold, Barnes and Critchley 25 – Reference Mazani, Fard and Baghi 27 , Reference Trexler, Smith-Ryan and Melvin 32 ). Nevertheless, it should be acknowledged that other dietary supplementation strategies have also exhibited similar ergogenic and protective effects during exercise. Conversely, supplementation with vitamin C or E does not influence strength performance and soreness post exercise( Reference Beaton, Allan and Tarnopolsky 90 ). Although dietary supplementation with acombination of tocopherols, flavonoids (i.e. hesperetin and quercetin), Se or DHA( Reference Phillips, Childs and Dreon 63 ), and the mixture of ascorbic acid, α-tocopherol, and Se( Reference Goldfarb, Bloomer and McKenzie 91 ), can attenuate systematic inflammation (CRP and IL-6) and oxidative stress after eccentric exercise, the effect of this nutrient combination on strength performance and DOMS has yet to be assessed. On the other hand, polyphenols specific to POM, including flavonols, ellagitannins and anthocyanins have demonstrated a positive effect on endothelial-dependent vasodilation, and importantly, this effect is greater than achieved with other fruits containing a different mix of polyphenols( Reference Chong, Macdonald and Lovegrove 92 ). Polyphenol supplementation from tart cherries has been shown to improve strength recovery following a bout of eccentric elbow flexion contractions (i.e. lower strength loss and pain( Reference Connolly, McHugh and Padilla-Zakour 93 )), completion of a marathon (i.e. faster recovery of isometric strength( Reference Howatson, McHugh and Hill 94 )) and prolonged, intermittent shuttle exercise (i.e. faster recovery of performance indices( Reference Bell, Stevenson and Davison 95 )). The enhanced recovery of muscle function after ingesting tart cherries was accompanied by increased TAC, and lower lipid peroxidation (TBARS) and attenuated inflammation markers (IL-6 and CRP)( Reference Howatson, McHugh and Hill 94 , Reference Bell, Stevenson and Davison 95 ). However, no other indices of muscle damage (CK and LDH), or oxidative stress (total lipid hydroperoxide (LOOH) and PC) were different between the PLA and the cherry juice groups( Reference Howatson, McHugh and Hill 94 , Reference Bell, Stevenson and Davison 95 ). In addition, consumption of multi-ingredient performance supplements 30 min before exercise for 8 weeks has been shown to improve bench press strength, lean body mass and quadriceps muscle thickness without impacting leg press strength( Reference Lowery, Joy and Dudeck 96 ). Collectively, these results suggest that POM supplementation could be an effective treatment to improve performance, muscle recovery and to reduce weakness and damage in responses to physical exercise. It also appears that POM supplementation compares favourably with other polyphenol-rich foods with regard to enhancing exercise performance and recovery, but further research is required to directly compare the efficacy of POM to enhance exercise performance and recovery compare to other polyphenol-rich foods.
Methodological considerations
It is important to stress that, while the polyphenol content of POM is positively associated with its protective effect against damage during exercise and with exercise performance( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 ), POM does not exhibit a uniform polyphenol content throughout the fruit( Reference Kalaycıoğlu and Erim 97 ). Indeed, higher levels of polyphenols are present in the inner and outer peels than in the seeds( Reference Aviram, Rosenblat and Gaitini 7 ). These observations underscore the importance of the juice manufacturing method and suggest that POMj/e which contains a mixture of seeds and peels( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 , Reference Fuster-Muñoz, Roche and Funes 26 ) is more likely to be beneficial for enhancing physiological and functional responses during and following physical exercise. Another important consideration that appears to influence the efficacy of POM supplementation is the supplementation regimen employed. Indeed, it has been reported that POM consumed 30 min before exercise improves intermittent capacity without impacting high-intensity anaerobic performance( Reference Trexler, Smith-Ryan and Melvin 32 ), while POM consumed 60 min prior exercise was able to improve high-intensity anaerobic performance as evidenced by increased total and maximal load lifted during weightlifting exercise( Reference Ammar, Turki and Chtourou 18 ). Therefore, it would appear advantageous to consume POM supplements at least 60 min before intensive anaerobic exercise to provide sufficient time to elicit a potential ergogenic effect on both aerobic and anaerobic performance. However, to optimise supplementation guidelines, the dose-response and pharmacokinetics of POM supplementation must be elucidated. Another important consideration for studies wishing to assess the efficacy of POM supplementation is that the beneficial effect of POM supplementation can persist for up to 3 weeks after consumption( Reference Matthaiou, Goutzourelas and Stagos 52 ). Accordingly, when a crossover experimental study design is adopted, the wash out period between supplements should be >3 weeks to avoid any potential confounding influence of the POM supplementation, if administered first, on the second supplementation arm of the study. Concerning the selection of biomarkers, it should be acknowledged that since exercise has been shown to provoke muscle damage, inflammation and oxidative modifications to several biological components( Reference Ammar, Chtourou and Trabelsi 20 – Reference Ammar, Chtourou and Hammouda 24 , Reference Radak, Zhao and Koltai 98 ) and since at least two or more biomarkers has been recommended to accurately infer oxidative, muscle or inflammatory damage( Reference Halliwell and Whiteman 99 , Reference Cobley, Close and Bailey 100 ), future studies should use multiple related biomarkers (e.g. at least: MDA and PC to measure oxidative stress; CK and LDH to measure muscle damage and hs-CRP, IL-6 and TNF to detect inflammation) to confirm the potential positive effects of POM supplementation on blunting exercise-induced oxidative stress, muscle damage or inflammation. Moreover, given that the effects of polyphenol derivatives (flavonols, ellagitannins, anthocyanins and resveratrol) on the biological response and adaptations to exercise training is controversial( Reference Ammar, Turki and Chtourou 18 , Reference Ammar, Turki and Hammouda 19 , Reference Barona, Aristizabal and Blesso 67 , Reference Tangney and Rasmussen 68 , Reference Gliemann, Schmidt and Olesen 85 ), it is also recommended that future studies investigating the potential synergistic or antagonistic link between exercise adaptations and POM supplementation present the exact composition of polyphenols in POM. This information could help elucidate the mechanisms for the synergistic or antagonistic effects of acute and long term POMj supplementation of exercise performance, recovery and adaptation. At last, given that existing findings evidenced the influence of exercise nature on the potential beneficial effect of POM, it should be also acknowledged that further research is required to address the exercise setting in which POM is more likely to be ergogenic.
Conclusion
The review indicates that POM has the potential to enhance endurance and strength performance and to expedite post-exercise recovery by conferring antioxidant and anti-inflammatory effects and improving cardiovascular responses during and following exercise. However, positive effects of POM supplementation are more likely when POMj contains >0·7 g total polyphenols/0·5 litre, when large muscle mass exercise is engaged and when POMj is ingested at least 60 min before exercise. Therefore, the inclusion (750 ml/d) of polyphenol-rich POM in the diet of active people prior (60 min) and after exercise (during 48 h) could be beneficial for their physical performance and muscle recovery during and following the physical tasks. However, further research is required to assess how chronic POM supplementation impacts the physiological and performance adaptations to exercise training to help optimise POM supplementation guidelines for a range of exercise settings.
Acknowledgements
The authors report no financial support/funding for this study and no specific acknowledgement. The authors alone are responsible for the content and writing of the paper.
A. A. drafted the article. S. J. B., H. C., K. T., M. T. and A. H. revised critically the article. N. S. revised and gave final approval. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
On behalf of all authors, the corresponding author states that there is no conflict of interest.








